Introduction
Common ragweed is a summer annual, native to North America, and commonly found in agricultural fields, roadsides, and other settings where soils are frequently disturbed. Common ragweed is a major agronomic weed in multiple cropping systems throughout the United States and Canada (Bassett and Crompton Reference Bassett and Crompton1975; Van Wychen Reference Van Wychen2019). It can be found in all U.S. states except Alaska, and in all major agricultural areas of Canada (USDA-NRCS 2023).
In a 2019 survey in Canada and the United States, common ragweed was ranked the seventh most common weed and most troubling weed in soybean fields (Van Wychen Reference Van Wychen2019). Common ragweed ranked tenth overall across all crops for being both the most common and most troublesome weed. Yield loss of soybean to competition from two common ragweed plants per meter row caused >40% reduction, while six plants per meter row caused >80% reduction (Barnes et al. Reference Barnes, Jhala, Knezevic, Sikkema and Lindquist2018).
Control of common ragweed at planting can be achieved with flumioxazin (a herbicide that inhibits protoporphyrinogen oxidase [PPO]; categorized as a Group 14 herbicide by the Weed Science Society of America [WSSA]) or with chlorimuron or cloransulam (herbicides that inhibit acetolactate synthase [ALS]; WSSA Group 2). Prior to the introduction of glyphosate-resistant (GR) soybean, farmers relied upon postemergence (POST) herbicides from the same herbicide groups, albeit different active ingredients (PPO-inhibiting herbicides acifluorfen or fomesafen, and ALS-inhibiting herbicides cloransulam or chlorimuron). No-till soybean systems were widely adopted in the Mid-Atlantic region and placed a greater emphasis on controlling weeds through herbicides than the traditional system of planting into a tilled and prepared seedbed. Successful no-till soybean systems require that both cover crops and weeds that are present at seeding be controlled, normally by use of a nonselective herbicide (PSU 2022). Common ragweed emergence begins in early spring, and a significant percentage of seedlings may be present at soybean planting time and are subsequently exposed to nonselective herbicides (Sweet et al. Reference Sweet, Veatch and Dunn1978).
In the United States, glyphosate (5-enolpyruvyl shikimate-3-phosphate synthase [EPSPS]; a WSSA Group 9 herbicide) is currently the most widely used herbicide. Glyphosate is registered for use in-crop and on non-crop sites with just over half (56%) applied to crops with GR traits, including soybean (Benbrook Reference Benbrook2016). In 1999, glyphosate was the sole herbicide used on 90% of the soybean hectares in the United States, leading to widespread glyphosate resistance. WSSA defines herbicide resistance as the inherited ability of a plant to survive and reproduce following exposure to a dose of herbicide normally lethal to the wild type (WSSA 2023).
The spread of GR weeds led to a significant increase in hectares incorporating ALS- and PPO-inhibiting herbicides in weed control programs beginning in 2010. Exclusive use of glyphosate dropped to 85% of planted soybean crops by 2011, and this was further reduced to 70% by 2014 (Benbrook Reference Benbrook2016).
Common ragweed resistance to ALS-inhibiting herbicides in the United States was first reported in 1998, and resistance to glyphosate was reported in 2004 (Heap Reference Heap2023). Select populations of common ragweed from Delaware were confirmed in 2005 with two-way resistance to ALS- and PPO-inhibiting herbicides (Heap Reference Heap2023). However, these populations remained localized and did not spread (MJV, personal communication).
Common ragweed with three-way resistance to glyphosate, cloransulam, and fomesafen herbicides was reported in Maryland and New Jersey in 2016 (Heap Reference Heap2023). Since then, reports of common ragweed infestations at harvest by farmers and crop advisors have been increasing in the region. The cause of poor control has not been thoroughly investigated. Therefore, this research was designed to determine whether the lack of common ragweed control in soybean fields in the Mid-Atlantic region was due to herbicide resistance. ALS- and PPO-inhibiting herbicides along with glyphosate were selected for these trials since they have been the most widely used POST herbicides applied to soybean in the region (USDA-NASS 2020).
Materials and Methods
Common ragweed seeds were collected from 40 field sites throughout the coastal mid-Atlantic region of the United States, from Virginia to New Jersey. Collection sites were selected on the basis of having a recent history of poor herbicidal control as described by extension personnel, industry representatives, or growers. None of the collection sites had previously been tested for herbicide resistance. Twenty-nine sites had enough viable seeds for testing (Figure 1).

Figure 1. Map representing common ragweed collection sites in Delaware, Maryland, and New Jersey and confirmed resistance at each location. The Virginia site is located near Lawrenceville, and was resistant to glyphosate only. Resistance is designated as follows: Sus = susceptible, ALS = resistance to acetolactate synthase-inhibiting herbicides; GLY = resistance to glyphosate; PPO = resistance to protoporphyrinogen oxidase-inhibiting herbicide.
Collection sites were soybean fields with common ragweed plants present in the fall. Seeds were collected from the surviving plants before or at soybean harvest. Common ragweed seeds were harvested by manually stripping plants or by separating them from the combine bin during harvest. All seeds from each site were combined into a single sample. Accessions within a state were numbered from south to north (Table 1). Collection sites were identified by the nearest municipality and the year collected (see Supplementary Table S1). Three accessions were chosen as susceptible checks: two were from field sites where herbicides of interest all provided excellent control; and the third sample was identified as being sensitive and was provided by FMC Corporation (Philadelphia, PA). All seeds were stored in a refrigerated facility at the University of Delaware, in Georgetown, or at the FMC Stine Research Center, Newark, DE, until the start of the experiment.
Table 1. Percent survival at 28 d after treatment of common ragweed accessions treated postemergence with cloransulam, fomesafen, or glyphosate (≤80% visual control). a,b

a An accession is designated resistant (R) if at least one plant survived (≤80% visual control) in both runs at either 2X or 4X application, susceptible (S) had no surviving plants (≤80% control) in all runs at either the 2x.
b Two runs of four plants each were completed with each treatment for all accessions with the following exceptions:
c one run, four repetitions;
d two runs, fewer than eight repetitions.
e Samples were collected from the same fields but at different times. MD10a and MD11a were collected in 2016 and MD10 and MD11 were collected in 2018.
Experiments were conducted in a greenhouse in Newark, DE (39.663°N, 75.785°W) set to provide a day temperature of 25 C (±2 C) and a night temperatures of 22 C (±2 C). Plants were watered daily. Supplemental light via high-pressure sodium lamps were set for a 16-h photoperiod, providing 250 µmol m–2 s−1. Fertilizer (Plantex 20-20-20; Master Plant-Prod Inc., Brampton, ON, Canada) was applied twice weekly through the watering system at 150 ppm nitrogen.
Postemergence Trial
Cloransulam (FirstRate®; Corteva Agriscience, Indianapolis, IN), glyphosate (Roundup Custom®; Bayer Crop Science, St. Louis, MO) and fomesafen (Reflex®; Syngenta Crop Protection, Greensboro, NC) were selected to evaluate resistance to POST herbicides. Herbicides were applied at 1×, 2×, and 4× rates with × corresponding to 17.5 and 350 g ai ha−1 for cloransulam and fomesafen, respectively, and 1,120 g ae ha−1 for glyphosate. A nontreated control was also included for each accession. Seeds for the POST trial were seeded into fiber pots that were 26 cm long, 16 cm wide, and 8 cm deep) (The HC Companies, Twinsburg, OH) filled with potting soil (Metro-Mix 360; SunGro Horticulture, Agawam, MA) and placed in the greenhouse and watered as needed. Seedlings were transplanted at the first true leaf stage into plastic pots that were 6 × 6 cm square and 7.5 cm deep (The HC Companies). Plastic pots were filled with the same potting mix as fiber pots. Each pot was considered a replicate and contained one seedling. Treatments were applied with a single 8001E TeeJet nozzle (Spraying Systems Co., Glendale Heights, IL) in a research track sprayer (DeVries Manufacturing Inc., Hollandale, MN) set to deliver 281 L ha−1 at 207 kPa. POST treatments were applied to common ragweed plants when they displayed at least four true leaves and were between 5 and 8 cm tall. All POST herbicide treatments included a nonionic surfactant (Activator 90; Loveland Products, Greeley, CO) at 0.25% v/v. Additionally, cloransulam treatments included urea ammonium nitrate, and glyphosate treatments included ammonium sulfate. The formulation of glyphosate we used did not contain an adjuvant, so the adjuvant type and amount were consistent for all glyphosate treatments (Burgos Reference Burgos2015). After herbicide application, plants were allowed to dry in the spray room, and then returned to the greenhouse. Plants were not watered for 24 h following application to ensure herbicides were properly absorbed by the seedlings. Thereafter, all plants were routinely watered and fertilized with the same procedure as described previously.
Individual plants were rated 7, 14, 21, and 28 d after treatment (DAT) on a scale of 0% (no visible control or herbicide injury) to 100% (complete plant death/no green tissue). The overall health of individual plants was rated, and the rating was a composite of symptoms of stunting, chlorosis, bleaching, and necrosis. For each accession, plants were compared with the nontreated plants of that accession.
After the final visual rating, aboveground plant biomass was collected by harvesting all plants at the soil line, placing them into individual paper bags, and drying them at 50 C for 48 to 72 h. Once drying was completed, the samples were weighed, and dry biomass was recorded.
The procedure was repeated in time, providing two runs for each accession; each run had four replications. Due to the number of accessions collected (40) and limited greenhouse space, planting and spraying was limited to six to 10 accessions at a time.
Preemergence Trial
PRE trials were conducted on common ragweed accessions that exhibited resistance in the POST trial. Those accessions were chosen based on results from the POST trial and availability of quality seed. Evaluations for ALS resistance included a susceptible accession, and five accessions that demonstrated resistance in the POST trial. Three accessions were selected for PPO resistance, including two of the most resistant accessions in the POST trial and a susceptible accession for comparison. Square plastic pots (10 ×10 wide, 8.5 cm deep, Sq Trad TW; The HC Companies) were filled with Matapeake silt loam (fine-silty, mixed, semiactive, mesic Typic Hapludults) field soil (pH 6.2 and 1.8% organic matter content). The soil was sifted before filling pots to remove stones and foreign debris. Each pot was seeded by volume with 1 mL of seed from the corresponding accession. Each pot was considered a replicate, with five replicates for each treatment. Filled and seeded pots were lightly watered and then placed in a freezer at −28 C for 4 to 6 wk to improve germination.
Before herbicide application, seeded pots were removed from the freezer and left at room temperature to thaw. Treatments were applied 1 d after removal from the freezer, and followed the same procedure as described above. In addition to the ALS and PPO herbicides used for the POST trial, a second herbicide for each mode of action was included. In each case, the second herbicide was of a different chemical family. ALS-inhibiting chlorimuron (Classic®; Corteva Agriscience) and PPO-inhibiting sulfentrazone (Spartan®; FMC Corporation) were included. The 1× rate for chlorimuron, cloransulam, fomesafen, and sulfentrazone was 35, 35, 420, and 280 g ai ha−1, respectively. All herbicides were applied at 0.25×, 0.5×, 1×, and 2× rates, and a nontreated control was included.
All pots were rated 14, 21, and 28 DAT for visual percent control based on a scale of 0% (no visible control or herbicide injury) to 100% (complete plant death). Additionally, emerged seedlings were counted for each pot at each rating date. At 28 DAT, the level of control of individual plants in all pots was visually assessed on a 1 to 4 scale, with 1 corresponding to ≤20% control, 2 indicated 21% to 50% control, 3 indicated 51% to 80% control, and 4 corresponded to >80% control.
Aboveground plant biomass was collected following the methodology previously described. The process was replicated in time to produce two runs, with 10 replicates in total for each treatment.
Data Analysis
Cumulative ragweed control was evaluated by calculating the area under the curve (AUC) as follows:
 $$AUC = \sum\limits_{i = 1}^{{N_i} - 1} {{{({y_i} + {y_{i + 1}})} \over 2}({t_{i + 1}} - {t_i})} $$
$$AUC = \sum\limits_{i = 1}^{{N_i} - 1} {{{({y_i} + {y_{i + 1}})} \over 2}({t_{i + 1}} - {t_i})} $$
where y i = common ragweed control at the ith observation, t i = days at the ith observation, and N i = total number of observations. This calculation provides a quantitative summary of common ragweed control over time for comparison across treatments (Ribeiro et al. Reference Ribeiro, Oliveira, Smith, Santos and Werle2021; VanGessel et al. Reference VanGessel, Johnson and Scott2016; Zhang et al. Reference Zhang, Johnson and Willenborg2016). Lower values result from lower initial common ragweed control and/or faster recovery rate.
Parameters were analyzed using a linear mixed effect model with SAS software (version 9.4; SAS Institute, Cary, NC). Run and replicate nested within run were considered random effects, while accession, herbicide, and rate were fixed effects. Comparisons between accession by rate combinations were adjusted for multiple comparisons using Tukey’s method (α = 0.05). Kenward-Rogers adjusted degrees of freedom were used. Plants deemed to be dead were not included in the dry-weight model.
Results and Discussion
Two accessions (DE3 and MD7) did not have adequate plants to carry out two full runs at each treatment rate, but they were included in the analysis and are noted as such in Table 1. Greenhouse growing conditions often allow severely injured plants to maintain some green tissue, but those plants would normally die under field conditions. So, restricting “dead or controlled” plants to only those with 100% control can misrepresent what would occur in the field. Thus, we characterized plants exhibiting 80% to 100% control as susceptible (“dead or controlled”). Other researchers have used a similar approach (Harre et al. Reference Harre, Nie, Robertson, Johnson, Weller and Young2017; Kruger et al. Reference Kruger, Davis, Weller, Stachler, Loux and Johnson2012; Singh et al. Reference Singh, Maity, Abugho, Swart, Drake and Bagavathiannan2020).
Postemergence Trial
Three susceptible accessions were included as checks. One or more plants from each susceptible accession (≤80% control) survived at least one of the treatments (Table 1). No plants from susceptible accessions survived two different herbicides. All these accessions were deemed susceptible to all three herbicides based on our criteria since no plants survived (≤80% visual control) in both runs of the tests for a given herbicide at either the 2× or the 4× application rate.
More accessions were resistant to glyphosate than either cloransulam or fomesafen (Table 1). Twenty-seven accessions were resistant to glyphosate (either alone or to multiple herbicides), followed by cloransulam with 25 resistant accessions. Nine accessions were resistant to fomesafen. Finding a high percentage of glyphosate resistance was not unexpected since seeds were collected from fields with common ragweed plants that are present late in the season, after all herbicides had been applied, and because use of glyphosate in the region is widespread.
Herbicide resistance was prevalent in the common ragweed accessions we collected. Plants were susceptible to all three herbicides at only one site, DE3. Eight accessions were resistant to all three modes of action that were tested, with at least two accessions from Delaware, Maryland, and New Jersey. Sixteen accessions were resistant to both glyphosate and cloransulam; one accession was resistant to both glyphosate and fomesafen.
The sole accession from Virginia was resistant to glyphosate (Table 1). However, accession VA1 was susceptible to both cloransulam and fomesafen, and no plant survived any application of those herbicides.
All accessions from Maryland were resistant to glyphosate, and no accession had fewer than five plants survive the 2× rate of glyphosate (Table 1). For the 16 accessions from Maryland with glyphosate resistance, only one (MD8) was susceptible to cloransulam. Four of the accessions were resistant to all three herbicide groups. The susceptible accessions from Delaware (DE-S1 and DE-S2) were susceptible to all three herbicides (Table 1). DE3 was also deemed to be susceptible but some of the plants survived all rates of glyphosate and the two lower rates of cloransulam. However, data were available for only one run, and based on our criteria (survivors in both runs), DE3 was considered to be susceptible.
There did not appear to be any geographical patterns related to the location of resistant accessions within or between states. Single-resistant accessions were often found near two-way or three-way resistant accessions. However, this study was not intended to be an exhaustive survey of regional resistance. A structured sampling may provide information on patterns and distribution of resistant populations.
Dry weights were collected for individual common ragweed plants (Supplementary Table S2). Only those plants that demonstrated resistance with a visual observation for control ≤80% at 28 DAT were included in the statistical analyses for dry weights. If fewer than three plants of an accession survived a specific dose of an herbicide, no statistical comparison was determined for that accession at that rate.
As expected, we could not analyze the biomass of the susceptible accessions (DE-S1, DE-S2, or MD-S1) at any rate for any herbicide, because none had more than a single plant survive any treatment. Average biomass per nontreated plant varied widely among the various accessions. The lowest biomass was 1.6 g plant−1 for DE1, while the greatest biomass was 5.1 g plant−1 for MD4. For each accession, the biomass of the nontreated plants and that of the surviving plants from each treatment were compared. The dry weight of treated plants was reduced compared with that of the nontreated plants from the same accession in all cases except DE1, when it was treated with cloransulam at the 1× or 2× rate (Supplementary Table S2). For those treatments, dry weight was nearly 10% greater than that of nontreated plants.
Area under the curve (AUC) was determined for all accessions at each treatment rate when three or more plants survived. The AUC value is unitless, yet it allows all accessions with surviving plants to be compared over all evaluation intervals. AUC is commonly used in plant pathology to describe the progression of disease epidemics (Madden et al. Reference Madden, Hughes and Van Den Bosch2007). Higher AUC values result from plants being severely injured or from injury symptoms that develop rapidly. Lower values can demonstrate a higher level of resistance. The maximum AUC value obtainable was 2,100, representing 100% control for all plants at all rating timings. Herbicides such as cloransulam or glyphosate, which kill weeds slowly, may not have the maximum value if susceptible plants have not died before the first rating. Accessions with AUC values lower than those of DE-S1 exhibited a lower level of control. DE-S1 was used as the comparison for statistical analyses for all other accessions at each corresponding rate.
Twenty-one accessions that received glyphosate treatments had different AUC values from that of the susceptible check (DE-S1) at P ≤ 0.05 with the 4× rate (Table 2). Twelve accessions had different AUC values at the 4× rate of cloransulam, whereas three accessions had different AUC values for fomesafen.
Table 2. Area under the curve for common ragweed accessions treated postemergence with cloransulam, fomesafen, or glyphosate. a – c

a Only plants that survived at the 80% threshold were included in the analysis. Individual accessions compared with the susceptible check (DE-S1); lower values indicate a higher resistance level.
b Values are the sum of visual control ratings with a maximum possible value of 2,100, which represents 100% control for all plants at each rating timing. Therefore, lower values for area under the curve represent higher levels of resistance for that population.
c Significance is designated with asterisks as follows: ***, P < 0.01; **, P = 0.05 to 0.01; *, P = 0.1 to 0.05. NS denotes P ≥ 0.1, and + denotes <3 surviving plants, no statistical comparison made.
The high frequency of resistance to both glyphosate and cloransulam is in line with the extended use of those herbicides for many years in soybean production. Before the release of GR soybean, ALS-inhibiting herbicides were the most widely used family of herbicides in soybean, and resistance to this family has been widely reported (Heap Reference Heap2023). Herbicides that inhibit ALS experienced a general decline in use on soybean crops during the period of 2002 through 2007, but have experienced an increase since then (USGS 2023).
Glyphosate has been used on many hectares of soybean both as a preplant burndown and as an in-crop POST treatment since the mid-1990s with the release of GR soybean. The use of fomesafen applied POST to soybean is a recent development in an effort to control GR biotypes. The number of soybean fields treated with PPO-inhibiting herbicides continues to increase as GR weed populations spread (USGS 2023).
Starting in 2005 and until 2017, 127 new reported cases of herbicide resistance were associated with soybean in the United States (Heap Reference Heap2023). Of those cases, 75 were of glyphosate resistance alone, with an additional 29 cases of resistance to herbicides with multiple modes of action, including glyphosate. The relationship between the increase in reported cases of GR weeds and the increase in both herbicides that inhibit ALS and PPO cannot be overlooked. Also of note, from 2010 to 2016, 24 new reports of ALS resistance in soybean and 14 cases of PPO resistance were reported.
At the 1× glyphosate field rate, all accessions exhibited >50% survival of common ragweed (Table 1). With the 1× rate of cloransulam, 24 accessions exhibited ≥50% survivorship. Six of the accessions exhibited ≥50% survivorship after the 1× application of fomesafen. This proportion of resistant plants in those populations will most certainly result in unacceptable levels of control and, likely, yield loss. The presence of just two common ragweed plants per meter of soybean row (soybean planted in 76-cm rows), resulted in a 40% to 76% yield reduction depending on the year (Barnes et al. Reference Barnes, Jhala, Knezevic, Sikkema and Lindquist2018).
Preemergence Trial
ALS-Inhibiting Herbicides
All accessions treated with ALS-inhibiting herbicides in the PRE trial demonstrated resistance (Figures 2 and 3). This was determined by all accessions having plants that emerged and survived at the 2× rates of chlorimuron or cloransulam. No plants of the susceptible accession survived the 0.5× rate of either herbicide. The number of plants that survived (<80% control 28 DAT) was similar to that of the nontreated control for all accessions, except the two highest rates of chlorimuron, yet >50% of the plants survived.

Figure 2. Common ragweed seedling emergence 28 d after treatment when treated preemergence with chlorimuron. The chlorimuron 1× rate is 35 g ha−1. Green segments = % of plants controlled 0% to 20% (healthy plants), yellow = % of plants controlled 21% to 50%, blue = % of plants controlled 51% to 80%, black = % of plants controlled 81% to 100% (considered severely damaged/dead).

Figure 3. Common ragweed seedling emergence 28 d after treatment when treated preemergence with cloransulam. The cloransulam 1× rate is 35 g ha−1. Green segments = % of plants controlled 0% to 20% (healthy plants), yellow = % of plants controlled 21% to 50%, blue = % of plants controlled 51% to 80%, black = % of plants controlled 81% to 100% (considered severely damaged/dead).
The dry weight per pot for accessions DE1, DE4, and DE6 treated with chlorimuron or cloransulam was similar to that of the nontreated pot for the respective accession (Table 3). The dry weight for MD4, which had been treated with chlorimuron, did not differ from that of the nontreated check at any rate, but plants from pots that had been treated with cloransulam at the 1× and 2× rates exhibited lower dry weights. The dry weight per pot for MD14, which was treated with chlorimuron at 0.5×, 1×, or 2× rates, was less than that of the nontreated pot, but the average weight was similar among these three treatments. MD14 accession, which was treated with cloransulam at the 1× and 2× rates, exhibited a lower dry weight than the nontreated check.
Table 3. Dry weight of common ragweed accessions at 28 d after preemergence application of chlorimuron or cloransulam. a,b

a Significance is designated with asterisks as follows: ***, P < 0.01; **, P = 0.05 to 0.01; *, P = 0.1 to 0.05. NS denotes P ≥ 0.1.
b Mean dry weight (in grams) per pot is presented for 0.25×, 0.5×, 1×, and 2× rates of the respective herbicide. Statistical comparison of differences from the nontreated check (0×) of same accession.
In comparing plants by level of control, DE1, DE4, and MD14 exhibited similar trends when treated with chlorimuron (Figure 2). At 1× or lower rates, less than 20% of all these accessions had plants that emerged with a visual control rating >80%. At the highest application rate, >40% of plants from all three accessions had ≤50% level of control. DE6 and MD4 both exhibited a stronger response to chlorimuron treatments than DE1, DE4, or MD14. Fewer plants from DE6 and MD4 exhibited <20% control compared with other accessions at all rates of chlorimuron.
The MD4 accession, which had been treated with cloransulam at 0.5×, 1×, or 2× rates, had <50% of plants with a control rating of 50% or less (Figure 3). The DE1 accession was similar, with nearly 40% of all plants at those application rates having a visual control level of 50% or more. A majority of plants from the DE4 and DE6 accessions exhibited <50% control after all application rates of cloransulam. At the 1× rate of cloransulam, approximately 60% of the plants from the MD14 accession exhibited <50% control, whereas at the 2× rate nearly 40% of the plants demonstrated that response.
PPO-Inhibiting Herbicides
Accessions selected for this trial were determined resistant to fomesafen in the POST study. The NJ5 accession, treated with fomesafen, had at least 20% of emerged plants survive with <80% control at 28 DAT (Figure 4). Results were similar between the four rates. The NJ6 accession had similar results when treated with the 0.5× or 1× rate of fomesafen. More than 70% of emerged plants exhibited <80% control at the 0.25× rate of fomesafen, while no plants demonstrated <80% control at the 2× rate.

Figure 4. Common ragweed seedling emergence 28 d after treatment when treated preemergence with fomesafen. The fomesafen 1× rate is 420 g ha−1. Green segments = % of plants controlled 0% to 20% (healthy plants), yellow = % of plants controlled 21% to 50%, blue = % of plants controlled 51% to 80%, black = % of plants controlled 81% to 100% (considered severely damaged/dead). No plants emerged for the DE-S2 accession at the 0.5×, 1×, or 2× rates of fomesafen.
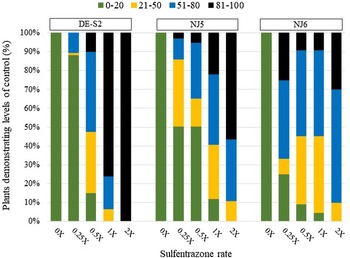
Sulfentrazone applied to the DE-S2 accession at the 1× rate resulted in 75% of plants exhibiting >80% control, and all plants of that accession were controlled (>80%) at the 2× rate of sulfentrazone (Figure 5). NJ5 accession at the 1× and 2× rates of sulfentrazone, had 80% and 40% of emerged plants, respectively, exhibited <80% control, demonstrating a high level of survivorship. More than 70% of emerged plants from the NJ6 accession had levels of control <80%.

Figure 5. Common ragweed seedling emergence 28 d after treatment when treated preemergence with sulfentrazone. The sulfentrazone 1× rate is 280 g ha−1. Green segments = % of plants controlled 0% to 20% (healthy plants), yellow = % of plants controlled 21% to 50%, blue = % of plants controlled 51% to 80%, black = % of plants controlled 81% to 100% (considered severely damaged/dead).
Accession NJ5 treated with fomesafen resulted in a dry weight reduction, with a clear rate response observed with mean dry weights of 0.30 g (0.25×), 0.21 g (0.5×), 0.12 g (1×), and 0.07 g (2×) (Table 4). The dry weight per pot from accession NJ6 treated with fomesafen was reduced compared with the nontreated check with very few plants emerging at the 1× or 2× rates. NJ5 had a dry weights that was similar to that of the nontreated plants when sulfentrazone was applied at the 0.25× or 0.5× rate, but dry weight was reduced at the 1× and 2× rates reduced dry weight. The dry weight of NJ6 treated with sulfentrazone had lower dry weights than the nontreated check, with no differences observed between rates.
Table 4. Dry weight of common ragweed accessions at 28 d after preemergence application of fomesafen or sulfentrazone. a,b

a Significance is designated with asterisks as follows: ***, P < 0.01; **, P = 0.05 to 0.01; *, P = 0.1 to 0.05. NS denotes P ≥ 0.1, and + denotes fewer than three pots with surviving plants, no statistical comparison made.
b Mean dry weight (in grams) per pot is presented for 0.25×, 0.5×, 1×, and 2× rates of the respective herbicide. Statistical comparison of differences from the nontreated check (0×) of same accession.
All the accessions collected at harvest throughout the Mid-Atlantic region were confirmed to be resistant to one or more of the herbicides in the tests, with one exception, DE3. Although DE3 was not classified as being resistant according to the criteria stated previously, multiple plants from this accession survived at the 2× rate of glyphosate, which could indicate a transition to a resistant population. Similar results were observed when NJ6 was treated with cloransulam.
Twenty-five accessions demonstrated two- or three-way resistance. Only nine accessions were resistant to fomesafen. The lower incidence of resistance to POST applications of the PPO-inhibiting herbicide fomesafen versus ALS-inhibiting herbicides corresponds with the more recent introduction and use of these herbicides as compared to glyphosate or ALS-inhibiting herbicides.
This is the first confirmation of common ragweed exhibiting resistance to PRE applications of ALS- or PPO-inhibiting herbicides. Levels of resistance appeared to be similar for a given accession when treated either PRE or POST with ALS-inhibiting herbicides. For instance, MD14 had a high AUC value (i.e., a high level of control and a lower level of resistance) (Table 2). When treated with chlorimuron or cloransulam PRE, MD14 exhibited the greatest reduction in dry weight compared with the nontreated pots (Table 3). In contrast, DE1, which was treated POST with cloransulam, had a lower AUC value, demonstrating a high resistance level (Table 2). The dry weights of DE1 when treated PRE with either chlorimuron or cloransulam were similar to those of the nontreated pots at all rates, also demonstrating a high level of resistance (Table 3). For PPO-inhibiting herbicides, insufficient data were available to make comparisons between PRE and POST applications.
Two accessions were separated by collection time: Milestown (MD10 and MD10a) and Chipatico (MD11 and MD11a) (Supplementary Table S1). Each was collected in 2016 and again in 2018. Both accessions demonstrated a change from two-way resistance with cloransulam and glyphosate in the 2016 collection, to three-way resistance in the 2018 collection. Such a small sample size would make any broad conclusion unwise; however, the results are concerning for area growers.
The loss of three commonly used POST herbicide modes of action due to ineffective control of common ragweed may prove costly for area growers and require the implementation of integrated weed management practices that focus on nonherbicidal approaches. POST herbicides used in other areas of the United States pose unique problems due to the nature of farming in the mid-Atlantic region, including proximity of high-value crops and neighborhoods. Volatile products such as dicamba may not be an option for many growers in the mid-Atlantic. The risk of off-target movement and injury to sensitive crops has been documented in the area (Wasacz et al. Reference Wasacz, Sosnoskie, VanGessel and Besançon2022). Current herbicide recommendations for soybean production include applying chlorimuron or cloransulam PRE, with a POST application of a Group 2, 9, or 14 herbicide, assuming resistance is not present. When GR weeds are suspected, recommendations include planting glufosinate-resistant soybean that allow for a POST application of glufosinate (PSU 2022).
Common ragweed plants that are present in a crop field at harvest are problematic. Common ragweed can impede combine operation during harvest, stems and seeds can contaminate the grain and cause dockage, and seeds can be distributed on the soil and contribute to weed populations in future seasons. However, even more troubling is the likelihood that a herbicide-resistant population of common ragweed exists in that field, and it may be resistant to several herbicide classes. In the mid-Atlantic region, common ragweed plants in the field at harvest are likely to be resistant to glyphosate and ALS-inhibiting herbicides. There is also a chance that those common ragweed populations are also resistant to PPO. These resistant traits not only provide resistance to POST applications of herbicides, but also to PRE applications of the same herbicide group as well. Additional research is required to characterize the nature of resistance for these accessions.
Practical Implications
The presence of large weeds at soybean harvest can lead to many issues, such as harvesting difficulty, foreign matter in the harvested grain, and adding weed seeds to the soil seedbank. Understanding why these plants are present is essential to developing a sustainable weed control program, because of several factors that allow the weeds to survive. Herbicide resistance is one factor. Our research shows that all of the fields we sampled were resistant to herbicides that are commonly used to control common ragweed. Furthermore, we sampled an additional 13 fields with common ragweed at harvest time, but we did not have enough viable seed to include in our trial. Eighty-six percent of the fields we sampled were resistant to two or more herbicides. This study demonstrates the need for farmers and crop advisors to immediately implement nonchemical weed control strategies to manage these biotypes, prevent further seed production, and prevent weed seed movement to uninfested fields.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/wet.2024.11
Acknowledgments
We thank William Bamka, Benjamin Beale, Todd Davis, Dylan Lynch, Matt Morris; Baylee Carr, and Dr. Sarah Hirsh for collecting and submitting samples. This research received no specific grant from any funding agency, commercial, or not-for-profit sectors. Mr. D’Amico and Dr. Ziegler are employed by FMC Corporation.