Autism spectrum disorder (ASD) is a lifelong neuro-developmental disorder with early childhood onset, which influences the individual’s functioning in a variety of ways. ASD is characterized by persistent difficulties in social communication and social interactions across multiple contexts, in addition to the presence of restricted, repetitive patterns of behavior, interests, or activities (DSM-5; American Psychiatric Association, 2013). ASD is among the most common neuro-developmental disorders, with recent findings indicating that more than 2% of children in the general population meet the criteria for ASD (Baio et al., Reference Baio, Wiggins, Christensen, Maenner, Daniels, Warren, Kurzius-Spencer, Zahorodny, Robinson, Rosenberg, White, Durkin, Imm, Nikolaou, Yeargin-Allsopp, Lee, Harrington, Lopez, Fitzgerald, Hewitt, Pettygrove, Constantino, Vehorn, Shenouda, Hall-Lande, Van, Naarden, Braun and Dowling2018; Maenner et al., Reference Maenner, Shaw, Bakian, Bilder, Durkin, Esler, Furnier, Hallas, Hall-Lande, Hudson, Hughes, Patrick, Pierce, Poynter, Salinas, Shenouda, Vehorn, Warren, Constantino, DiRienzo, Fitzgerald, Grzybowski, Spivey, Pettygrove, Zahorodny, Ali, Andrews, Baroud, Gutierrez, Hewitt, Lee, Lopez, Mancilla, McArthur, Schwenk, Washington, Williams and Cogswell2021). Although ASD symptoms can start manifesting during the first year of life, diagnosis is not typically given before children reach the second year of life, by which time better communicative abilities are expected to be shown (Ozonoff et al., Reference Ozonoff, Iosif, Baguio, Cook, Hill, Hutman, Rogers, Rozga, Sangha, Sigman, Steinfeld and Young2010; Webb & Jones, Reference Webb and Jones2009). Currently, mean age of diagnosis is around 3–4 years, and in many cases even later (Clifford et al., Reference Clifford, Hudry, Elsabbagh, Charman and Johnson2013; van ’t Hof et al., Reference van ’t Hof, van Berckelear-Onnes, van Nieuwenhuyzen, Daniels, Deen, Hoek and Ester2021).
Extensive intervention, administered as early as possible, has the potential to reduce autistic symptoms and improve children’s functioning (Green et al., Reference Green, Charman, Pickles, Wan, Elsabbagh, Slonims, Taylor, McNally, Booth, Gliga, Jones, Harrop, Bedford and Johnson2015; Zwaigenbaum et al., Reference Zwaigenbaum, Bauman, Choueiri, Kasari, Carter, Granpeesheh, Mailloux, Smith Roley, Wagner, Fein, Pierce, Buie, Davis, Newschaffer, Robins, Wetherby, Stone, Yirmiya, Estes, Hansen, McPartland and Natowicz2015). Thus, there is interest in identifying young infants who are at elevated risk of developing ASD, in order to provide them with such early care. Many studies have focused on identifying early prodromal markers of ASD, that is, indicators of the disorder that are evident before the full symptoms are manifested. The present paper adds to this endeavor, by examining whether infants’ empathic responses to others’ distress and joy, long before autism is typically diagnosed, could predict subsequent diagnosis of the disorder.
Prodromal signs of ASD diagnosis
The preferable methodology for identifying early diagnostic markers of ASD is a longitudinal prospective design, in which infants are followed from an early age until diagnosis age. Such studies are less vulnerable to hindsight bias than retrospective designs, in which information about the child’s early development is sought only after ASD diagnosis has already been given (Ozonoff & Iosif, Reference Ozonoff and Iosif2019; Yirmiya & Charman, Reference Yirmiya and Charman2010). Prospective studies typically follow infants who have at least one older sibling diagnosed with ASD, and a matched control group. Because ASD is highly hereditable (Bailey et al., Reference Bailey, Le Couteur, Gottesman, Bolton, Simonoff, Yuzda and Rutter1995), infants whose older sibling has autism have a substantially higher probability of being diagnosed with ASD, as well as with other neurodevelopmental difficulties, compared to infants in the general population (Jokiranta-Olkoniemi et al., Reference Jokiranta-Olkoniemi, Cheslack-Postava, Sucksdorff, Suominen, Gyllenberg, Chudal, Leivonen, Gissler, Brown and Sourander2016; Ozonoff et al., Reference Ozonoff, Young, Carter, Messinger, Yirmiya, Zwaigenbaum, Bryson, Carver, Constantino, Dobkins, Hutman, Iverson, Landa, Rogers, Sigman and Stone2011)
Many prospective sibling studies have found behavioral differences between children who were later diagnosed, versus not diagnosed, with ASD (Canu et al., Reference Canu, Van der Paelt, Canal-Bedia, Posada, Vanvuchelen and Roeyers2021; Jones et al., Reference Jones, Gliga, Bedford, Charman and Johnson2014; Szatmari et al., Reference Szatmari, Chawarska, Dawson, Georgiades, Landa, Lord, Messinger, Thurm and Halladay2016). Importantly, most of the prospective studies that identified early markers during the first year of life, and especially during the first 6 months of life, have used neuroscience methods (e.g., EEG, MRI, DTI; Wolf et al., Reference Wolf, Freisthler and Chadwick2021) or eye-tracking technology to measure subtle differences in infants’ attention patterns (Bedford et al., Reference Bedford, Gliga, Shephard, Elsabbagh, Pickles, Charman and Johnson2017; Bussu et al., Reference Bussu, Jones, Charman, Johnson and Buitelaar2018; Chawarska et al., Reference Chawarska, Macari and Shic2013; Jones & Klin, Reference Jones and Klin2013; Shic et al., Reference Shic, Macari and Chawarska2014; Varcin & Jeste, Reference Varcin and Jeste2017; Wass et al., Reference Wass, Jones, Gliga, Smith, Charman, Johnson, Baron-Cohen, Bedford, Bolton, Chandler, Davies, Fernandes, Garwood, Hudry, Maris, Pasco, Pickles, Ribiero, Tucker and Volein2015). In comparison, prospective studies that examined simpler behavioral markers of reduced social communication, that is, markers that can be observed without specialized equipment, have generated a more complex pattern of findings. Many of these studies have identified predictive markers of autism– including reduced levels of eye contact, imitation, joint attention, or response to name – but only during the second year of life, that is, from the age of 12 months onward, but not prior (Franchini et al., Reference Franchini, Hamodat, Armstrong, Sacrey, Brian, Bryson, Garon, Roberts, Zwaigenbaum and Smith2019; Jones et al., Reference Jones, Gliga, Bedford, Charman and Johnson2014; Nadig et al., Reference Nadig, Ozonoff, Young, Rozga, Sigman and Rogers2007; Rozga et al., Reference Rozga, Hutman, Young, Rogers, Ozonoff, Dapretto and Sigman2011; Zwaigenbaum et al., Reference Zwaigenbaum, Bryson, Rogers, Roberts, Brian and Szatmari2005; Varcin & Jeste, Reference Varcin and Jeste2017). More recently, a few studies have also found significant predictive indicators at 9 months (lower levels of orientation to name, imitation, or other-directed vocalization; Ibañez et al., Reference Ibañez, Grantz and Messinger2013; Miller et al., Reference Miller, Iosif, Hill, Young, Schwichtenberg and Ozonoff2017; Sacrey et al., Reference Sacrey, Zwaigenbaum, Bryson, Brian, Smith, Roberts, Szatmari, Vaillancourt, Roncadin and Garon2020). In addition, earlier markers of subsequent ASD, from age 6 months or prior, were reported in some studies that used parental reports of infants’ social, emotional, and behavioral responses (e.g., Del Rosario et al., Reference Del Rosario, Gillespie-Lynch, Johnson, Sigman and Hutman2014; Feldman et al., Reference Feldman, Ward, Savona, Regehr, Parker, Hudson, Penning and Holden2012), and occasionally in studies that observed non-social behaviors, such as motor skills or learning (e.g., Canu et al., Reference Canu, Van der Paelt, Canal-Bedia, Posada, Vanvuchelen and Roeyers2021; Flanagan et al., Reference Flanagan, Landa, Bhat and Bauman2012).
Therefore, it is of continued interest to try and identify very early prodromal signs, particularly those that can be assessed in the community without the use of specialized equipment. The current study addressed this goal, by examining empathic responding to others’ distress and to others’ happiness during the first year of life (6–12 months) as potential behavioral markers of subsequent ASD diagnosis. Moreover, beyond the potential clinical utility of empathy as an early marker of ASD, a focus on empathy is also conceptually warranted. Thus, because empathy difficulties are ubiquitous in ASD (see next section for review), prospectively studying differences in early empathic responses can advance understanding of the nature of autism and its developmental progression.
Empathy and ASD
Empathy – the ability to feel what another is feeling – is an important socio-emotional skill, promoting adaptive social functioning (e.g., Eisenberg et al., Reference Eisenberg, Fabes, Spinrad, Damon and Eisenberg2006). Empathic feelings can be evoked by various emotions, both negative and positive (Light et al., Reference Light, Coan, Zahn-Waxler, Frye, Goldsmith and Davidson2009). However, most of the literature on empathy to date—in typically developing samples, as well as at-risk samples—focuses on empathy in response to others’ distress (Decety & Meyer, Reference Decety and Meyer2008; Eisenberg et al., Reference Eisenberg, Fabes, Spinrad, Damon and Eisenberg2006; Knafo et al., Reference Knafo, Zahn-Waxler, Van Hulle, Robinson and Rhee2008).
The empathic response includes two components – cognitive and affective (e.g., Knafo et al., Reference Knafo, Zahn-Waxler, Van Hulle, Robinson and Rhee2008). The former refers to cognitively comprehending what the other person is experiencing, for example, understanding that the other is in pain, sad, or happy. The affective component, which was the focus of this work, refers to the emotional response evoked in the observer, when exposed to the other person’s emotional state; of particular interest are other-oriented emotional reactions, felt on behalf of, or for the other person. Thus, if another person is distressed, affective empathy, known as ‘empathic concern’ (or sympathy) refers to caring about and having tender feeling on behalf of the other; and when the other is happy, affective empathy refers to feeling happy for the other and participating in their joy, known as ‘empathic happiness’ or ‘positive empathy’ (Light et al., Reference Light, Coan, Zahn-Waxler, Frye, Goldsmith and Davidson2009; Morelli et al., Reference Morelli, Lieberman and Zaki2015).
Impairments in empathy to distress are frequent among individuals with ASD, including young children, and some researchers refer to deficits in empathy for others in distress as one of the core features of ASD (Baron-Cohen, Reference Baron-Cohen2009; Gillberg, Reference Gillberg1992; Harmsen, Reference Harmsen2019). Importantly, young children with ASD can certainly show empathy. For example, 3–5 year-olds with ASD showed greater interest and concern toward a distressed person (an experimenter simulating pain) than toward an emotionally neutral stimulus (Corona et al., Reference Corona, Dissanayake, Arbelle, Wellington and Sigman1998). Nevertheless, young children with ASD usually show less intense empathic responses to simulations of distress by the parent or experimenter, compared to typically developing children of the same age (chronological or mental), as well as compared to children with other developmental delays (Butean et al., Reference Butean, Costescu and Dobrean2014; Corona et al., Reference Corona, Dissanayake, Arbelle, Wellington and Sigman1998; Sigman et al., Reference Sigman, Kasari, Kwon and Yirmiya1992). These findings have also been replicated with toddlers (ages 20–24 months), in their responses to experimenter/parent distress simulations or to a realistic crying doll (Campbell et al., Reference Campbell, Leezenbaum, Schmidt, Day and Brownell2015; Charman et al., Reference Charman, Swettenham, Baron-Cohen, Cox, Baird and Drew1997; McDonald & Messinger, Reference McDonald and Messinger2012).
As noted, there is a dearth of prior work in general on empathy for others’ positive emotions (Light et al., Reference Light, Coan, Zahn-Waxler, Frye, Goldsmith and Davidson2009). Nevertheless, some work exists regarding a basic form of positive empathy – contagious positive emotion. The latter denotes instances in which perceiving the happiness of the other evokes positive emotion (smiling, laughter) in the observer. Contagious positive emotion is seen as an empathic response because the emotion of the other vicariously evokes a similar emotion in the perceiver (even though it was not directed at the perceiver). However, this is a rudimentary empathic response, because the perceiver’s positive affect is not necessarily other-oriented in nature, that is, the observer is happy because of the other’s happiness, but is not necessarily happy for the other. Pertinent to the current investigation, some studies found that older children and adolescents with ASD show lower levels of emotional contagion when exposed to a stranger’s laughter compared to typically developing controls (Helt et al., Reference Helt, Fein and Vargas2019, Reference Helt, de Marchena, Schineller, Kirk, Scheub and Sorensen2021; Scambler et al., Reference Scambler, Hepburn, Rutherford, Wehner and Rogers2007). However, other work suggests that adolescents with ASD can share in others’ positive emotions to the same degree as typically developing controls, evidencing reduced emotional empathy only for others’ negative emotions (Mazza et al., Reference Mazza, Pino, Mariano, Tempesta, Ferrara, Berardis, Masedu and Valenti2014).
Studies have also found negative associations between autism and the cognitive components of empathy, such as perspective taking and emotional knowledge. Thus, children and adults with ASD typically struggle with theory of mind (ToM) and false belief tasks, which require taking the perspective of another person and examining the situation from their viewpoint (Baron-Cohen et al., Reference Baron-Cohen, Leslie and Frith1985, Reference Baron-Cohen, Wheelwright, Hill, Raste and Plumb2001). Similarly, high-functioning children with ASD had difficulties with emotion recognition and labeling tasks, compared to typically developing children with matching mental age and verbal abilities (Yirmiya et al., Reference Yirmiya, Sigman, Kasari and Mundy1992). These difficulties with cognitive empathy pertain to both negative and positive emotions (Mazza et al., Reference Mazza, Pino, Mariano, Tempesta, Ferrara, Berardis, Masedu and Valenti2014).
Overall, these findings raise the likelihood that decreased empathic responses in infancy may constitute useful prodromal signs of ASD development. To date, only one prospective study examined empathic responses in infancy as potential prodromal signs of ASD (Hutman et al., Reference Hutman, Rozga, Delaurentis, Barnwell, Sugar and Sigman2010). This study followed siblings of children diagnosed with ASD from 12 to 36 months of age and examined their responses to experimenter’s simulation of distress. Infants subsequently diagnosed with ASD showed weaker attention and affective reactions at 12 and 18 months (both other-oriented empathic concern and self-oriented responses, like self-distress and seeking comfort from the parent), compared to infants who were not diagnosed with ASD (low-risk infants from the control group or non-ASD infants from the siblings group). In addition, although the infants who subsequently developed ASD did shift their attention from the toys to the distressed person; the increase in their social interest as a result of the distress simulation was limited, compared to that shown by the typically developing children (but not different from that of children with other developmental concerns), and they continued to be engaged with toys to a greater extent (Hutman et al., Reference Hutman, Chela, Gillespie-Lynch and Sigman2012). Interestingly, there was no significant difference in the amount of social interest demonstrated by the infants later diagnosed with ASD compared to typically developing infants during the free play period which preceded the distress, underscoring the uniqueness of the distress situation.
The present study sought to replicate and extend some of the findings by Hutman et al., (Reference Hutman, Rozga, Delaurentis, Barnwell, Sugar and Sigman2010, Reference Hutman, Chela, Gillespie-Lynch and Sigman2012), by examining infants’ responses to both the distress and the happiness of others, at even younger ages – during the first year of life.
Empathy in the first year of life
Despite the links between empathy and autism reviewed above, the associations between individual differences in empathy during the first year of life and a subsequent ASD diagnosis have never been investigated. This is likely because until recently, it has been generally assumed that infants are incapable of empathy for others during the first year of life (e.g., Hoffman, Reference Hoffman1984, Reference Hoffman2000). More specifically, it has been thought that prior to the second year, infants are only capable of responding to another’s distress with self-focused distress (e.g., crying). However, this view has been challenged on both theoretical and empirical grounds (Davidov et al., Reference Davidov, Zahn-Waxler, Roth-Hanania and Knafo2013).
Specifically, the assumption that young infants cannot distinguish between the distress of others and that of the self (Hoffman, Reference Hoffman2000) was called into question in light of extensive evidence that infants can implicitly differentiate between self and other from birth, and perhaps even earlier (Castiello et al., Reference Castiello, Becchio, Zoia, Nelini, Sartori, Blason, D’Ottavio, Bulgheroni and Gallese2010; Dondi et al., Reference Dondi, Simion and Caltran1999; Rochat & Hespos, Reference Rochat and Hespos1997); such implicit self-other distinction is hypothesized to enable empathic concern for a distressed other, so long as the others’ distress is expressed clearly and in a manner suitable to the infant’s regulatory abilities (Davidov et al., Reference Davidov, Zahn-Waxler, Roth-Hanania and Knafo2013). Supporting this alternative view of early empathy development, there is growing evidence that expressions of empathic concern can already be seen during the first year of life (Abramson et al., Reference Abramson, Paz, Knafo-Noam and Knafo-Noam2019; Davidov et al., Reference Davidov, Paz, Roth-Hanania, Uzefovsky, Orlitsky, Mankuta and Zahn-Waxler2021; Liddle et al., Reference Liddle, Bradley and Mcgrath2015; Roth-Hanania et al., Reference Roth-Hanania, Davidov and Zahn-Waxler2011). Davidov and colleagues followed a large sample of typically developing infants from age 3 to 18 months and systematically assessed their empathic responses to others’ distress (Davidov et al., Reference Davidov, Paz, Roth-Hanania, Uzefovsky, Orlitsky, Mankuta and Zahn-Waxler2021). Empathic responses, in the form of concerned affect and exploration of the other’s distress, were observed as early as 3 months, and showed moderate consistency across situations and age. Moreover, empathic responses to distress during the first year of life predicted subsequent prosocial behavior, greater social competence, and reduced aggression at 18 and 36 months (Davidov et al., Reference Davidov, Paz, Roth-Hanania, Uzefovsky, Orlitsky, Mankuta and Zahn-Waxler2021; Paz et al., Reference Paz, Orlitsky, Roth-Hanania, Zahn-Waxler and Davidov2021, Reference Paz, Davidov, Orlitsky, Roth-Hanania and Zahn-Waxler2022).
As for empathy for others’ joy, typically developing infants have also been shown to participate in others’ happiness at ages 5 and 10 months (Jordan & Thomas, Reference Jordan and Thomas2017). The infants exhibited greater smiling in response to positive emotional stimuli than to neutral stimuli. Another study found that infants can participate in others’ joy by showing contagious positive affect from the age of 3 months, a response that is moderately consistent across situations and age (Davidov et al., Reference Davidov, Paz, Orlitsky, Roth-Hanania, Uzefovsky, Mankuta and Zahn-Waxlerin preparation).
Taken together, these findings point to the early appearance of empathic responses in low-risk samples. They therefore highlight the utility of targeting early empathic responses, during the first year of life, as potential prodromal signs of ASD, a condition marked by impaired empathy.
The present study
This study examined whether early empathy for others’ distress and happiness from 6–12 months can predict a later ASD diagnosis. The question was examined in a sample of high-risk infants, siblings of children diagnosed with ASD, and a low-risk control group. Based on the literature reviewed above, we hypothesized that infants later diagnosed with ASD will show lower empathic concern for others in distress at 6–12 months, compared to infants not subsequently diagnosed with ASD (both low-risk infants and non-ASD siblings). Given the consistency of early individual differences in empathic responses (Davidov et al., Reference Davidov, Paz, Roth-Hanania, Uzefovsky, Orlitsky, Mankuta and Zahn-Waxler2021), we hypothesized that reduced empathic reactions to others in distress, observed from 6 to 12 months, would predict subsequent ASD diagnosis. Due to the scarce and inconsistent literature regarding empathic happiness, we did not have a priori hypothesis as to whether infants’ vicarious affective responses to others’ joy would predict a subsequent ASD diagnosis.
The study examined children’s responses to others’ expressions of distress and joy presented in two different mediums: in-person simulations and video recordings. Studies examining empathy development in typically developing infants found that empathic responses to video stimuli and in-person simulations typically converge (Davidov et al., Reference Davidov, Paz, Roth-Hanania, Uzefovsky, Orlitsky, Mankuta and Zahn-Waxler2021, Reference Davidov, Paz, Orlitsky, Roth-Hanania, Uzefovsky, Mankuta and Zahn-Waxlerin preparation). However, previous studies examining the links between empathic responses and ASD diagnosis used mainly simulations by parent/experimenter (e.g., Hutman et al., Reference Hutman, Rozga, Delaurentis, Barnwell, Sugar and Sigman2010; Sigman et al., Reference Sigman, Kasari, Kwon and Yirmiya1992) or a realistic substitute (Campbell et al., Reference Campbell, Leezenbaum, Schmidt, Day and Brownell2015), but not reactions to filmed emotional expressions.
Moreover, preoccupation with screens and media is common among older children on the autistic spectrum (Mazurek et al., Reference Mazurek, Shattuck, Wagner and Cooper2012; Montes, Reference Montes2016); if such preference is already present in infancy then it might affect the results. For example, if infants later diagnosed with ASD are selectively drawn to video stimuli, then they may not show reduced empathy in response to filmed distress/joy compared to their typically developing counterparts. At the same time, ASD studies examining the cognitive aspect of empathy often used computerized tasks and nevertheless found differences in the performance of ASD children (Golan et al., Reference Golan, Baron-Cohen and Golan2008; Yirmiya et al., Reference Yirmiya, Sigman, Kasari and Mundy1992). Therefore, our statistical models included the target of the child’s empathic response (an actual person performing a simulation in front of the child, or a filmed expression of emotion observed on a screen); we had no a priori hypothesis regarding this variable.
Finally, because the early detection of infants at elevated risk of ASD is essential in order to provide them with early suitable care, we also examined the predictive utility of empathy measures assessed only in the earliest time-point of the study. Specifically, we explored whether any empathy measures assessed at 6 months can significantly improve the prediction of subsequent ASD diagnosis, above and beyond known preexisting risk factors (familial risk and male gender), and the sensitivity and accuracy of such prediction.
Methods
Participants
The sample consisted of 60 Israeli infants: 39 high-risk infants (33% girls) and 20 low-risk infants (35% girls), recruited when infants were 6 months old, and one additional infant originally recruited for a different study. In the high-risk group (HR), infants had at least one older sibling diagnosed with ASD. The low-risk infants (LR) all had at least one older sibling and no family history of autism. The additional infant was recruited as part of another study examining empathy development in infancy (Davidov et al., Reference Davidov, Paz, Roth-Hanania, Uzefovsky, Orlitsky, Mankuta and Zahn-Waxler2021, Reference Davidov, Paz, Orlitsky, Roth-Hanania, Uzefovsky, Mankuta and Zahn-Waxlerin preparation) and was later diagnosed with ASD; his data was transferred, with parental consent, to the current project (he did not have older siblings). One infant (a high-risk boy) passed away from Sudden Infant Death Syndrome (SIDS) several weeks after the first meeting at 6 months; his data was therefore excluded from analysis, resulting in a final sample of N = 59 (with 38 high-risk children).
The low-risk group was matched to the high-risk group in gender, χ 2(2) = 0.53, p = .769, and family income, t(57) = 0.42, p = .678. As well, in both groups most of the mothers were married (HR: 97%; LR: 95%), Jewish (HR: 100%; LR: 95%) and were born in Israel (HR: 62%; LR: 90%, χ 2(1) = 3.80, p = .051). The sample was predominantly of middle to upper-middle class socio-economic background (38% of the families belonged to the 70th–100th income percentiles, 52% were in the 30th–70th income percentiles, and 10% were in the 0th–30th percentiles, according to Israel Central Bureau of Statistics, 2017).
The high-risk infants were recruited via social media, forums related to ASD, through advertisements at the child developmental center of Sheba Academic Medical Center in Israel, and through word of mouth. The low-risk sample was recruited via social media and word of mouth. The study received ethics approval from the Sheba Academic Medical Center Hospital’s Helsinki committee, the Hebrew University of Jerusalem IRB, and Tel Aviv-Yaffo Academic College IRB.
Procedure
Only the procedures and measures relevant to the current paper are detailed below. Measures of infants’ empathy and early development were administered by a trained experimenter (the first author) at three time points during the first year of life, when infants were 6 months (N = 60, M = 6.45, SD = .54), 9 months (N = 56, 93%, M = 9.38, SD = .33), and 12 months of age (N = 59, 98%, M = 12.44, SD = .40). These included three tasks assessing empathy for others’ distress and two tasks assessing empathy for others’ happiness (see below). Infants’ responses were filmed for subsequent coding. The tasks were not counterbalanced, but rather administered in a standard order, designed to streamline the procedure and maximize usable data (e.g., more stressful tasks were interspersed with easier ones; see Supplementary Material for the order of the tasks).
The 6 months meeting was a lab visit, held at one of three possible locations, depending on the family’s preference (the Sheba Academic Medical Center Hospital’s Helsinki, the Hebrew University of Jerusalem, or Tel Aviv-Yaffo Academic College). At the other two ages the assessments were conducted at the family’s home or (less frequently) at the lab, depending on the family’s preference (5% of the meetings at 9 months and 25% at 12 months were lab visits, and the remainder were home visits). Empathy responses did not differ based on the location of their assessment (all |ts| < 1.43, ps > .159). During each visit, the parent also completed some questionnaires, including a demographic questionnaire at 6 months.
All infants were screened for early signs of ASD between 9 and 18 months, using experimenter and parent ratings: at the 9 and 12 months visits the experimenter completed a measure monitoring early signs of ASD (SACS; Barbaro et al., Reference Barbaro, Ridgway and Dissanayake2011), and at age 17–18 months parents completed the Q-CHAT and M-CHAT questionnaires screening for early signs of ASD (Allison et al., Reference Allison, Baron-Cohen, Wheelwrite, Charman, Richler and Brayne2008; Kleinman et al., Reference Kleinman, Robins, Ventola, Pandey, Boorstein, Esser, Wilson, Rosenthal, Sutera, Verbalis, Barton, Hodgson, Green, Dumont-Mathieu, Volkmar, Chawarska, Klin and Fein2008; see below). If any of these screeners indicated risk of ASD, or if concern was raised by the parents, the family was invited for diagnostic meetings at 18 months. If no clear decision could be made at 18 months regarding the child’s ASD status, the family was invited for a follow-up diagnostic meeting at age 24 months. Finally, after children turned 36 months old, all the families were contacted by the research team to document any changes that may have occurred in the meantime in the child’s diagnostic status. With the exception of four children, the diagnoses did not change (see below).
At the end of each lab and home visit, the family received a gift (toy) for the infant. The family also received a gift card of 100 NIS (approximately 30$) at the end of the 6 months lab visit. Finally, families of infants who participated in diagnostic sessions as part of the study received a report regarding the child’s diagnosis.
Measures
Empathic responses to others in distress
At 6, 9, and 12 months, infants’ reactions to others in distress were observed using three tasks: two simulations (parent and experimenter) and a video of a distressed peer. The video stimulus was administered in between the two in-person simulations, in order to reduce the confounding between target of empathy (simulation versus video) and order of task.
Parent and experimenter distress simulations
In each simulation, the parent (typically the mother; 91%) and the experimenter pretended to have hurt themselves – the experimenter by bumping her knee and the parent by hitting their finger with a pounding toy – and feigned distress for 60 s. The first 30 s consisted of moderate crying, and in the last 30 s the crying subsided gradually. No eye-contact with the infant was made during the enactment of distress, so as not to invite a response. At the end of the simulation the experimenter/parent resumed eye contact with the infant, smiled, and assured the infant that everything was now alright. Infants were seated in the parent’s/experimenter’s lap or sat independently (once able to) and were free to move about. Similar procedures have been used in prior work on empathy development in infants and young children (e.g., Davidov et al., Reference Davidov, Paz, Roth-Hanania, Uzefovsky, Orlitsky, Mankuta and Zahn-Waxler2021; Roth-Hanania et al., Reference Roth-Hanania, Davidov and Zahn-Waxler2011; Zahn-Waxler, et al., Reference Zahn-Waxler, Radke-Yarrow, Wagner and Chapman1992).
Crying peer video
A 50 s video of a distressed (crying) baby was presented to the infant on an eye-tracker screen (tobii 60XL) when the infants were 6 months old, and on a tablet screen when they were 9 and 12 months old. Video presentations of another’s distress have been used in prior work with typically developing samples to assess empathic responses (Davidov et al., Reference Davidov, Paz, Roth-Hanania, Uzefovsky, Orlitsky, Mankuta and Zahn-Waxler2021; Roth-Hanania et al., Reference Roth-Hanania, Davidov and Zahn-Waxler2011; Ungerer et al., Reference Ungerer, Dolby, Waters, Barnett, Kelk and Lewin1990).
Coding
Infants’ responses were filmed and coded using the empathy coding scheme from the McArthur Longitudinal Twin Study (Zahn-Waxler et al., Reference Zahn-Waxler, Robinson and Emde1992). The scheme was adapted to fit the younger ages (half-points were added, to increase sensitivity, and coding relied less on verbalization; Davidov et al., Reference Davidov, Paz, Roth-Hanania, Uzefovsky, Orlitsky, Mankuta and Zahn-Waxler2021). The coder (fourth author) had extensive experience in coding infant empathy and was blind to the infants’ risk status and diagnostic outcomes. To minimize halo effects, the coder first completed one task at 6 months, before moving on the second and then third tasks, and only then coded each of the 9 months tasks, and so on; thus, children’s responses to multiple tasks at the same age and/or across multiple ages were not directly compared during coding.
The main code used in the current report is empathic concern, which reflects affective empathy. It assesses the level of concerned affect expressed by the infants, while looking at the distressed other, based on their facial expressions, vocalizations, body language and gestures. The rating scale ranges from 0 to 3, and includes half-points for greater sensitivity, with 0 = concern absent, 1 = slight/brief concern (slight or brief change in affect, such as sobering, accompanied by body tension), 2 = moderate (more extended and pronounced sobering expression or sympathy face, along with change in body posture and/or gestures), and 3 = strong concern (full recruitment of sympathy expression for a substantial duration, accompanied by concerned vocalizations or gestures).
Two additional measures, which reflect low empathy, were also included, in order to probe any significant effects of empathic concern. Specifically, infants may show low empathy for a distressed other because they ignore the other, and/or because they become overly aroused and distressed themselves. Accordingly, we coded both avoidance and self-distress. Avoidance assesses the degree to which the infant disengaged from the distressed other, by looking away. However, short gaze aversions, under 2 sec in duration, are viewed as a regulation strategy (Kopp, Reference Kopp1989) and were therefore not coded as disengagement/avoidance. Avoidance was rated on a 0–4 scale, with 0 = absent (no avoidance), 1 = slight (at least 8–10 s in total), 2 = moderate (at least for a third of the episode, 20 s in total), 3 = strong (the infant disengaged for a substantial time and did not re-engage), 4 = never engaged. Self-distress assesses infant’s distress that is focused on the self. It was scored on a 0–3 scale, with 0= does not occur; 1= visible distress manifested non-vocally, but through the body (irritability, jerkiness) or through facial expressions (wariness or fear; e.g., eyes wide, mouth open), clearly expressed for several seconds; 2 = whimpering (expressed vocally); 3 = full blown crying. (Another measure often used in empathy research—inquiry behavior or hypothesis testing, which reflects exploration of the others’ distress—was also coded, but not used in the current report, because it was highly correlated with and produces virtually identical results to empathic concern).
Reliability
For each task (mother, experimenter, video), at each age (6, 9, 12 months), a randomly selected subset of 20%–30% of the videos was independently coded by a second coder. Inter-rater reliabilities, examined using interclass correlations (ICC, two-way random, absolute agreement), were good across all measures: ICCs ranging from 0.72 to 0.92 for empathic concern, 0.73–1.00 for avoidance, and 0.73–0.95 for self-distress scores. In case of conflict, the ratings of the main coder were always preferred.
Empathic responses to others’ happiness
At 6, 9, and 12 months, infants’ reactions to others’ happiness were observed using two tasks: a simulation by the experimenter and a video of a laughing peer (laughter simulation by the parent was not included, because in our experience it is quite challenging for parents to perform this simulation reliably).
Experimenter laughter simulation
The experimenter pretended to watch a funny movie clip on the tablet while wearing headphones, and feigned laughter for 60 s. The first 15 s were of moderate giggling, followed by 30 s of higher rolling laughter, and finally 15 s of giggling subsiding gradually. No eye-contact with the infant was made during the simulation; when it ended, the experimenter resumed eye contact and said it was a very funny movie. Infants were seated in the parent’s lap or sat independently (once able to) and were free to move about.
Laughing peer video
A 50 s video of a laughing baby was presented to the infant on an eye-tracker screen (tobii 60XL) when the infants were 6 months old, and on a tablet screen when they were 9 and 12 months old.
Coding
Infants’ responses were filmed and coded using parallel dimensions to those used for coding responses to distress. A different coder (fifth author) with extensive experience in coding infants’ empathic happiness served as the main coder and was blind to the infants’ risk status and diagnostic outcomes. The main dimension used in the current study was contagious happiness, which as noted earlier reflects a basic form of empathic happiness – that is, positive affect evoked in the child by observing someone else’s joy. To capture contagious positive emotion that is more other-oriented in nature, we focused on positive affect expressed by the infant while looking at the joyous other, which reflects the infants’ participation in the other’s joy. This was manifested by smiling, positive vocalizations (e.g., laughter), and happy gestures (e.g., clapping hands, pointing with excitement), while looking at the laughing other (experimenter or peer video). Ratings were assigned on a 0–3 scale, with half-points included for greater sensitivity. Ratings were based on both the duration and intensity of the child’s response, with 0 = contagious happiness does not occur, 1 = slight (brief smile), 2 = moderate (longer or more pronounced expressions of shared enjoyment/positive affect), and 3 = strong contagious happiness (very clear/intense expression, and longer in duration).
Similar to the coding of responses to others’ distress (see above), avoidance was also coded on the same 0–4 scale, reflecting the degree to which infants turned their gaze away from the happy other, and self-distress was coded on the same 0–3 scale, reflecting the degree to which the infant expressed distress.
Reliability
Inter-rater reliabilities, calculated for a randomly selected set of 20% of the videos, coded by a second independent coder, were high for the two tasks at all ages, ICCs ranging from .85–.97 for contagious happiness, .78–.91 for avoidance, and .95–1.00 for self-distress.
ASD screening instruments
Between the ages 9 and 18 months three screeners were used to determine which of the infants will be invited to the diagnosis meetings (budgetary limitations and concern for parents’ time precluded inviting all the infants to diagnostic sessions). The three screeners we used were the following:
Social attention and communication study
(SACS; Barbaro et al., Reference Barbaro, Ridgway and Dissanayake2011). This is a survey check-list (yes/no questions) for health care professionals developed to identify infants at high risk of ASD before the age of 24 months. The instrument is completed during an interaction with the infant, and has been shown to be a sensitive screener for ASD from the age of 12 months (Barbaro et al., Reference Barbaro, Ridgway and Dissanayake2011). In the current study, a trained research experimenter (the first author, PhD student with MA in child clinical and educational psychology, and relevant practical training) filled out the SACS during the visits at 9 months (version for 8 months, 8 items) and 12 months (version for 12 months, 12 items).
Quantitative checklist for Autism in toddlers
(Q-CHAT; Allison et al., Reference Allison, Baron-Cohen, Wheelwrite, Charman, Richler and Brayne2008). A parent-report screening tool in which parents rate the frequency of characteristic ASD behaviors, including sensory issues, behavior problems, and social skills. The Q-CHAT contains 25 items, scored on a 5 points scale (0–4), designed for toddlers age 18–24 with a cutoff of more than 39 points linked to increased likelihood of ASD. This measure was found to have good sensitivity for ASD including differentiation from other developmental delays (Ruta et al., Reference Ruta, Chiarotti, Arduino, Apicella, Leonardi, Maggio, Carrozza, Chericoni, Costanzo, Turco, Tartarisco, Gagliano, Allison, Baron-Cohen, Pioggia and Muratori2019). In the current study it was completed by parents at age 17–18 months.
The modified checklist for Autism in toddlers
(M-CHAT; Kleinman et al., Reference Kleinman, Robins, Ventola, Pandey, Boorstein, Esser, Wilson, Rosenthal, Sutera, Verbalis, Barton, Hodgson, Green, Dumont-Mathieu, Volkmar, Chawarska, Klin and Fein2008). A screening tool for early ASD related behaviors, for detection of high-risk toddlers at ages 16–30 months. The M-CHAT contains 20 yes/no questions for parental report. It is a widely used tool recommended by the American Academy of Pediatrics, with good specificity (Duby et al., Reference Duby, Lipkin, Macias, Wegner, Duncan, Hagan, Cooley, Swigonski, Biondich, Lollar, Ackermann, Brin, Crane, Gibson, Skipper, Steinberg-Hastings and Capers2006). In the current study it was completed by parents at age 17–18 months.
ASD diagnosis
As noted above, children who showed elevated risk on any of the screeners, or whose parents expressed concerns (at any point following the 6 months assessment), were seen for full diagnostic sessions. Overall, 18 children participated in diagnostic sessions as part of the study; additional four children underwent diagnostic procedures in the community (see below).
Children’s diagnostic status was determined by an experienced applied developmental psychologist and a specialized child neurologist, at 18 months, and if needed at 24 months or 36 months as well. ASD diagnoses were made in accordance with DSM-5 criteria (American Psychiatric Association, 2013) and were based on: neuro-developmental examination and psychological assessment including administration of the ADOS-2 (Lord et al., Reference Lord, Rutter, DiLavore, Risi, Gotham and Bishop2012), as well as standardized measures of development using the MSEL (Mullen, Reference Mullen1995), and the Adaptive Behavior Assessment System-II (ABAS-II; gray & Carter, Reference Gray and Carter2013) (see below).
Four of the children were diagnosed in the community, by professionals outside our research team (but they too were diagnosed by a specialized psychologist as well as a neurodevelopmental physician and using the same diagnostic tools as required for formal diagnosis, in keeping with the regulations of the Israeli ministry of health; Davidovitch et al., Reference Davidovitch, Gazit, Patalon, Leitner and Rotem2023). One was the child whose data was added to the sample from another study (see above); the other three were infants who had received an ASD diagnosis through services in the community a short while before they were scheduled to come for diagnostic meetings as part of the study (based on their screeners), and their parents did not want to go through the diagnostic process again.
The Autism diagnostic observation schedule-2
(ADOS-2; Lord et al., Reference Lord, Rutter, DiLavore, Risi, Gotham and Bishop2012). A structural observational measure comprised of play-based activities that elicit behaviors relevant to the diagnosis of ASD. The ADOS is considered the “gold standard” procedure for diagnosing ASD (Reaven et al., Reference Reaven, Hepburn and Ross2008).
The Mullen scales of early learning
(MSEL; Mullen, Reference Mullen1995). An assessment of cognitive functioning for young children from birth through 68 months. The MSEL yields standard scores in five developmental domains, including gross and fine motor skills, visual perception skills, and receptive and expressive language.
Adaptive behavior assessment system-II
(ABAS-II; gray & Carter, Reference Gray and Carter2013). A survey for ages 0–5 used to assess the child’s personal and social skills necessary for daily living in a number of domains: conceptual, social, and practical, along with a general adaptive scale. The questionnaire was completed by the child’s parent for the diagnostic meeting.
36 months follow up
As noted above, all families were contacted by phone and/or email after children turned 36 months old, to ask about any changes that may have occurred in children’s diagnosis status since they were last seen for the study and up to the present point. The large majority of the children (94.8%, 55 of 58; one family could not be reached) did not change in their diagnosis status (similar to previous studies; Ozonoff et al., Reference Ozonoff, Young, Landa, Brian, Bryson, Charman, Chawarska, Macari, Messinger, Stone, Zwaigenbaum and Iosif2015). Four children changed in status: one boy who was classified earlier as having mild delay and one boy with global developmental delay (GDD; see below) were diagnosed with ASD, one girl previously diagnosed with GDD changed to mild delay, and one girl with earlier mild delay changed status to GDD. Importantly, Israel has a public healthcare system that provides free ASD diagnosis to children showing risk. The diagnosis is performed according to strict guidelines, and parents of children with confirmed diagnosis are eligible for various benefits, including a monthly stipend (Davidovitch et al., Reference Davidovitch, Slobodin, Weisskopf and Rotem2020). As a result, the majority of children in Israel with ASD diagnosis are currently diagnosed in the community by the public health system at the relatively early age of around 3 years (Davidovitch et al., Reference Davidovitch, Gazit, Patalon, Leitner and Rotem2023).
Distribution of diagnostic status
Of the 38 high-risk children in the sample, eight children – all male – met criteria for ASD. With the addition of the infant (boy) who was added from another sample, nine children altogether received ASD diagnosis by 36 months. None of the 20 low-risk infants was diagnosed with ASD.
For those children who received ASD diagnosis by our research team, the overall level of adaptive behavior on the ABAS-II was in the low range (M = 66.6, SD = 4.51, range 62–73; regretfully we did not have this information for the children diagnosed in the community). Their ADOS total scores, on and Module 1 or the Toddler Module (depending on the child’s age and development), were above the cut-off score in each module respectively. As well, their general DQ scores measured using the Mullen, M = 78.33, SD = 19.86, range 64–101, tended to be lower than those of the infants who participated in the diagnostic sessions but did not receive ASD diagnosis, M = 96.66, SD = 15.37, range 67–119, although the difference fell short of significance (likely due to the small sample size), t(11) = 1.71, p = .116.
In addition, by 36 months, two children (one male; both from the high-risk group) demonstrated global developmental delay (GDD). Their Mullen general scale score was more than 2 SDs below the mean compared to the norm, and/or two or more Mullen scale scores fell more than 1.5 SDs below the mean. These children also had delays in their communication skills, although their communication level matched their mental age, and they did not meet criteria for ASD diagnosis.
The remaining 48 children were in the broad normative range (NR) of development. This group included 31 children with no developmental delays or difficulties (11 from the high-risk group and the 20 infants of the low-risk group), and 17 children with relatively mild difficulties (all from the high-risk group). The mild difficulties classification was based on the diagnostic assessments conducted for the study and/or on parental reports regarding the assessments and interventions received by the child in the community. Of the 17 children in this group, nine (5 girls) had mild speech delays, seven (3 girls) had regulatory difficulties in attention and/or emotion (anxiety or disruptive temper tantrums that required intervention), and one child (male) had both speech delay and emotional difficulties.
Analytic approach
Creating diagnosis status scores (criterion)
Preliminary analysis indicated that low risk children, high risk children with no developmental problems, and high risk children with relatively mild difficulties, did not differ in any of the empathy measures, Wilk’s lambda = .48, F(30, 62) = .91, p = .608. Thus, all these children, whose development was considered within the NR, were dummy-coded “0”, while children diagnosed with ASD were dummy-coded “1”. The global developmental delay (GDD) group included only two children and therefore could not be examined as a separate group.
We debated how to treat the two GDD children in the analysis. On the one hand, GDD children were not diagnosed with ASD, which was the pertinent outcome of the current study. But on the other hand, their development was atypical, including delays in their communication abilities. Moreover, other studies found that for many prodromal signs, GDD children had similar characteristics to the ASD group (Hutman et al., Reference Hutman, Chela, Gillespie-Lynch and Sigman2012; Nadig et al., Reference Nadig, Ozonoff, Young, Rozga, Sigman and Rogers2007). We therefore opted for the following approach: our main analysis was conservative, with the two GDD children included in the non-ASD group (dummy-coded “0”), as frequently done in prior work (e.g., Hutman et al., Reference Hutman, Rozga, Delaurentis, Barnwell, Sugar and Sigman2010). Notably, omitting the two GDD children from the analysis yielded virtually identical results. In addition, we conducted an auxiliary analysis, presented in the supplementary material online, in which the two GDD children were dummy coded “1” together with the ASD children; this model compared the children with atypical development (ASD and GDD) to all the other children, whose development was typical, namely, within the normative range.
Analytic strategy
To test our hypothesis regarding reduced empathic response among infants with subsequent ASD diagnosis, we conducted a mixed-effects linear model using the R package lme4 (Bates et al., Reference Bates, Mächler, Bolker and Walker2014). The dependent variable, infants’ empathic response, was regressed on children’s risk level (see details below), and the main effects and interactions of the following four factors, all mean-centered: one between-subjects variable – subsequent ASD diagnosis (2 levels: 0 = no ASD, 1 = ASD diagnosis), and three within-subjects variables – infant age (3 levels: 6, 9, and 12 months), target of response (2 levels: 0 = in-person simulation by experimenter and/or mother, 1 = video stimulus), and the emotion expressed by the other (2 levels: 0 = distress, 1 = joy). The model included by-participant random intercept and by-participant random slope. Emmeans R package was used to prob significant interactions (Searle et al., Reference Searle, Speed and Milliken2023).
Our secondary question, regarding the possibility of predicting subsequent ASD diagnosis only from the 6 months measures, was examined in two stages. First, we repeated the same mixed-model described above using only the observations at age 6 months (i.e., the age variable was excluded from this analysis, and the interaction variables included diagnosis, target, and emotion). Second, based on the results of this model, the relevant empathy measures from 6 months were examined in a hierarchical logistic regression model, to predict subsequent ASD diagnosis beyond known preexisting risk. The logistic regressions included two steps: Step 1 always included the known risk level (reflecting familial risk and gender, as detailed below), and Step 2 included the empathy measure being tested (selected based on the results of the mixed-linear model).
For the best logistic regression model, we also examined how accurately the model classified children as non-ASD or ASD vis-à-vis their actual diagnosis (i.e., predicted vs. observed). For this purpose, the probability of ASD diagnosis was set to 15%, which reflects the actual rate of ASD diagnoses in the current study (9 out of 59). Notably, this rate also approximates the weighted average odds of being diagnosed with ASD in the current sample, taking into account both the proportion of high-risk versus low-risk siblings in the current sample (approximately two thirds vs. third, respectively) and the known probability of ASD diagnosis for each group (20% vs 2%, respectively).
Finally, for the best logistic regression model, we also conducted follow-up exploratory analyses using the additional responses to others’ distress or happiness (avoidance, self-distress); this enabled us to clarify whether the utility of the relevant empathy task in predicting subsequent ASD was specific to the empathic concern code, or also extended to other relevant behaviors (the non-empathic behaviors of self-distress and/or avoidance).
Results
Descriptive information and construction of known risk-level score
Table S1 presents the means and standard deviations of all empathy measures by diagnostic group, and Figure S1 presents empathic response means as a function of age, diagnosis, target, and emotion. Screening of the data revealed no outliers in any of the empathy measures across the different tasks. Zero-order correlations are presented in Table S2.
As expected, familial history of ASD (having at least one sibling with ASD diagnosis) was a significant risk of ASD diagnosis, χ 2(1) = 4.88, p = .027, as was male gender, χ2(1) = 5.45, p = .020, with boys over-represented in the ASD group (100% boys) compared to the non-ASD group (60% boys). No other demographic variable (maternal education, family income, number of siblings, or religiosity level), was significantly associated with ASD diagnosis. To maximize the degrees of freedom available for the main analyses, a single score of known risk factors was created: familiar risk (having a sibling with ASD) was counted as one risk point, as was male gender, and these factors were tallied to yield a known preexisting risk score ranging from 0 to 2. Notably, the findings remained highly similar when gender and familial risk were entered into the models as two separate dummy variables.
Mixed linear model examining early empathy as a function of diagnosis, risk level, age, target, and emotion
The results of the mixed linear model are presented in Table 1. None of the main effects of risk level, diagnosis, age, target, or emotion was significant, and neither was the quadratic interaction. There was a significant diagnosis-by-emotion two-way interaction, B = .43, SE = .12, p < .001; 95% CI = .20, .67, and a significant target-by-emotion two-way interactions, B = .66, SE = .09, p < .001; 95% CI = .49, .83, which were qualified by a significant three-way interaction between diagnosis, target (in-person simulation vs. video), and the portrayed emotion (distress vs. joy), B = −.62, SE = .24, p = .011; 95% CI = −1.09, −.15. The three-way interaction is presented in Figure 1.
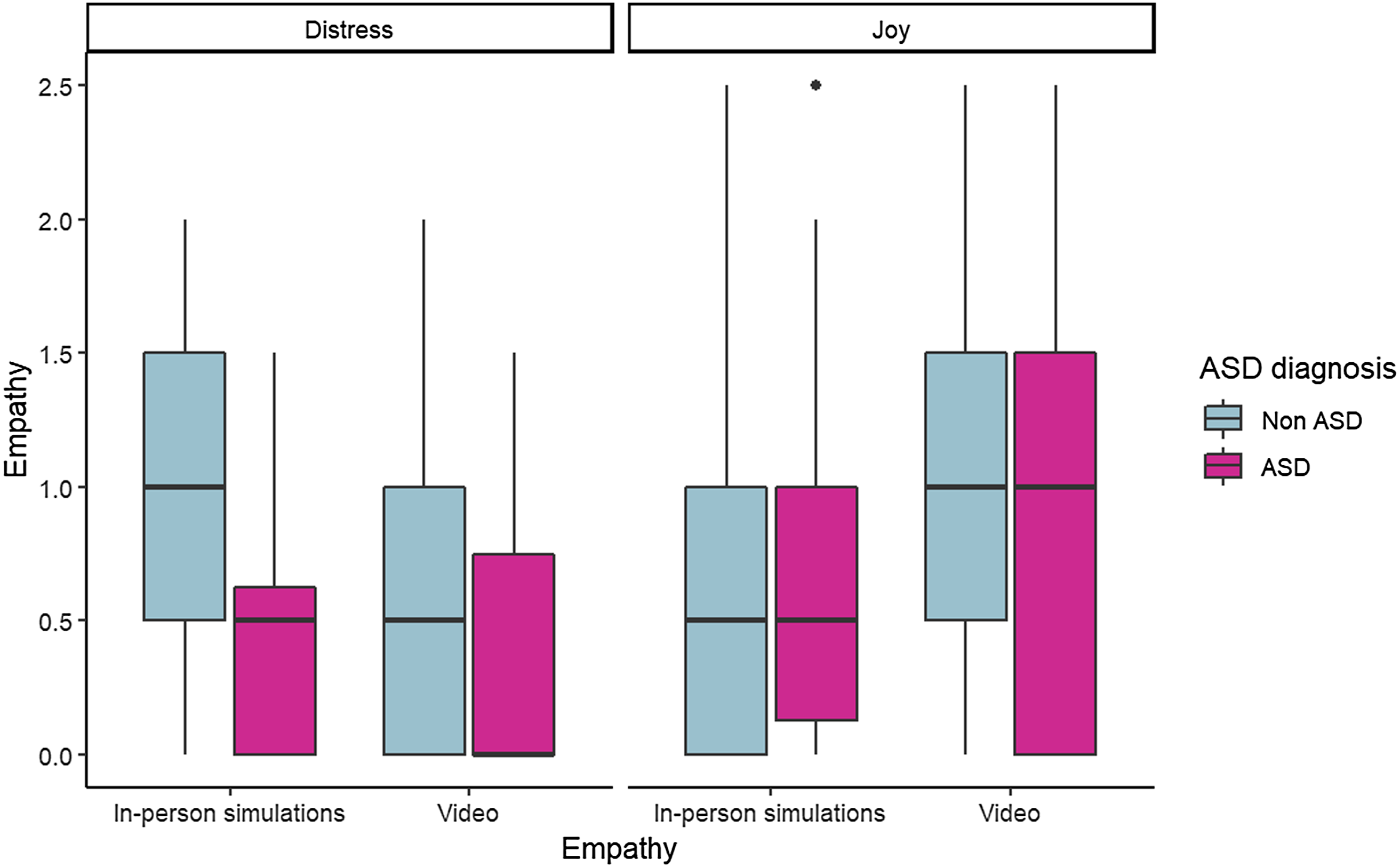
Figure 1. Infants, who were subsequently diagnosed in ASD, show lower empathic concern to human targets than infants without subsequent ASD diagnosis.
Table 1. Fixed effects from the mixed-effects linear model predicting empathic response
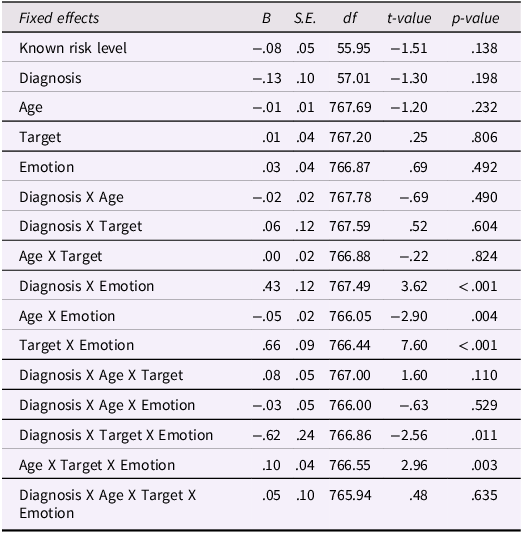
Notes. Known risk level is the sum score of familial risk (0 = low risk, 1 = high risk) and infants gender (0 = girls, 1 = boys). Diagnosis is subsequent ASD diagnosis (0 = non-ASD, 1 = ASD). Age is infants age in empathy assessment (6, 9, and 12 months). Target is the target of empathic response (0 = in-person simulation of experimenter or mother, 1 = video stimuli). Emotion is the emotion portrayed by the target (0 = distress, 1 = joy). The analysis included 59 participants and 838 observations.
Probing this interaction revealed that a significant difference between diagnosis groups was apparent for others’ distress yet not for others’ joy (i.e., in empathic concern but not contagious happiness), and only in response to in-person simulations but not video stimuli. Consistent with our prediction, infants who were subsequently diagnosed with ASD had lower empathic concern toward another person simulating distress, M = .52, SE = .12, compared to infants who were not diagnosed with ASD, M = .95, SE = .05, t(130) = 3.42, p = .019. Age did not significantly moderate this effect, indicating it was consistent from 6 to 12 months.
As well, within the non-ASD group, empathy for a distressed other was stronger in response to in-person simulations compared to video, M IPS = .95, SE = .05; M vid = .65, SE = .06; t(767) = 4.88, p < .001, whereas empathy for a joyful other was stronger in response to the video than to the simulation, M IPS = .61, SE = .06; M vid = 1.07, SE = .06; t(766) = −6.41, p < .001. None of these differences were significant for the ASD group.
Notably, the results were virtually identical when the maternal distress simulation was removed from analysis, that is, when both empathy for others’ distress and others’ joy were assessed using one experimenter simulation and one video stimulus each (see Table S3). Moreover, the same pattern of results emerged when the diagnosis variable was atypical development (ASD + GDD) compared to typical development (i.e., all other children; see Table S4).
Finally, there was also a significant age-by-emotion two-way interaction, B = −.05, SE = .02, p = .004; 95% CI = −.09, −.02, which was qualified by a significant tree-way interaction between infants' age, target of response, and portrayed emotion, B = −.10, SE = .04, p = .003; 95% CI = .04, .17. Diagnosis group did not moderate this interaction hence it refers to the entire sample. Probing the three-way interaction revealed that empathic responses to distress were stable across age, with no significant age differences for either target (video or in-person simulations), whereas the pattern for contagious happiness was more complex: Infants’ responses to the laughing video were stable across age, whereas their contagious happiness in response to the experimenter was higher at age 6 months, M = 1.04, SE = .12, than at ages 9 months, M = .76, SE = .08, and 12 months, M = .49, SE = .11, for both comparisons t(768) = 3.44, p = .031.
Can empathy measures from 6 months alone improve prediction of subsequent ASD above known risk level?
To examine this secondary question, the mixed-effects linear model was first repeated using only the observations from age 6 months. The results are presented in Table S5. At this age, the three-way interaction between diagnosis group, emotion and target of response fell short of significance, B = −.66, SE = .42, p = .125; 95% CI = −1.50, .18. However, there was a significant two-way interactions between diagnosis group and the portrayed emotion, B = .61, SE = .21, p = .004; 95% CI = .20, 1.04. This interaction reflected the fact that, compared to children who did not develop ASD, infants with subsequent ASD diagnosis showed reduced empathic concern for a distressed other at 6 months, regardless of the target – that is, in response to in-person simulations and video stimulus alike – yet they did not show reduced empathic happiness in response to others’ joy, regardless of the target.
Based on these results of the mixed linear model, all three empathic concern tasks at age 6 months (experimenter simulation, mother simulation, and crying baby video) may potentially improve the prediction of later ASD diagnosis. We therefore conducted three hierarchical logistic regressions, to predict ASD diagnosis separately from each individual task. The first step in all three regressions, which included the child’s known risk level (i.e., familial history and gender), was significant, χ 2(1) = 10.46, p = .001, Nagelkerke’s R 2 = .28. As expected, infants with higher risk, mainly boys with familial history of ASD, were more likely to be later diagnosed with ASD, B = 2.55, S.E. = 1.08, OR = 12.81, p = .018, 95% CI = 1.58, 106.07.
Table 2 summarizes the results of Step 2 of the logistic regressions, which included the empathic concern score from each individual task. As can be seen, infants' empathic concern to the experimenter was the best predictor of later ASD diagnosis, χ 2(1) = 5.11, p = .024, Nagelkerke’s ΔR 2 = .12. Low concern toward the distressed experiment predicted subsequent diagnosis above known risk, B = −1.85, S.E. = .91, OR = .16, p = .042, 95% CI = .03, .94. with each additional standard deviation in empathy decreasing the odds of diagnosis by 6.37. In contrast, neither concern toward the mother, B = −.78, S.E. = .98, OR = .46, p = .426, 95% CI = .07, 3.14, nor concern toward the video stimulus, B = −1.74, S.E. = .98, OR = .18, p = .077, 95% CI = .03, 1.20, were significant predictors of subsequent diagnosis on their own. As well, including more than one distress task in the regression (for example, experimenter and video) did not improve the prediction beyond the results of the model using only the experimenter distress simulation.
Table 2. Summary of logistic regressions predicting ASD diagnostic status at 36 m from early empathic concern at 6 months
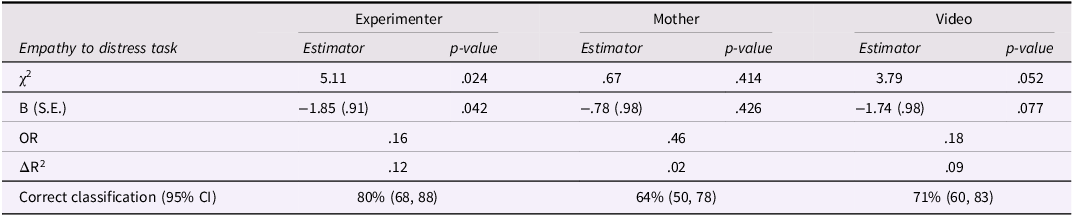
Notes. Step 1 included known risk level is the sum score of familial risk (0 = low risk, 1 = high risk) and infants gender (0 = girls, 1 = boys), χ2(1) = 10.46, p = .001, Nagelkerke’s R2 = .28 (slight changes between regression for mother and video simulation due to differences in missingness).
OR = odds ratio; ASD = autism spectrum disorder (n = 9); non-ASD total = all non-ASD children (including GDD; n = 50).
The model using the experimenter simulation correctly classified 80% of the sample (95% CI = 68, 88), including 78% (7 of 9) of the children with ASD, and 80% of the non-ASD children (‘correct rejections’ was 40 of 50). The overall classification was better than the demographic model in Step 1, which correctly identified 70% of the sample, mainly because of its high ‘false alarm’ rate (66% correct classification of non-ASD children, mis-classifying 17 of 50 infants as having subsequent ASD).
Finally, to shed light on whether the predictive utility of the experimenter simulation was specific to empathic concern, we also examined infants’ tendency to show two non-empathic behaviors in this task – avoidance and self-distress. Each of these scores were thus included in Step 2 of the regression analysis, instead of empathic concern. Self-distress was not a significant predictor, χ 2(1) = .01, p = .922, Nagelkerke’s ΔR2 = .00. Moreover, when both self-distress and empathic concern were entered together into the model, self-distress was not significant, B = −.08, S.E. = .68, OR = .92, p = .906, whereas the effect of empathic concern remained significant, B = −1.86, S.E. = .91, OR = .16, p = .042. In contrast, avoidance was a significant predictor when included alone in Step 2, χ 2(1) = 7.81, p = .005, Nagelkerke’s ΔR2 = .18, B = 1.21, S.E. = .49, OR = 3.34, p = .013, 95% CI = 1.29, 8.65. The model with avoidance of the experimenter’s distress correctly classified 86% of the children (95% CI = 75, 93), including 7 of 9 of those later diagnosed with ASD and 44 of 50 of the non-ASD children. These findings suggest that avoidance can potentially be used as a predictor of ASD at 6 months, instead of empathic concern. Moreover, when both avoidance and empathic concern were included together on Step 2, neither empathic concern nor avoidance were significant predictors of subsequent ASD diagnosis (empathic concern: B = −.73, S.E. = 1.11, OR = .48, p = .509, 95% CI = .05, 4.24; avoidance: B = .96, S.E. = .59, OR = 2.61, p = .103, 95% CI = .82, 8.28), suggesting that at 6 month, the effect of reduced empathic concern may have been due to infants’ tendency to turn away from the victim. Notably, exploratory analyses indicated that at ages 9 and 12 months, avoidance of the distressed experimenter was no longer a significant predictor of ASD diagnosis.
Discussion
This study examined early empathic responses during the first year of life as potential behavioral markers of subsequent ASD diagnosis. Using a prospective design, infants at elevated risk of ASD (siblings of children with ASD) and a matched sample of low-risk infants were followed from age 6 months until diagnosis at 18–36 months. Consistent with our hypothesis, empathic concern to others’ distress was a significant negative predictor of ASD diagnosis: Already at the early age of six months (and thereafter), infants with lower empathic concern scores were more likely to be later diagnosed than infant who exhibited greater empathy. Prior work showed the utility of empathy assessed at 12 months or later, as a prodromal marker of ASD (Hutman et al., Reference Hutman, Rozga, Delaurentis, Barnwell, Sugar and Sigman2010); the current study is the first to show this link at younger ages, and to examine both empathy to others’ distress and others’ joy as predictors of ASD. Notably, only empathic response to distress but not contagious happiness, a rudimentary form of empathy for others’ joy, predicted later ASD diagnosis.
The present study indicates that difficulties in empathy, a core features of ASD, are not only characteristic of children and adults with ASD (Baron-Cohen, Reference Baron-Cohen2009; Harmsen, Reference Harmsen2019), but can also help foretell which infants are more likely to develop, or perhaps have already begun to develop, the disorder. Notably, many other behavioral markers, considered central to the ASD phenotype, were found to predict ASD diagnosis from age 12 months onward, but rarely at 6 months (Jones et al., Reference Jones, Gliga, Bedford, Charman and Johnson2014; Szatmari et al., Reference Szatmari, Chawarska, Dawson, Georgiades, Landa, Lord, Messinger, Thurm and Halladay2016). Empathic responses may be especially early indicators of the risk of ASD, because empathy is a very early appearing individual characteristic – it can be reliably assessed in young infants (Abramson et al., Reference Abramson, Paz, Knafo-Noam and Knafo-Noam2019; Davidov et al., Reference Davidov, Zahn-Waxler, Roth-Hanania and Knafo2013; Roth-Hanania et al., Reference Roth-Hanania, Davidov and Zahn-Waxler2011), and it is moderately stable across early development (Davidov et al., Reference Davidov, Paz, Roth-Hanania, Uzefovsky, Orlitsky, Mankuta and Zahn-Waxler2021). Features of social communication that emerge later, or are unstable, may be less effective as prodromal markers. Empathic responses are also relatively easy to observe – they can be assessed multiple times and without special equipment (only a camera and some training), highlighting their potential utility.
Why do infants later diagnosed with ASD exhibit less concern for others in distress? A person can show less empathic concern for different reasons; for example, because they do not understand what is happening to the other, or they understand but do not care, or are too overwhelmed by the other’s distress, to name a few possibilities. To understand the behavior of infants later diagnosed with ASD in the present study, several features of their early responses must be considered together: they showed less empathic concern for a distressed other, yet equal levels of participation in another’s happiness, did not evidence elevated self-distress, and at 6 months (but not at later ages) looked away more from the distressed other. While we cannot infer the exact reasons behind infants’ responses in this study, this overall pattern of results appears to support certain interpretations more than others. First, it is unlikely that infants later diagnosed with ASD were less concerned with the distressed other because they were too distraught and overwhelmed. As noted, our exploratory analysis indicated that, at least at 6 months, self-distress did not account for later diagnosis, and did not overlap with the predictive effect of empathy.
Second, it does not appear that infants later diagnosed were simply not interested in other people in general. At first glance, low affective concern combined with looking away might suggest reduced social interest, and prior findings have likewise shown reduced attention to social stimuli among infants later diagnosed with ASD. For example, compared to infants who did not subsequently develop autism, infants later diagnosed with ASD looked less the face of a female actor at 6 months, (Chawarska et al., Reference Chawarska, Macari and Shic2013), and their attention to an actor’s eyes declined from 2 to 6 months (Jones & Klin, Reference Jones and Klin2013). Nevertheless, as noted above, infants in the current study who were later diagnosed with ASD did not show reduced response to others’ positive emotions, which are arguably equally social in nature. Indeed, they showed similar levels of contagious happiness in response to the simulation and video portraying others’ joy as did the typically developing infants. Thus, their lower empathic concern likely does not reflect a general lack of social interest.
An explanation that fits better with the present pattern of results is that infants later diagnosed did not understand the situation in the same way as the typically developing infants did. For example, they may not have understood as clearly that the other is suffering or in pain, a prerequisite for feeling concern for the other. Because our assessment focused on infants’ affective empathy and not their cognitive empathy, this explanation awaits future research. But it does appear consistent with prior work and theorizing. For example, weaker activation of the amygdala was found among ASD participants while processing negative faces (Ashwin et al., Reference Ashwin, Baron-Cohen, Wheelwright, O’Riordan and Bullmore2007). Moreover, autism has often been linked with reduced cognitive empathy relative to affective empathy (e.g., Shalev et al., Reference Shalev, Warrier, Greenberg, Smith, Allison, Baron-Cohen, Eran and Uzefovsky2022).
If the reduced empathic concern of infants later diagnosed with ASD stems from their difficulties in processing and understanding the situation, then why were their responses to others’ joy not similarly affected? One possibility is that ASD may impact the processing of distress and happiness in different ways. Despite the overlap in some brain regions that are relevant to all emotions (Light et al., Reference Light, Coan, Zahn-Waxler, Frye, Goldsmith and Davidson2009; Morelli & Lieberman, Reference Morelli and Lieberman2013), there are also different patterns of brain activation unique to specific emotions (Jimura et al., Reference Jimura, Konishi and Miyashita2009), and these may be differentially affected in ASD. Another explanation is that the form of empathic happiness we examined in the present study – emotional contagion, is much simpler than the form of empathy we examined for another’s distress – empathic concern. The former involves merely catching another’s person’s emotion, whereas the latter requires a great deal more (e.g., understanding that another person is suffering, regulating one’s own arousal, wanting the other person to feel better; see Davidov et al., Reference Davidov, Vaish, Knafo-Noam and Hastings2016). Higher forms of empathic happiness, that are more clearly other-oriented in nature – being happy for the other – may therefore reveal similar association with ASD. However, such forms are difficult to measure in infancy, as they require the child to understand that something good has happened to another person, without that person portraying any positive emotion – to rule out emotional contagion. Future work could therefore begin by examining the association between higher forms of empathic happiness and ASD diagnosis in older children, to clarify whether the two are unrelated (similar to the findings for contagious happiness in the present study) or are negatively associated (similar to current and prior findings regarding empathic concern).
Notably, findings regarding patterns of brain activity in adults/older children with ASD can also shed light on the lower levels of empathic concern shown by the infants later diagnosed with ASD in this study. Thus, there is evidence that neural systems central for the processing of others’ emotions and actions underperform among individuals with ASD. These include the “pain matrix” (Di Martino et al., Reference Di Martino, Ross, Uddin, Sklar, Castellanos and Milham2009; Ebisch et al., Reference Ebisch, Gallese, Willems, Mantini, Groen, Romani, Buitelaar and Bekkering2011) – a neural network that is activated both when people feel pain and when they perceive the pain of another person (Shamay-Tsoory, Reference Shamay-Tsoory2011), as well as mirror neurons (Hadjikhani et al., Reference Hadjikhani, Joseph, Snyder and Tager-Flusberg2006; Oberman & Ramachandran, Reference Oberman and Ramachandran2007) – a group of neurons in the motor cortex that likewise fire when the individual either preforms or observes a certain action (Iacoboni & Dapretto, Reference Iacoboni and Dapretto2006). These systems have been linked with empathic responding in adults/older children (Iacoboni & Dapretto, Reference Iacoboni and Dapretto2006; Shamay-Tsoory, Reference Shamay-Tsoory2011; Silani et al., Reference Silani, Bird, Brindley, Singer, Frith and Frith2008; Singer et al., Reference Singer, Seymour, O’Doherty, Kaube, Dolan and Frith2004). It is therefore important for future work to uncover whether similar differences in brain functioning underlying empathic concern can also be seen in infancy, and may predict ASD diagnosis. Understanding how such neural differences may reduce empathic concern is also essential; for example, they may do so by hindering the person’s ability to understand the situation (cognitive empathy), or because they weaken the level of autonomic arousal evoked by the other’s state (which may evolve into affective empathy), or both.
Importantly, reduced empathic concern, that may stem from neural variations, can have cascading effects on children’s subsequent social development (Bradshaw et al., Reference Bradshaw, Schwichtenberg and Iverson2022). For example, when infants show muted reactions to others’ distress, their interaction partners might become less inclined to share their needs and emotions with the child, thereby reducing the child’s opportunities to learn about these social situations and to develop more sophisticated processing and communicative skills; this can also affect the child’s brain development in return (Vivanti et al., Reference Vivanti, Dawson and Rogers2017). Better understanding of these processes can help design early interventions that could reduce these cascading effects, by providing children with the learning experiences that they need but are apt to miss (Bradshaw et al., Reference Bradshaw, Schwichtenberg and Iverson2022).
Our secondary question examined whether any early empathy measures, from 6 months alone, can improve the prediction of ASD. The experimenter’s distress simulation added the most information, contributing significantly to the model beyond the known risk associated with having a sibling with ASD and male gender. Thus, it may be useful to include a distress simulation measure at 6 months as part of baby-health screening batteries administered in the community, to examine whether this measure can contribute useful diagnostic information. It is also notable that not only empathic concern toward the experimenter predicted subsequent ASD at 6 months, but so did avoidance of the distressed experimenter (looking away for a substantial amount of time) – a behavior that is substantially easier to code. Because the effect of avoidance was less consistent over time, however, future studies should include both empathy and avoidance measures, to examine replication.
The classification model of the logistic regression using the experimenter simulation had high sensitivity, identifying most of the children who were later diagnosed (i.e., there were few 'misses'). Specificity was also high, although a notable minority of the infants were misclassified as likely to be in the ASD group, yet they did not actually develop autism (i.e., ‘false positive’ errors). Taken together, when infants exhibited high empathy, later ASD diagnosis was very unlikely, whereas low empathy was less conclusive – although it was associated with elevated risk, there was variability in outcomes, as many infants with low empathy do not develop ASD. A similar pattern of results was found in a previous study with toddlers (McDonald & Messinger, Reference McDonald and Messinger2012). Low empathy seen at one point in time may not reflect the child’s true potential for empathy – the child’s behavior may be affected by transient factors, and/or their empathy level may increase over time (Paz et al., Reference Paz, Davidov, Orlitsky, Roth-Hanania and Zahn-Waxler2022). Moreover, some children (and adults) express less empathy even though their communication skills are intact, a reflection of the wide individual differences in empathic responding seen from an early age in typically developing samples (Davidov et al., Reference Davidov, Paz, Roth-Hanania, Uzefovsky, Orlitsky, Mankuta and Zahn-Waxler2021; Knafo et al., Reference Knafo, Zahn-Waxler, Van Hulle, Robinson and Rhee2008). As well, low empathic abilities in early empathy are not exclusive to ASD, and may predict other developmental difficulties, including aggression and callous-unemotional traits (Paz et al., Reference Paz, Orlitsky, Roth-Hanania, Zahn-Waxler and Davidov2021; Waller et al., Reference Waller, Wagner, Barstead, Subar, Petersen, Hyde and Hyde2020). Thus, whereas early empathy may be seen as a protective factor in the context of emerging ASD, low empathy should be interpreted with caution.
The replication of our finding with the broader atypical versus typical development comparison (i.e., ASD and GDD combined), suggest that early difficulties in empathic concern might be indicative of a more general delay in communication skills, rather than autism per se. Yet, because only two children received the GDD diagnosis in the current study, it is hard to draw firm conclusions regarding this issue. A different picture emerged for children with milder delays or problems, such as speech delay or regulation difficulties. Our preliminary analyses indicated that the latter children did not differ in their early empathic responses from the control group or from the high-risk infants without developmental problems, as was previously found for high risk siblings without ASD diagnosis (Orm et al., Reference Orm, Vatne, Tomeny and Fjermestad2022).
Finally, one of the features we examined was the target of empathy – whether infants responded to an actual person present in the room simulating the emotion, or a video of a person (infant) expressing the emotion. Overall, only responses to in-person simulations (of distress) significantly differentiated between the infants who were later diagnosed versus not diagnosed with ASD. Perhaps infants’ fascination with the screen itself obscures some of the potential differences in their responses to the portrayed emotion. Thus, it may be preferable to use in-person simulation in future prospective studies or screening procedures of ASD, making sure to administer it appropriately (e.g., in terms of length, intensity, eye-contact, and so on), as methodological deviations can substantially influence infants' responses (Thompson & Newton, Reference Thompson and Newton2013). Importantly however, in the current study the method of stimuli administration (simulation, video) was confounded with the age of the target (adults, infant), making it impossible to distinguish their effects. It is thus essential to separate these two features in future work, to clarify what crucial characteristics distress stimuli should have in order to best predict subsequent ASD diagnosis. Other features should also be systematically examined. For example, in the present study the experimenter simulation was more predictive than the mother simulation at 6 months, but it is unclear whether this is because the experimenter is unfamiliar to the child, or due to other differences between the mother and experimenter (e.g., which body part they hurt, their relative experience in administering the simulation, and so on).
There are limitations to this study. First, the sample was small, rendering the results preliminary in nature, and the need for replication essential. The small sample also precluded differentiating between small subgroups of children with specific problems. The ASD group included only boys, and thus the generalizability of the findings to girls with ASD is uncertain. As well, diagnostic tests were administered only to the subgroup that was identified at higher risk by the screening instruments rather than to the whole sample. It is thus possible that children with milder presentations of ASD may have been missed (notably, the absence of diagnosed girls in the present sample increases this concern). Moreover, our focus was on a thorough assessment of empathy, and thus we did not include measures of other socio-communication abilities, that might contribute to, or account for, the present findings. Empathy tasks were also administered in a preset order, and thus order effects could not be ruled out. Finally, to maximize sample retention, we gave families a choice of location (home or lab, at 9 and 12 months), resulting in some loss of standardization. Nevertheless, the study has important strengths, particularly the prospective design beginning from an early age, the collection of observational data regarding different subtypes of empathy in different situations, and the blind coding of empathy.
This study identifies one of the earliest behavioral markers of subsequent ASD diagnosis. The findings shed light on the central role of empathy difficulties in ASD, and have applied implications for the development of early screening procedures for autism, which could facilitate earlier and thus more effective interventions.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579424001226.
Acknowledgments
This research was supported by the Anita Morawetz Fund for Research on Children at Risk, in the School of Social Work and Social welfare, the Hebrew University of Jerusalem, awarded to Maayan Davidov, Carolyn Zahn-Waxler, and Ronit Roth-Hanania, and by the Dissertation Funding Award given to Yael Paz by the Student and Early Career Council of SRCD. Yael Paz was also supported by the Ariane de Rothschild Women Doctoral Fellowship. We are grateful to the families for their participation, and to the team of the Weinberg Child Development Center at Sheba Medical Center for their assistance with data collection and recruitment.
Funding statement
This work was supported by the Anita Morawetz Fund for Research on Children at Risk, School of Social Work and Social welfare, the Hebrew University of Jerusalem, awarded to Maayan Davidov, Carolyn Zahn-Waxler, and Ronit Roth-Hanania, and by the Dissertation Funding Award given to Yael Paz by the Student and Early Career Council of SRCD.
Competing interests
None.






