To the Editor—Antimicrobial resistance (AMR) poses a significant threat to health and human development worldwide. The overuse and misuse of antimicrobials has increased the risk of emergence and spread of AMR globally. Reference Aldeyab, López-Lozano, Gould and Babar2 Recent developments in antifungal resistance have highlighted the importance of implementing effective antifungal stewardship in hospitals and the need to do so. Reference Urbancic, Thursky, Kong, Johnson and Slavin3 Whereas significant work has been done to describe and evaluate antibiotic stewardship interventions in hospitals, experience with antifungal stewardship interventions is limited. In February 2018, King Abdullah University Hospital (KAUH), a 533-bed tertiary teaching hospital in Jordan, implemented an overarching antimicrobial stewardship program (ASP) that aimed toward optimizing antibiotic and antifungal use. Reference Yusef, Hayajneh and Bani Issa4 The impact of the ASP on antibiotic consumption was evaluated separately. Reference Yusef, Hayajneh and Bani Issa4 The objective of this study was to evaluate the impact of an ASP on reducing antifungal use in hospitalized patients.
This retrospective ecological evaluation took place at KAUH using data from January 2014–December 2019. The ASP in February 2018 was considered and evaluated as an intervention, and 2 study periods were defined: before the ASP intervention (January 2014–January 2018) and after the ASP intervention (February 2018–December 2019). The study population included all adult inpatients admitted to KAUH during the study period. Approval of the institutional review board (IRB) at Jordan University of Science and Technology and KAUH was obtained for this study. Monthly quantities of antifungals used (ie, conventional amphotericin B, caspofungin, anidulafungin, voriconazole, and fluconazole) were converted into a number of defined daily doses (DDD; 2019 WHO/ATC index) and normalized per 100 occupied bed days (OBD). The ASP involved several components including awareness and education, an antimicrobial restriction policy that included restricted antibacterial and antifungal drugs with prior approval, and tracking via audit of compliance to the restriction policy and feedback. Reference Yusef, Hayajneh and Bani Issa4
A key intervention was the strict antifungal approval policy, which required that a prescriber seek approval from an infectious diseases consultant before using conventional amphotericin B, caspofungin, anidulafungin, or voriconazole. The usual uses of antifungals in our hospital were mainly for prophylactic, empiric, or definitive therapies, with no or little role for pre-emptive therapy. We followed the Infectious Diseases Society of America (IDSA) guidelines for therapy.Reference Patterson, Thompson and Denning1,Reference Pappas, Kauffman and Andes5 Education and awareness about appropriate use of antifungals comprised a major part of this ASP; these measures included grand rounds and regular lectures directed mainly toward healthcare providers in the hospital. They also included direct, easily accessible links integrated into the electronic medical system for information about different antifungals including dosing and dosing adjustment.
The impact of the ASP on the restricted antifungal consumption was evaluated using a segmented regression of interrupted time series.Reference Yusef, Hayajneh and Bani Issa4,Reference Jirjees, Al-Obaidi and Sartaj6 Caspofungin and anidulafungin use were grouped under the echinocandins category. In addition, the potential overall increase in awareness of the issue of antimicrobial use and resistance due to the introduced ASP interventions on the use of fluconazole was assessed. The final model was simplified by removing insignificant terms using a backward stepwise approach based on the Bayesian full information criteria (BIC). Any outliers discovered during residual diagnostics were adjusted for in the model as additive outliers.Reference Yusef, Hayajneh and Bani Issa4 Analyses were performed using SCA Statistical System version 8.1 software (Scientific Computing Associates, River Forest, IL).
The average total restricted antifungal use was 0.78 DDDs per 100 OBD, and the total antifungal use (including fluconazole) was 1.81 DDDs per 100 OBD. The average conventional amphotericin B use was 0.09 DDDs per 100 OBD; echinocandin use was 0.45 DDDs per 100 OBD; voriconazole use was 0.25 DDDs per 100 OBD; and fluconazole use was 1.04 DDDs per 100 OBD. The results of our analysis of the impact of the ASP on antifungal use are presented in Table 1 (a and b). Statistically significant decreases in the level of fluconazole (regression coefficient, −0.3793; P = 0.0091) and total antifungal use (regression coefficient, −0.5735; P = 0.0108) were observed after the introduction of the ASP (Table 1b). A statistically significant decrease in the slope after intervention for fluconazole (regression coefficient, −0.0241; P = .0131) was observed (Table 1b). For echinocandins and total restricted antifungals, significantly increasing trends were observed before the intervention (P ≤ .001 and P = .0002, respectively). This trend was halted in the post-intervention period (P = .1462 and P = .5695, respectively) (Table 1a). A significant increase in the level of conventional amphotericin B (regression coefficient, 0.0870; P = .001) was observed after the introduction of the ASP. We detected no change in voriconazole use (Table 1).
Table 1. Changes in Antifungal Use After the Intervention Using Segmented Regression Analysis, January 2014–December 2019
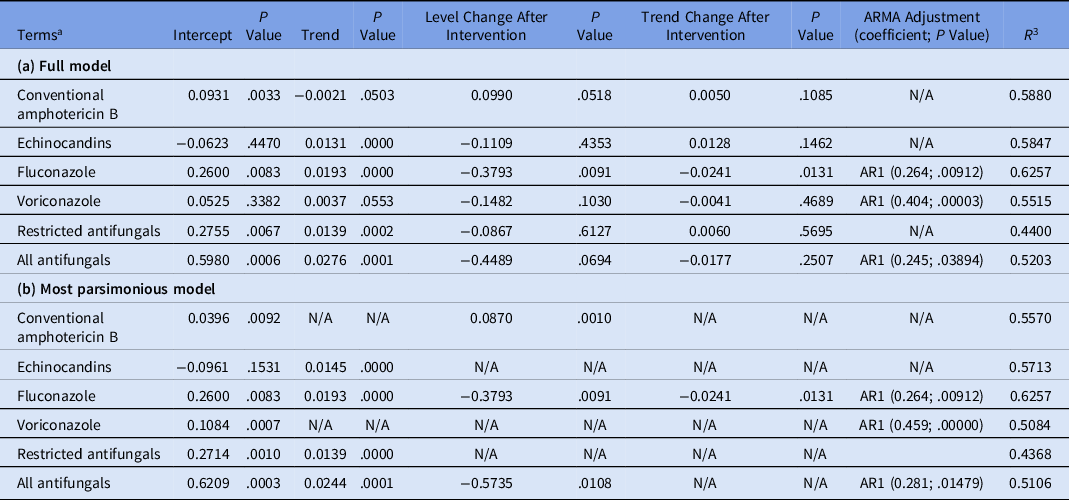
Note. ARMA, autoregressive moving average. N/A, not applicable; AR1, first-order autocorrelation coefficient; DDD, defined daily dose; OBD, occupied bed days.
a Antibiotic use expressed as DDD/100 OBD.
The assessment demonstrated the impact of the ASP in a hospital with low use of antifungal agents, on average 1.8 DDDs per 100 OBD, compared with other studies. Reference Apisarnthanarak, Yatrasert and Mundy7–Reference Standiford, Chan, Tripoli, Weekes and Forrest9 Although fluconazole was not on the restricted antifungals list, our analysis of these data revealed a decrease in its use in the post-ASP period, possibly as the result of the overall impact of the ASP and the increased awareness among prescribers. Other studies have reported reduced use of nontargeted drugs, such as fluconazole, as a result of their educational and bedside intervention. Reference Valerio, Muñoz and Rodríguez10 Notably, we detected a significant increase in trend during the preintervention period for echinocandins (caspofungin and anidulafungin); however, this trend stopped after the intervention. The reduction in echinocandin use had a significant impact on cost reduction in our general tertiary-care teaching hospital after the implementation of antifungal stewardship measures. Reference Valerio, Muñoz and Rodríguez10 The use of conventional amphotericin B increased after the intervention; however, the overall use of conventional amphotericin B throughout the study was low (5% of total antifungals used). In conclusion, a multifaceted ASP contributed to a reduction in the use of antifungals in hospitalized patients. This study provides evidence supporting the efficacy of ASPs to optimize the use of antifungal agents in hospitals, and our findings emphasize the need to promote antifungal stewardship alongside antibiotic stewardship.
Acknowledgments
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.




