Introduction
Our knowledge of the fishes of the Hawaiian Islands is broad compared with that of many other subtropical and tropical islands of the central Pacific. Bruce Mundy's (Reference Mundy2005) masterful Checklist of the Fishes of the Hawaiian Archipelago compiles taxonomic data and records for 1250 species of fishes, native and introduced, within the 200-nautical mile Exclusive Economic Zone. Modern illustrated taxonomic summaries of the ichthyofauna are led by Jack Randall's (Reference Randall2007) Reef and Shore Fishes of the Hawaiian Islands and his 2010 Shore Fishes of Hawaii. The well-illustrated guide of Hoover (Reference Hoover2016) provides information on the fishes most commonly encountered by snorkellers and divers, and those of interest to aquarists throughout the Hawaiian Islands.
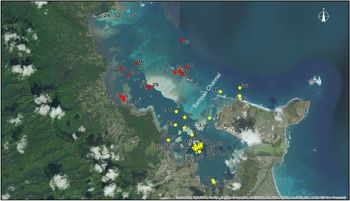
Even with these comprehensive treatments of the fishes of Hawaii, there have been few attempts at a thorough systematic inventory and assessment of the fish species of Kāne‘ohe Bay, O‘ahu, illustrated in Figure 1, the largest sheltered body of water in the Hawaiian Islands (Jokiel, Reference Jokiel1991). Since the late 1940s, Kāne‘ohe Bay has been the site of the University of Hawaii's marine field station, now known as the Hawai‘i Institute of Marine Biology (HIMB), on Moku o Lo‘e, or Coconut Island, in the south-east portion of the Bay (Gordon & Helfrich, Reference Gordon and Helfrich1970). Then HIMB ichthyologist Wayne J. Baldwin compiled ‘a list of 71 families and 211 species found in the waters of Kaneohe Bay and its tributaries from October 5, 1967 to the summer of 1970. A continuing study …’ as it was described by Gordon & Helfrich (Reference Gordon and Helfrich1970: 12) and known by the working title ‘Tentative List of Fishes of Kaneohe Bay and Tributaries Field Notes and Work List. unpubl[ished]’. When Baldwin (now deceased) ‘left Hawai‘i he discarded the list and there are no copies. Thus, there is no information on the species known to occur in the bay’ (Greenfield, Reference Greenfield2003: 47).

Fig. 1. Map of Kāne‘ohe Bay with the 2017 MarineGEO fish collecting localities numbered 1 to 61, as described in text. Blue dots = North-west bay; Red dots = Central bay; Yellow dots = South-east bay.
Two detailed technical reports provided baseline data on marine species occurrence and relative abundance in the Bay. Watson & Leis (Reference Watson and Leis1974) summarized data from a one-year study of fish eggs and larvae collected from two localities, the Sampan Channel, seen in Figure 1, and the south-east Bay. Coles et al. (Reference Coles, DeFelice and Eldredge2002) reported on a survey of the non-native marine biota and summarized literature records for native and non-native species reported from throughout the Bay including museum catalogue numbers of voucher specimens for many, but not all, species. The most recent comprehensive systematic study of fishes in Kāne‘ohe Bay is that of Greenfield (Reference Greenfield2003) who surveyed small reef fishes from 1990 to 1995 using the ichthyocide rotenone. We compare the results of these studies with ours, below.
In 2017, we conducted a survey of the fishes of Kāne‘ohe Bay as part of a broader Smithsonian Institution MarineGEO Hawai‘i bioassessment: Ola I Ke Kai, or Life Comes from the Sea. The Marine Global Earth Observatory (MarineGEO), directed by the Smithsonian's Tennenbaum Marine Observatories Network (TMON), is a global network dedicated to describing marine ecosystems and understanding how they function. Kāne‘ohe Bay was chosen for intensive study because of the long history (~800 years) of use by Hawaii's peoples living around the Bay (Devaney et al., Reference Devaney, Kelly, Lee and Motteler1982; Bahr et al., Reference Bahr, Jokiel and Toonen2015). The Bay has a total surface area of about 41.4 km2 at mean surface levels; it is ~4.3 km wide and 12.8 km long, in a south-west to south-east axis (Jokiel, Reference Jokiel1991; Bahr et al., Reference Bahr, Jokiel and Toonen2015). Its average depth is 10 m, with its deepest point about 19 m (Bahr et al., Reference Bahr, Jokiel and Toonen2015). Native Hawaiians divided its surrounding lands into nine ahupua‘a each of which spans the area from the uplands to the sea. They built walled fishponds throughout the Bay as a reliable source of food. Many of these have been filled in, but the fishpond of the He‘eia ahupua‘a, has been restored and is maintained by the Paepae o He‘eia, a non-profit cultural and educational organization. The He‘eia National Estuarine Research Reserve comprising 1385 acres from upland forests to coral reefs, was designated in 2017 to aid in restoration and recovery of natural habitat as well as historic and cultural sites. HIMB is the site of vigorous research and educational activities focused on the natural history of the Bay and its biota. Despite hundreds of years of stress from human activity, the fringing and patch coral reefs of the Bay remain among the most resilient in the world (Bahr et al., Reference Bahr, Jokiel and Toonen2015).
The Hawaiian shorefish fauna is notable for its high level of endemism, estimated at 25% (Randall, Reference Randall2007). Systematists have recognized the uniqueness of this fish biota by describing populations as new species or subspecies. Here we use DNA barcode sequences from the 5′-end of the mitochondrial cytochrome oxidase I gene (mtCOI) to measure genetic distances between conspecifics from remote regions to verify the hypothesis that Hawaiian populations of species broadly distributed throughout the Indo-Pacific may be genetically distinct from other populations throughout the species' range. We present case studies to demonstrate the potential for undiscovered endemism in the Hawaiian fish biota.
The objectives of this report are to: (1) compile an annotated systematic inventory of the fish species collected during the 2017 survey and document the occurrence of each species with an archived voucher specimen or a verified field observation, ethanol-preserved tissue sample, and a colour photograph of a freshly dead specimen; (2) to sequence the mitochondrial DNA barcode region (see below; Hebert et al., Reference Hebert, Cywinska, Ball and Dewaard2003; Ward et al., Reference Ward, Zemlak, Innes, Last and Hebert2005) of as many species as possible to confirm their taxonomic identification; (3) to compare the barcode sequences of select species with those previously reported for other specimen vouchered collections from the Pacific; and (4) to develop a working list of the fish species that live in Kāne‘ohe Bay.
Materials and methods
Fish specimen collection
We collected fish specimens using methods approved by the Hawaii Department of Land and Natural Resources (DLNR) under Special Activity Permit 2018–35. Ichthyocides such as rotenone or quinaldine were not approved for use, which limited our collection of cryptobenthic species. Sampling gear included dip nets, hand nets, cast nets, spears, and hook and line, on a pole or by hand, while wading, snorkelling or on scuba. We also collected by hand specimens stranded on the beach or in the mud during ebb tide. Other teams that were collecting algae and invertebrates during the 2017 MarineGEO survey also contributed fish specimens that they took incidentally. Specimens were collected and transported back to the laboratory at HIMB; a few were processed in the field. Live specimens were euthanized in buffered MS-222 (Argent Chemical Laboratories, Inc., Redmond, WA) until gill movements ceased. A representative of nearly every species was photographed and one or more tissue samples (dorsal muscle, fin clip and/or eyeball) were dissected from the right side of the specimen and fixed in 95% ethanol overnight. The ethanol was changed the next day and the samples were frozen in cryovials and held at −80°C. The vials were placed in liquid nitrogen-charged dry shippers, transferred to and subsequently deposited in the Smithsonian's National Museum of Natural History (NMNH) Biorepository. Voucher specimens were fixed in 10% formalin following dissection and transported to the NMNH where they were transferred to 75% ethanol for long-term storage. Randall's (Reference Randall2007, Reference Randall2010) volumes served as general guides for the morphological identification of specimens. Specimens we identified only to genus were too small to identify morphologically to species with confidence and the mtCOI barcode sequences were insufficient to resolve their identification further (see below). Additional publications used for identification are cited in the Remarks sections for each family or species. Data for all specimens are available from the online catalogue of the Division of Fishes: http://collections.mnh.si.edu/search/fishes/. Catalogue numbers for the Kāne‘ohe Bay specimens are preceded by the abbreviation USNM (former United States National Museum). Catalogue numbers for comparative material from French Polynesia are preceded by the abbreviation MNHN (Muséum national d'Histoire naturelle, Paris). Higher classification follows Mundy (Reference Mundy2005) and/or Fricke et al. (Reference Fricke, Eschmeyer and Van der Laan2019). Family-group names follow van der Laan et al. (Reference Van der Laan, Eschmeyer and Fricke2014).
DNA barcode
DNA extraction, amplification and sequencing were performed in the Laboratories of Analytical Biology (LAB), NMNH. Total genomic DNA was extracted from ethanol-fixed tissue samples using the AutoGenPrep 965 high-throughput DNA extractor (AutoGen) using the manufacturer's instructions for animal tissue extraction. This included two ethanol wash steps and final elution in 100 μl of AutoGen R9 reagent solution. A 655 bp region at the 5′-end of cytochrome oxidase-c subunit I (mtCOI) was amplified using the Fish-BCL/Fish-HCL primer set (Baldwin et al., Reference Baldwin, Mounts, Smith and Weigt2009). Polymerase chain reaction (PCR) was carried out in 10 μl reactions for each sample. Two PCR approaches were employed, utilizing different Taq polymerases and thermocycling conditions. Method #1 used a GoTaq® Mastermix (2X; Promega, Madison, WI), and each 10 μl sample reaction comprised the following: 5 μl of GoTaq® Mastermix, 3.15 μl of sterile water, 0.3 μl each of forward and reverse primers (10 mM concentration), 0.25 μl BSA (New England Biolabs, 20 mg ml−1) and 1 μl of template DNA. The PCR thermocycling protocol consisted of the following steps: initial denaturation at 95°C for 5 min; 34 cycles of 45 s at 95°C, 45 s at 50°C and 60 s at 72°C; and the final extension at 72°C for 5 min. Method #2 used BIOLASE DNA polymerase (Bioline USA Inc., Taunton, MA) and each 10 μl sample reaction comprised the following: 0.1 μl BIOLASE polymerase (5 units μl−1), 6.4 μl of sterile water, 1 μl of 10× reaction buffer (Bioline), 0.3 μl of each forward and reverse primers (10 mM concentration), 0.5 μl of dNTPs (10 mM, Bioline), 0.4 μl magnesium chloride (50 mM, Bioline) and 1 μl of template DNA. The PCR thermocycling protocol consisted of the following steps: initial denaturation at 95°C for 5 min; 35 cycles of 30 s at 95°C, 30 s at 52°C and 45 s at 72°C; and the final extension at 72°C for 7 min. PCR products were visualized using 1.5% agarose gel electrophoresis and purified with USB ExoSAP-IT following the manufacturer's protocol (Affymetrix, Santa Clara, CA). Purified PCR products were sequenced using a 3730xl DNA analyser (Applied Biosystems, Inc., Waltham, MA). Geneious v9 and v11 (Biomatters Ltd, Auckland, New Zealand) were used for sequence management and quality control. Forward and reverse raw traces were assembled into contigs using the ‘De Novo Assemble’ function in the software, where primers and poor quality 3′- and 5′-ends were automatically trimmed. Barcode sequences of selected species with widespread distribution were aligned in MAFFT v7.4 (Katoh & Standley, Reference Katoh and Standley2013) using the default parameters against the mtCOI datasets obtained from the NCBI GenBank (Benson et al., Reference Benson, Cavanaugh, Clark, Karsch-Mizrachi, Lipman, Ostell and Sayers2017). Using the BLASTn search algorithm (Altschul et al., Reference Altschul, Gish, Miller, Myers and Lipman1990), we revealed sequences of conspecific individuals collected at different localities outside Hawaii and measured their uncorrected genetic distances (p-distances) from our samples representing Hawaiian populations with the dist.seqs module in mothur v1.43 (Schloss et al., Reference Schloss, Westcott, Ryabin, Hall, Hartmann, Hollister, Lesniewski, Oakley, Parks, Robinson and Sahl2009). All mtCOI sequences produced in the current study were uploaded to the NCBI GenBank (Supplementary material T1) and may be found in the NCBI BioProject PRJNA437657. All data are also included in the public BOLD project KANB. We also compared sequences with those in BOLD (The Barcode of Life Data System; Ratnasingham & Hebert, Reference Ratnasingham and Hebert2007) to confirm species identification.
Collecting localities
Here we report our collecting localities in one of three general sectors that correspond to the divisions of Kāne‘ohe Bay based on circulation patterns in the lagoon and the relative degree of oceanic influence: south-east, central and north-west (Bahr et al., Reference Bahr, Jokiel and Toonen2015). We surveyed a variety of habitats throughout the Bay with a concentration in the south-east sector near our laboratory facilities at HIMB, as illustrated in Figure 2. Each locality is mapped in Figure 1. Time of collection was not recorded for specimens received from other field teams.

Fig. 2. Select localities throughout Kāne‘ohe Bay. (A) View of the bay looking north from Laenani Beach. The small island to the right of the Ko‘olau mountain range is Mokoli‘i (Chinaman's Hat). Photo by L.R. Parenti. (B) Exposed lavarock tidepools on Kapapa Island. Photo by D.E. Pitassy. (C) Laenani Beach looking north. Photo by L.R. Parenti. (D) Wall of the He‘eia fishpond; enclosed fishpond waters are to the left of the wall, open bay to the right. Photo by Z. Jaafar. (E) Moku o Lo‘e, or Coconut Island, home of the Hawai’i Institute of Marine Biology. Photo by L.R. Parenti. (F) The Ko‘olau mountain range looking north at Kahalu‘u. Photo by L.R. Parenti.
South-east Kāne‘ohe Bay
Station LRP 17–1
Coconut Is., sandflat fore reef beach, 21°26′38.5″N 157°47′47.2″W. Collection depth 0.3–1 m. Coarse sand, coral bommies, reef front. 1 May 2017. 1015–1115 h. Method: dip nets. Collectors: L.R. Parenti, C. Lager and V. Carter.
Station LRP 17–2
Coconut Is., sandflat fore reef beach, 21°26′38.5″N 157°47′47.2″W. Collection depth 0.3–1 m. Coarse sand, coral bommies, reef front. 2 May 2017. 1015–1115 h. Method: dip nets. Collectors: L.R. Parenti, C. Lager and M. Henley.
Station LRP 17–3
Coconut Is., sandflat fore reef beach, 21°26′38.5″N 157°47′47.2″W. Collection depth 0.3–1 m. Coarse sand, coral bommies, reef front. 4 May 2017. 1015–1115 h. Method: dip nets. Collectors: C. Lager, M. Henley and L.R. Parenti.
Station LRP 17–4
Coconut Is., sandflat fore reef beach, 21°26′38.5″N 157°47′47.2″W. Collection depth 0.3–1 m. Coarse sand, coral bommies, reef front. 10 May 2017. 1015–1115 h. Method: dip nets. Collectors: M. Henley and L.R. Parenti.
Station LRP 17–5
Coconut Is., sandflat fore reef beach, 21°26′38.5″N 157°47′47.2″W. Collection depth 0.3–1 m. Coarse sand, coral bommies, reef front. 15 May 2017. 1015–1115 h. Method: dip nets. Collectors: M. Henley and L. R. Parenti.
Station LRP 17–7
Coconut Is., sandflat fore reef beach. 21°26′38.5″N 157°47′47.2″W. Collection depth: 0–5 m. Coarse sand, coral bommies, reef front. 22 May 2017. 1230–1400 h. Method: dip nets and hand spear. Collectors: L.R. Parenti, K. Cole, Z. Jaafar and D.E. Pitassy.
Station LRP 17–8
Patch reef 14. 21°27′11″N 157°48′4″W. Collection depth: 1–2 m. Coral rubble. 22 May 2017. Method: by hand. Collector: G. Pauley.
Station LRP 17–9
Coconut Is., fringing reef off southern portion of island. 21°25′51″N 157°47′14.7″W. Collection depth: 0–1 m. Inner fringing reef flat with coarse sand and rubble. 23 May 2017. 0815–1115 h. Method: dip nets while snorkelling. Collectors: Z. Jaafar, D.E. Pitassy, J.P. Daly and K. Vinnikov.
Station LRP 17–10
Coconut Is., west side, coral community near mangroves. 21°26′41″N 157°47′24.3″W. Collection depth: 0–5 m. Coral, silty bottom. 23 May 2017. 1045–1115 h. Method: dip nets while snorkelling. Collector: M. Henley.
Station LRP 17–11
Patch reef 14, windward side. 21°27′11″N 157°48′4″W. Collection depth: 1–12 m. 22 May 2017. Method: scuba diving. Collector: G. Pauley.
Station LRP 17–13
He‘eia fishpond. 21°26′14.0″N 157°48′21.1″W. Fishpond, mud bottom with emergent vegetation. 23 May 2017. 1000–1500 h. Methods: hook and line, cast net, dip nets. Collectors: H. Kawelo, L.K.C. Morgan, K. Kotubetey, K. Bishop, K. Piiohia, K. Pizarro, T.S. Ramoy, L.R. Parenti, J.P. Daly, K. Vinnikov, Z. Jaafar, K. Cole and D.E. Pitassy.
Station LRP 17–15
Coconut Is., lagoon in front of Lanai suites (HIMB visiting scientist quarters). 21°25′59.0″N 157°47′25.8″W. Collection depth: 0–0.3 m. 25 May 2017. Method: dip net. Collector: D. Gross.
Station LRP 17–22
Marine Corps Base Hawaii, Pyramid Beach near pyramid at the water line at low tide. 21°27′41.5″N 157°45′49.0″W. Collection depth: 0–0.3 m. Intertidal sediment. 26 May 2017. 0855 h. Method: sediment scooped into one-gallon, resealable plastic bag. Collector: J.L. Norenburg.
Station LRP 17–25
Patch reef 9. 21°26′44.2″N 157°48′6″W. Coral. Collection depth: 3 m. 27 May 2017. Method: Autonomous Reef Monitoring Structure. Collector: A. Anand.
Station LRP 17–26
Coconut Is., leeward side. 21°26′1.64″N 157°47′23″W. Collection depth: 1–4 m. 28 May 2017. 1200–1330 h. Method: hand spear while snorkelling. Collectors: J. Zill and F. Carvalho.
Station LRP 17–27
Coconut Is., windward side. 21°26′4.40″N 157°47′14.51″W. Collection depth: 1–4 m. 28 May 2017. 1400–1800 h. Method: hand spear while snorkelling. Collectors: J. Zill and F. Carvalho.
Station LRP 17–28
Coconut Is., near shark pen. 21°25′59.0″N 157°47′13.6″W. Collection depth: 0–1.5 m. 28 May 2017. 2130 h. Method: hand spear while snorkelling. Collector: D. Gross.
Station LRP 17–32
Coconut Is., southern end, off Point Lab. 21°25′58.4″N 157°47′10.8″W. Collection depth: 0–5 m. 29 May 2017. 0950–1245 h. Method: hand spear while scuba diving. Collector: D. Gross.
Station LRP 17–33
Coconut Is., off Point Lab and Lighthouse. 21°25′56.4″N -157°47′25″W. Collection depth: 0–3 m. 29 May 2017. 1000–1600 h. Method: hand spear while scuba diving. Collector: L. Weaver.
Station LRP 17–35
Patch reef 1. 21°25′57.8″N 157°47′28″W. Collection depth: 3 m. Coral. 30 May 2017. Method: Autonomous Reef Monitoring Structure. Collector: M. Henley.
Station LRP 17–36
Black Rock Point near Marine Corps Base Hawaii. 21°27′58″N 157°45′48.7″W. Collection depth: 12–14 m. 30 May 2017. 1445 h. Method: hand spear while scuba diving. Collectors: L. Weaver and D. Uyeno.
Station LRP 17–37
Coconut Is., leeward side, sand flat in front of Lanai suites (HIMB visiting scientist quarters). 21°26′2″N 157°47′23″W. Collection depth: 0–1 m. Sandy silt flat. 30 May 2017. Method: sand sieve. Collector: G. Pauley.
Station LRP 17–38
Coconut Is., leeward side, sand flat in front of Lanai suites (HIMB visiting scientist quarters). 21°26′2″N 157°47′23″W. Collection depth: 0–1 m. Sandy silt flat. 30 May 2017. Method: yabby pump (used to extract crayfish or shrimp from burrows within the substrate). Collector: G. Pauley.
Station LRP 17–40
Kekepa Is., off windward side. 21°27′49.8″N 157°46′30.5″W. Collection depth: 15–25 m. Coral reef and sand. 31 May 2017. 1030–1130 h. Method: hand spear while scuba diving. Collectors: D. Gross and L. Weaver.
Station LRP 17–41
Coconut Is., southern end, off Point Lab. 21°25′58.4″N 157°47′10.8″W. Collection depth: 0–5 m. 31 May 2017. 0800–0900 h. Method: hook and line. Collector: D. Gross.
Station LRP 17–42
South-east bay fringing reef. 21°24′53.9″N 157°46′46.3″W. Coral. Collection depth: 3 m. 31 May 2017. Method: Autonomous Reef Monitoring Structure. Collector: M. Henley.
Station LRP 17–43
Coconut Is., windward side near Point Lab. 21°26′0.38″N 157°47′10.64″W. Collection depth: 1–4 m. 29 May 2017. 1100–1230 h. Method: hand spear while snorkelling. Collectors: J. Zill and F. Carvalho.
Station LRP 17–44
South-east bay fringing reef. 21°24′50.3″N 157°46′44.2″W. Collection depth: 0.5–1 m. 29 May 2017. 1330–1430 h. Method: hand spear while snorkelling. Collectors: J. Zill and F. Carvalho.
Station LRP 17–45
Near the Sunken City. 21°26′24.25″N 157°47′30.39″W. Collection depth: 1–4 m. 29 May 2017. 1500–1700 h. Method: hand spear while snorkelling. Collectors: J. Zill and F. Carvalho.
Station LRP 17–46
Coconut Is., leeward side, sand flat in front of Lanai suites (HIMB visiting scientist quarters). 21°26′2.0″N 157°47′23.0″W. Collection depth: 0–1 m. 31 May 2017. Method: yabby pump (used to extract crayfish or shrimp from burrows within the substrate). Collectors: G. Pauley and macroinvertebrates team.
Station LRP 17–47
Coconut Is. 21°25′59.8″N 157°47′13.1″W. Collection depth: 0–3 m. 30 May 2017. Method: hand spear. Collector: L. Weaver.
Station LRP 17–48
Coconut Is., southern end, off Point Lab. 21°25′58.4″N 157°47′10.8″W. Collection depth: 0–5 m. 31 May 2017. 1815–1845 h. Method: hook and line. Collector: D. Gross.
Station LRP 17–49
Patch reef 11. 21°26′59.2″N 157°47′46.2″W. 29 May 2017. Collection depth: 3 m. Method: Autonomous Reef Monitoring Structure. Collector: M. Henley.
Station LRP 17–51
Near Marine Corps Base Hawaii. 21°26′57.8″N 157°46′53.4″W. 30 May 2017. Collection depth: 5 m. Method: by hand while scuba diving. Collector: B. Brooks.
Station LRP 17–53
Coconut Is., shuttle boat lagoon. 21°25′54.9″N 157°47′21.9″W. Collection depth: 0–5 m. 31 May 2017. 1740 h. Method: handline. Collector: D. Gross.
Station LRP 17–54
Coconut Is. (no coordinates reported). 31 May 2017. Method: hand spear while snorkelling. Collector: D. Gross.
Station LRP 17–56
Coconut Is., leeward side, mangrove channel and sandflat near boat launch. 21°25′57.3″N 157°47′21″W. Collection depth: 0–2 m. 31 May 2017. Method: hand spear and dip net while snorkelling. Collector: L. Weaver.
Station LRP 17–57
Coconut Is., windward side, off edge of fringing reef. 21°26′6.3″N 157°47′11.4″W. Collection depth: 0–3 m. 1 June 2017. Method: hand spear while snorkelling. Collector: L. Weaver.
Station LRP 17–58
South-east Kāne‘ohe Bay. 31 May 2017. Collector: Miscellaneous material from the macroinvertebrates team or Autonomous Reef Monitoring System team.
Station LRP 17–59
Near Coconut Is. 21°25′59.8″N 157°47′13.1″W. April 2017. Collector: HIMB Shark Lab.
Station LRP 17–60
Coconut Is., windward side, sandflat, fore reef beach. 21°26′38.5″N 157°47′47.2″W. Collection depth: 0.3–1 m. Sandy beach with coral bommies. 19 June 2017. 0830–1030 h. Method: dip nets while snorkelling. Collectors: M. Henley and C. Lager.
Station LRP 17–61
Coconut Is., windward side, sandflat, fore reef beach. 21°26′38.5″N 157°47′47.2″W. Collection depth: 0.3–1 m. Sandy beach with coral bommies. 22 June 2017. 0845–0930 h. Method: dip nets while snorkelling. Collectors: C. Lager and N. Zuchowicz.
Central Kāne‘ohe Bay
Station LRP 17–6
Kahalu‘u, Laenani Beach. 21°27′38.7″N 157°49′55.9″W. Collection depth 0 m. Sandy beach with debris. 17 May 2017. 1130 h. Method: by hand (freshly dead specimen on beach). Collectors: L.R. Parenti and T.S. Ramoy.
Station LRP 17–12
Patch reef 26. 21°28′0.0″N 157°49′5.2″W. Collection depth: 4–5 m. Patch reef. 22 May 2017. Methods: by hand while scuba diving. Collector: D. Gross.
Station LRP 17–14
Kahalu‘u, Lihikai Dock at the end of Lihikai Drive. 21°27′41.0″N 157°50′1.6″W. Collection depth: at surface, 0 m. 24 May 2017. 0615–0715 h. Method: dip nets. Collector: L.R. Parenti.
Station LRP 17–16
Sandbar near patch reef 14. 21°28′46.8″N 157°49′28.9″W. Collection depth: 4–7 m. 23 May 2017. 0842–0958 h. Method: By hand while scuba diving. Collector: D. Gross.
Station LRP 17–17
South of Kapapa Is. 21°28′30.0″N 157°47′56.0″W. Collection depth: 1–2 m. Limestone bench, patchy coral and rubble. 25 May 2017. Method: dip nets while snorkelling. Collectors: G. Pauley and macroinvertebrates team.
Station LRP 17–18
Kapapa Is., north-east side. 21°28′38.9″N 157°47′41.6″W. Collection depth: 4–6 m. Fore reef. 25 May 2017. Method: dipnets on scuba. Collectors: G. Pauley and macroinvertebrates team.
Station LRP 17–19
Kahalu‘u, Lihikai Dock at the end of Lihikai Drive. 21°27′41.0″N 157°50′1.6″W. Collection depth: 0–1 m. Inlet with mangroves and sponge-encrusted pontoon. 26 May 2017. 1000–1230 h. Method: dip nets, hand net, and by hand. Collectors: L.R. Parenti, Z. Jaafar, D.E. Pitassy, and H. Bashaw.
Station LRP 17–20
Patch reef 44. 21°28′35.8″N 157°50′1.0″W. 26 May 2017. Collection depth: 3 m. Method: Autonomous Reef Monitoring Structure. Collector: M. Timmers.
Station LRP 17–21
Patch reef 38. 21°28′24.1″N 157°49′46.0″W. Collection depth: 2–4 m. 27 May 2017. 1030–1200 h. Method: hand spear while scuba diving. Collectors: Z. Jaafar and E. Nalley.
Station LRP 17–23
Outer reef slope. 21°29′34.2″N 157°47′52.5″W. Collection depth: 10.7 m. Outer reef slope, subtidal sand. 28 May 2017. 1100 h. Method: collection bag while scuba diving. Collectors: F. Goetz and K. Jorger.
Station LRP 17–29
Patch Reef 29, south-west side. 21°28′00.0″N 157°49′00.0″W. Collection depth: 1–2 m. 27 May 2017. Method: by hand. Collector: G. Pauley.
Station LRP 17–30
Kahalu‘u, just north of Laenani Beach park. 21°27′34″N 157°49′56″W. Collection depth: 0–0.3 m. Tidal pools in exposed basalt, smooth lava rock formation and sandy shore. 28 May 2017. 1200 h. Method: by hand. Collector: T.S. Ramoy.
Station LRP 17–31
Kahalu‘u, just north of Laenani Beach park. 21°27′34″N 157°49′56″W. Collection depth: 0–0.3 m. Tidal pools in exposed basalt, smooth lava rock formation and sandy shore. 29 May 2017. 1010–1130 h. Methods: dip nets and by hand. Collectors: L. R. Parenti and T. S. Ramoy.
Station LRP 17–34
Kapapa Is., leeward rocky reef flat. 21°28′32.7″N 157°47′56.4″W. Collection depth: 0–1.5 m. Rocky reef flat, windward tidal surge area and tide pools. 30 May 2017. 0930–1115 h. Methods: Spear, dip nets and by hand. Collectors: L.R. Parenti, Z. Jaafar, D.E. Pitassy and D. Gross.
Station LRP 17–39
Kapapa Is., leeward side 21°28′28.1″N 157°48′6.4″W. Collection depth: 2–2.5 m. 31 May 2017. Method: by hand. Collector: G. Pauley.
Station LRP 17–50
Kapapa Is., leeward side. 21°28′24.6″N 157°47′50.2″W. Rubble field of barrier reef. 29 May 2017. Method: hand spear while scuba diving. Collector: D. Uyeno.
North-west Kāne‘ohe Bay
Station LRP 17–24
Kualoa Regional Park. 21°30′31″N 157°50′19.8″W. Collection depth: 0–0.3 m. Rocky intertidal area off sandy beach. 28 May 2017. 1000–1200 h. Method: dip nets. Collectors: L.R. Parenti, Z. Jaafar and D.E. Pitassy.
Station LRP 17–52
Kualoa Regional Park. 21°30′30.3″N 157°50′19.0″W. Sandy shoreline and rocky intertidal. Collection depth: 0–0.5 m. 1 June 2017. Method: dip nets while snorkelling. Collectors: L.R. Parenti, Z. Jaafar and D.E. Pitassy.
Station LRP 17–55
South of Mokoli‘i Is. (Chinaman's Hat). 21°30′31″N 157°49′46″W. Collection depth: 0–4 m. 31 May 2017. Method: hand spear while scuba diving. Collector: L. Weaver.
Results
We recorded 109 species in 43 families from Kāne‘ohe Bay (Table 1). The most speciose families were Acanthuridae (11 species), Gobiidae (11 species), Pomacentridae (10 species) and Chaetodontidae (9 species). Nine of the species that we collected are known or suspected to be introduced to the Hawaiian Islands: Herklotsichthys quadrimaculatus, Osteomugil engeli, Gambusia affinis, Poecilia sp., Omobranchus obliquus, Parablennius thysanius, Sarotherodon melanotheron, Tilapia sp. and Lutjanus fulvus. Two species, Bathytoshia lata and Novaculichthys taeniourus, were seen but not taken. Sequences of mtCOI gene were successfully produced for a total of 151 samples, representing 94 species. The sequence length ranged from 547 to 658 bp, with an average length of 650 bp.
Table 1. List of species collected in a classification

*Known or suspected introduced species.
Systematics
Class Chondrichthyes Huxley, 1880
Order Carcharhiniformes Compagno, 1977
Family Sphyrnidae Bonaparte, 1840
Sphyrna lewini (Griffith & Smith, 1834)
Remarks
Kāne‘ohe Bay is well documented as a breeding ground of the scalloped hammerhead (Clarke, Reference Clarke1971). We obtained one juvenile specimen as part of the 2017 survey. It was collected alive in April 2017 by the HIMB Shark Lab and frozen following its death in the lab's shark tank. We received the frozen specimen on 2 June 2017 and thawed it sufficiently to take several tissue samples which we fixed in 95% ethanol and ultimately froze in liquid nitrogen. We fixed the specimen in formalin and transferred it to 75% ethanol.
Material
South-east bay: USNM 442494 (1).
Order Myliobatiformes Compagno, 1973
Family Dasyatidae Jordan & Gilbert, 1879
Bathytoshia lata (Garman, 1880)
Remarks
One adult was seen, but not taken, in the He‘eia fishpond on 24 May 2017. Classification of this species in the genus Bathytoshia follows Last et al. (Reference Last, Naylor and Manjaji-Matsumoto2016a, Reference Last, White, de Carvalho, Séret, Stehmann and Naylor2016b).
Class Actinopterygii Klein, 1885
Division Teleostei Müller, 1845
Order Anguilliformes Berg, 1943
Family Congridae Kaup, 1856
Ariosoma bowersi (Jenkins, 1903)
Remarks
We collected one of our two specimens by hand; it was a larva stranded in the mud at low tide near the wall of the Kahalu‘u fishpond. Mundy (Reference Mundy2005: 139) lists this species as Ariosoma marginatum (Vaillant & Sauvage, 1875), a Hawaiian endemic. That name was pre-occupied by Leptocephalus marginatus Kaup, 1856 when it was placed in Ariosoma by Bertin (Reference Bertin1935). Leptocephalus marginatus Kaup, 1856 is a synonym of Ariosoma balearicum (Delaroche, 1809), an Atlantic species. Therefore, the next available name for the Hawaiian species is Ariosoma bowersi (Jenkins, 1903) (DG Smith personal communication).
Material
Central bay: USNM 442311 (1); USNM 442312 (1).
Family Muraenidae Rafinesque, 1815
Echidna nebulosa (Ahl, 1789)
Material
Central bay: USNM 442412 (1).
Gymnothorax eurostus (Abbott, 1860)
Figure 3

Fig. 3. Gymnothorax eurostus (Abbott, 1860), family Muraenidae. USNM 442426. Photo by L.R. Parenti.
Remarks
We collected one individual, by hand, stranded on the beach at low tide.
Material
Central bay: USNM 442426 (1).
Gymnothorax undulatus (Lacépède, 1803)
Remarks
We collected one individual of this common moray eel. A second individual was seen and photographed outside the Kahalu‘u fishpond in the central portion of the Bay on 5 April 2017, but not collected.
Material
Central bay: USNM 442319 (1).
Order Clupeiformes Goodrich, 1909
Family Clupeidae Cuvier, 1816
Herklotsichthys quadrimaculatus (Rüppell, 1837)
Remarks
The natural distribution of this species of herring is broadly throughout the Indo-Pacific from South Africa and the Red Sea to Japan, Australia and Samoa (Mundy, Reference Mundy2005: 149). It was introduced unintentionally to the Hawaiian Islands in 1972 (Mundy, Reference Mundy2005). We collected two specimens in the He‘eia fishpond.
Material
South-east bay: USNM 442242 (1); USNM 442243 (1).
Order Aulopiformes Rosen, 1973
Family Synodontidae Gill, 1861
Note: Randall (Reference Randall2007) recognized three species in the genus Saurida from Hawaii: S. flamma, S. gracilis and S. nebulosa. Our specimens of Saurida lack the distinctive orange colouration on the mouth that is diagnostic of S. flamma and keyed out to S. gracilis and S. nebulosa. Saurida gracilis differs from S. nebulosa by having relatively long pectoral-fin rays that extend past the point of insertion of the pelvic fins as opposed to reaching the point of insertion of the pelvic fins, teeth on the vomer as opposed to no vomerine teeth, and three as opposed to two rows of palatine teeth, among other characters. We sequenced the mtCOI DNA barcode (see below) of our specimens to confirm these species identifications.
Saurida gracilis (Quoy & Gaimard, 1824)
Material
South-east bay: USNM 442221 (1); USNM 442397 (1); USNM 442485 (1); USNM 442475 (1).
Saurida nebulosa Valenciennes in Cuvier & Valenciennes, 1850
Material
Central bay: USNM 442308 (1); USNM 442427 (1). North-west bay: USNM 442473 (1), USNM 442471 (1).
Order Mugiliformes Berg, 1940
Family Mugilidae Jarocki, 1822
Mugil cephalus Linnaeus, 1758
Material
South-east bay: USNM 442273 (1); USNM 442275 (1); USNM 442277 (1); USNM 442279 (1); USNM 442281 (1); USNM 441983 (2); USNM 442163 (5).
Osteomugil engeli (Bleeker, 1858)
Remarks
This species of mullet was introduced from the Marquesas Islands to O‘ahu in 1955 where it is now well established (Mundy, Reference Mundy2005). It has also been classified as Moolgarda engeli and Valamugil engeli; we follow the recent classifications of Durand et al. (Reference Durand, Chen, Shen, Fu and Borsa2012), Schemmel et al. (Reference Schemmel, Kamikawa, Shimoda and Peyton2019) and Fricke et al. (Reference Fricke, Eschmeyer and Van der Laan2019).
Material
South-east bay: USNM 442245 (1); USNM 442247 (1); USNM 442249 (1); USNM 442282 (1); USNM 442284 (1); USNM 442286 (1); USNM 442288 (1).
Order Atheriniformes Rosen, 1964
Family Atherinidae Risso, 1827
Atherinomorus insularum (Jordan & Evermann, 1903)
Material
South-east bay: USNM 442290 (1); USNM 442292 (1); USNM 442162 (5); USNM 442482 (1). Central bay: USNM 442309 (1).
Order Beloniformes Berg, 1937
Family Belonidae Bonaparte, 1835
Tylosurus crocodilus (Péron & Lesueur, 1821)
Material
North-west bay: USNM 442362 (1). Unspecified locality in Kāne‘ohe Bay: USNM 442493 (1).
Order Cyprinodontiformes Berg, 1940
Family Poeciliidae Bonaparte, 1831
Note: All species of poeciliids in the Hawaiian Islands are introduced. Our specimens are from the brackish He‘eia fishpond.
Gambusia affinis (Baird & Girard, 1853)
Material
South-east bay: USNM 441930 (1).
Poecilia sp.
Remarks
Our specimen is morphologically comparable to species in the closely related Poecilia sphenops and Poecilia mexicana complexes (see Alda et al., Reference Alda, Reina, Doadrio and Bermingham2013), but we cannot identify it to species with confidence. Mundy (Reference Mundy2005) concluded that Hawaii has an exotic hybrid poeciliid of unknown origin. Our specimen may be a hybrid. It is a female that clusters with other specimens identified as Poecilia mexicana Steindachner, 1863, along with Poecilia gillii (Kner, 1863) and Poecilia orri Fowler, 1943, in a Neighbour-joining tree obtained from a general BLAST against the BOLD database. These are all members of the Poecilia mexicana complex of Alda et al. (Reference Alda, Reina, Doadrio and Bermingham2013). Additional specimens of this taxon are needed to confirm its identification to species.
Material
South-east bay: USNM 442278 (1).
Order Holocentriformes Betancur-R. et al. 2014
Family Holocentridae Bonaparte, 1833
Neoniphon sammara (Forsskål, 1775)
Material
South-east bay: USNM 442370 (1); USNM 442478 (1); USNM 442483 (1).
Order Syngnathiformes Berg, 1940
Family Aulostomidae Rafinesque, 1815
Aulostomus chinensis (Linnaeus, 1766)
Material
South-east bay: USNM 442387 (1); USNM 442439 (1).
Family Fistulariidae Stark, 1828
Fistularia commersonii Rüppell, 1838
Material
South-east bay: USNM 442207 (1).
Family Syngnathidae Bonaparte, 1831
Hippocampus kuda Bleeker, 1852
Figure 4

Fig. 4. Hippocampus kuda Bleeker, 1852, family Syngnathidae. USNM 442322. Photo by D.E. Pitassy.
Remarks
Our single specimen of H. kuda in Figure 4 was collected by hand from among algae on exposed pilings at low tide outside the Kahalu‘u fishpond.
Material
Central bay: USNM 442322 (1).
Order Scorpaeniformes Greenwood, Rosen Weitzman & Myers, 1966
Family Scorpaenidae Risso, 1827
Dendrochirus barberi (Steindachner, 1900)
Material
South-east bay: USNM 442423 (1); USNM 442266 (1). Central bay: USNM 442280 (1).
Sebastapistes coniorta Jenkins, 1903
Figure 5

Fig. 5. Sebastapistes coniorta Jenkins, 1903, family Scorpaenidae. USNM 442298. Photo by D.E. Pitassy.
Remarks
Once reported as a Hawaiian Islands and Johnston Atoll endemic (Randall, Reference Randall2007), this species is now known also from the Wake Atoll and New Caledonia (Fricke et al., Reference Fricke, Kulbicki and Wantiez2011). We report on its mtCOI DNA barcode, below. Our single specimen is illustrated in Figure 5.
Material
Central bay: USNM 442298 (1).
Sebastapistes fowleri (Pietschmann, 1934)
Material
Central bay: USNM 442383 (1).
Scorpaenopsis cacopsis Jenkins, 1901
Material
South-east bay: USNM 442452 (1).
Taenianotus triacanthus Lacépède, 1802
Material
Central bay: USNM 442240 (1).
Order Gobiiformes Günther, 1880
Family Gobiidae Cuvier, 1816
Note: Greenfield & Randall (Reference Greenfield and Randall2004) summarized the taxonomy of the marine gobies of Hawaii.
Asterropteryx semipunctata Rüppell, 1830
Figure 6

Fig. 6. Asterropteryx semipunctata Rüppell, 1830, family Gobiidae. USNM 421677. Photo by Z. Jaafar.
Remarks
This species was targeted for research on cryopreservation of fish spermatogonial cells because it is one of the most common species living in varied marine microhabitats around Coconut Island (Hagedorn et al., Reference Hagedorn, Daly, Carter, Cole, Jaafar, Lager and Parenti2018). We collected specimens for these studies beginning in 2013; here we report just those collected in 2017. We report on the mtCOI DNA barcode of this species, below. A representative adult male is illustrated in Figure 6.
Material
South-east bay: USNM 441795–441809 (15); USNM 441931 (1); USNM 441978 (6); USNM 441979 (6); USNM 441980 (5); USNM 441933 (1); USNM 442149 (12); USNM 442227–442232 (6); USNM 442151 (1); USNM 442153 (1); USNM 442158–442161 (4); USNM 442175–442205 (31). Central bay: USNM 442152 (1).
Bathygobius sp.
Material
South-east bay: USNM 441974 (1). Central bay: USNM 442156 (2).
Bathygobius coalitus (Bennett, 1832)
Material
South-east bay: USNM 442350 (1); Central bay: USNM 442419 (1); North-west bay: USNM 442363 (1).
Bathygobius cocosensis (Bleeker, 1854)
Material
South-east bay: USNM 441972 (1); USNM 441973 (1); USNM 442236 (1); USNM 442238 (1); USNM 442467 (1). Central bay: USNM 442146 (3); USNM 442170 (3); USNM 442350 (1); USNM 442351 (1); USNM 442352 (1); USNM 442353 (1); USNM 442433 (1); USNM 442434 (1); USNM 442435 (1). Unspecified locality in Kāne‘ohe Bay: USNM 442492 (1).
Eviota epiphanes Jenkins, 1903
Material
South-east bay: USNM 441971 (1); USNM 442166 (1); USNM 442171 (1); USNM 442224 (1); USNM 442225 (1); USNM 442226 (1); USNM 442239 (1). Central bay: USNM 442173 (3).
Eviota susanae Greenfield & Randall, Reference Greenfield and Randall1999
Remarks
This species was described from Kāne‘ohe Bay (Greenfield & Randall, Reference Greenfield and Randall1999: 439).
Material
South-east bay: USNM 442165 (1).
Gnatholepis anjerensis (Bleeker, 1851)
Material
South-east bay: USNM 442233 (1); USNM 442235 (1); USNM 442237 (1); USNM 442174 (1); USNM 442235 (1).
Central bay: USNM 442172 (1).
Remarks
We classify this species following Greenfield & Randall (Reference Greenfield and Randall2004) and Randall & Greenfield (Reference Randall and Greenfield2007). In contrast, Larson & Buckle (Reference Larson and Buckle2012) use G. knighti for the Hawaiian taxon. In the mtCOI DNA barcode analysis, our specimens cluster with those identified as G. anjerensis (USNM 402522) and G. knighti (USNM 435610). Larson & Buckle (Reference Larson and Buckle2012: 12) commented on the state of this taxonomic confusion: ‘Four species (G. anjerensis, G. thompsoni, G. cauerensis and G. knighti) remain problematic for reliable identification using meristic counts, morphometric proportions, body and fin pigment and geographic location …While current information does not reliably and easily distinguish all species, it is possible that a deeper understanding of live specimen colour and genetic differences in combination with taxonomic information may provide better identification of species within Gnatholepis.’
Pleurosicya sp.
Material
South-east bay: USNM 442436 (1).
Remarks
The mtCOI barcode sequence of this single specimen clusters with those of specimens from the Marquesas identified as Pleurosicya labiata (Weber, 1913), a species that not been reported from the Hawaiian Islands (Mundy, Reference Mundy2005). Our specimen is too small to confirm its identification via morphology. We await collection of additional specimens of this taxon to confirm its identification.
Priolepis eugenius (Jordan & Evermann, 1903)
Material
Central bay: USNM 442463 (1); USNM 442465 (1).
Priolepis farcimen (Jordan & Evermann, 1903)
Material
South-east bay: USNM 442406 (1); USNM 442468 (1); USNM 442469 (1); USNM 442470 (1).
Psilogobius mainlandi Baldwin, Reference Baldwin1972
Remarks
This species was described from Kāne‘ohe Bay (Baldwin, Reference Baldwin1972: 126).
Material
South-east bay: USNM 442466 (1); USNM 442464 (1).
Central bay: USNM 442337 (1); USNM 442339 (1); USNM 442341 (1).
Unspecified locality in Kāne‘ohe Bay: USNM 442491 (1).
Family Kraemeriidae Whitley, 1935
Kraemeria bryani Schultz, 1941
Remarks
Our single specimen was taken on the outer reef slope of Kāne‘ohe Bay on subtidal sand. No tissue sample was taken.
Material
Central bay: USNM 442150 (1).
Order Blenniiformes Wiley & Johnson, 2010
Family Blenniidae Rafinesque, 1810
Blenniella gibbifrons (Quoy & Gaimard, 1824)
Material
Central bay: USNM 442164 (1); USNM 442425 (1); USNM 442421 (1); USNM 442422 (1); USNM 442431 (1); USNM 442432 (1).
Entomacrodus strasburgi Springer, 1967
Material
Central bay: USNM 442420 (1).
Omobranchus obliquus (Garman, 1903)
Figure 7.

Fig. 7. Omobranchus obliquus (Garman, 1903), family Blenniidae. USNM 442345. Photo by D.E. Pitassy.
Remarks
This species was classified as Omobranchus rotundiceps obliquus (Garman, 1903) by Springer & Gomon (Reference Springer and Gomon1975) and Mundy (Reference Mundy2005). We recognize this taxon as Omobranchus obliquus (Garman, 1903), following Randall (Reference Randall2007) and others. This species is known in Hawaii only from Kāne‘ohe Bay where it was first collected in 1953; it is considered to be introduced (Randall, Reference Randall2007). One specimen from the Central bay is illustrated in Figure 7.
Material
South-east bay: USNM 442453 (1); Central bay: USNM 442154 (2); USNM 442343 (1): USNM 442345 (1); USNM 442347 (1); USNM 442349 (1).
Parablennius thysanius (Jordan & Seale, 1907)
Remarks
This species was introduced into Hawaii, likely from the Philippines, via the shipping industry (Mundy, Reference Mundy2005).
Material
Central bay: USNM 442155 (19); USNM 442313 (1); USNM 442316 (1); USNM 442334 (1); USNM 442336 (1); USNM 442338 (1); USNM 442340 (1); USNM 442342 (1); USNM 442344 (1); USNM 442346 (1); USNM 442348 (1).
Order Perciformes Bleeker, 1863
Family Acanthuridae Bonaparte, 1835
Acanthurus sp.
Material
Central bay: USNM 442168 (7).
Acanthurus blochii Valenciennes, 1835
Material
South-east bay: USNM 442218 (1); USNM 442382 (1). Central bay: USNM 442167 (10); USNM 442306 (1); USNM 442310 (1); USNM 442323 (1); USNM 442325 (1); USNM 442326 (1); USNM 442329 (1).
Acanthurus guttatus Forster in Bloch & Schneider, 1801
Material
Central bay: USNM 442411 (1).
Acanthurus leucopareius (Jenkins, 1903)
Material
South-east bay: USNM 442459 (1). Central bay: USNM 442414 (1).
Acanthurus triostegus (Linnaeus, 1758)
Remarks
The Hawaiian Islands and Johnston Atoll populations of A. triostegus have been classified as the endemic subspecies Acanthurus triostegus sandvicensis Streets, 1877. Recent compilations of the Hawaiian biota either follow that classification (e.g. Mundy, Reference Mundy2005) or use the more broadly applied A. triostegus (e.g. Randall, Reference Randall2007). We report on its mtCOI DNA barcode below.
Material
South-east bay: USNM 442219 (1); USNM 442256 (1); USNM 442391 (1). Central bay: USNM 442307 (1); USNM 442321 (1); USNM 441975 (2).
Acanthurus xanthopterus Valenciennes in Cuvier & Valenciennes, 1835
Material
South-east bay: USNM 441928 (2); USNM 442212 (1); USNM 442258 (1); USNM 442264 (1); USNM 442267 (1); USNM 442381 (1); USNM 442386 (1). Central bay: USNM 442304 (1).
Ctenochaetus strigosus (Bennett, 1828)
Material
South-east bay: USNM 442217 (1); USNM 442376 (1); USNM 442378 (1). Central bay: USNM 441976 (5).
Naso lituratus (Forster in Bloch & Schneider, 1801)
Material
Central bay: USNM 442407 (1).
Naso unicornis (Forsskål, 1775)
Material
South-east bay: USNM 442255 (1); USNM 442257 (1); USNM 442259 (1). Central bay: USNM 442415 (1).
Zebrasoma flavescens (Bennett, 1828)
Material
South-east bay: USNM 442208 (1); USNM 442372 (1).
Zebrasoma veliferum (Bloch, 1795)
Material
South-east bay: USNM 442220 (1); USNM 442379 (1); USNM 442388 (1).
Family Apogonidae Günther, 1859
Foa brachygramma (Jenkins, 1903)
Material
South-east bay: USNM 442296 (1). Central bay: USNM 442428 (1); USNM 442299 (1); USNM 442331 (1); USNM 442333 (1); USNM 442335 (1). USNM 442157 (1).
Family Carangidae Rafinesque, 1815
Caranx ignobilis (Forsskål, 1775)
Material
South-east bay: USNM 442474 (1); USNM 442283 (1).
Caranx melampygus Cuvier in Cuvier & Valenciennes, 1833
Material
South-east bay: USNM 442254 (1); USNM 442481 (1).
Gnathanodon speciosus (Forsskål 1775)
Material
South-east bay: USNM 442424 (1).
Remarks
This specimen could not be identified with confidence using morphology alone. Sequence data for this specimen matches that of other sequences identified in BOLD as G. speciosus.
Scomberoides lysan (Forsskål, 1775)
Material
South-east bay: USNM 442260 (1); USNM 442261 (1); USNM 442297 (1). North-west bay: USNM 442472 (1).
Family Chaetodontidae Rafinesque, 1815
Chaetodon sp.
Material
Central bay: USNM 442301 (1).
Chaetodon auriga Forsskål, 1775
Material: South-east bay: USNM 442364 (1). Central bay: USNM 442324 (1).
Chaetodon lunula (Lacépède, 1802)
Figure 8

Fig. 8. Chaetodon lunula (Lacépède, 1802), family Chaetodontidae. USNM 442377. Photo by D.E. Pitassy.
Remarks
Our specimen from the South-east bay is illustrated in Figure 8.
Material
South-east bay: USNM 442377 (1). Central bay: USNM 441977 (7); USNM 442300 (1); USNM 442302 (1); USNM 442303 (1); USNM 442314 (1); USNM 442317 (1).
Chaetodon lunulatus Quoy & Gaimard, 1825
Material
South-east bay: USNM 442209 (1); USNM 442389 (1); USNM 442404 (1). Central bay: USNM 442355 (1).
Chaetodon multicinctus Garrett, 1863
Material
South-east bay: USNM 442440 (1); USNM 442443 (1); USNM 442449 (1).
Chaetodon ornatissimus Cuvier in Cuvier & Valenciennes, 1831
Material
South-east bay: USNM 442457 (1).
Chaetodon quadrimaculatus Gray, 1831
Material
Central bay: USNM 442409 (1).
Chaetodon unimaculatus Bloch, 1787
Material
South-east bay: USNM 442437 (1).
Forcipiger flavissimus Jordan & McGregor in Jordan & Evermann, 1898
Material
South-east bay: USNM 442394 (1).
Family Cichlidae Bonaparte, 1835
Note: All species of cichlids in the Hawaiian Islands are introduced. Species in three genera – Oreochromis, Sarotherodon and Tilapia – have been recorded and many specimens likely represent hybrids (B. Mundy personal communication).
Sarotherodon melanotheron Rüppell, 1852
Material
South-east bay: USNM 441929 (1).
Tilapia sp.
Material
Central bay: USNM 442354 (1).
Family Cirrhitidae Macleay, 1841
Cirrhitus pinnulatus (Forster in Bloch & Schneider, 1801)
Material
South-east bay: USNM 442456 (1); USNM 442460 (1).
Family Creediidae Waite, 1899
Crystallodytes cookei Fowler, 1923
Material
South-east bay: USNM 442356 – 442360 (5). Central bay: USNM 442384 (1).
Family Kuhliidae Jordan & Evermann, 1896
Kuhlia xenura (Jordan & Gilbert, 1882)
Figure 9

Fig. 9. Kuhlia xenura (Jordan & Gilbert, 1882), family Kuhliidae. USNM 442361. Photo by D.E. Pitassy.
Remarks
Randall & Randall (Reference Randall and Randall2001) reviewed the species of the genus Kuhlia from the Central Pacific and concluded that there are two species in Hawaii: the widespread Kuhlia sandvicensis (Steindachner, 1876) and K. xenura, a Hawaiian endemic, seen in Figure 9. Some reports of K. sandvicensis from Kāne‘ohe Bay (e.g. Tester & Takata, Reference Tester and Takata1953) are of K. xenura, as noted by Randall & Randall (Reference Randall and Randall2001). This is the only species of Kuhlia recorded from the Bay by Greenfield (Reference Greenfield2003), the only species in the collection of fishes from Kāne‘ohe Bay in the Bishop Museum, and the only species that we collected, with certainty, in 2017. Other reports of K. sandvicensis from the Bay (e.g. Watson & Leis, Reference Watson and Leis1974; Coles et al., Reference Coles, DeFelice and Eldredge2002; ×Appendix 1) need to be confirmed. We report on its mtCOI DNA barcode, below.
Material
South-east bay: USNM 441981 (12); USNM 442285 (1); USNM 442287 (1); USNM 442289 (1); USNM 442291 (1); USNM 442293 (1); USNM 442294 (1); USNM 442295 (1). USNM 442400 (1); USNM 442479 (1); USNM 442402 (1); USNM 441934 (1). Central bay: USNM 442430 (1). North-west bay: USNM 442361 (1); USNM 442148 (2).
Family Kyphosidae Jordan, 1887
Note: Identification of the species of Kyphosus follows Knudsen & Clements (Reference Knudsen and Clements2013).
Kyphosus hawaiiensis Sakai & Nakabo, Reference Sakai and Nakabo2004
Material
Central bay: USNM 442416 (1).
Kyphosus vaigiensis (Quoy & Gaimard, 1825)
Material
South-east bay: USNM 442396 (1); USNM 442399 (1).
Family Microcanthidae Bleeker, 1876
Microcanthus strigatus (Cuvier, 1831)
Material
Central bay: USNM 442318 (1).
Family Labridae Cuvier, 1816
Bodianus albotaeniatus (Valenciennes in Cuvier & Valenciennes, 1839)
Remarks
As the subspecies Bodianus bilunulatus albotaeniatus in Mundy (Reference Mundy2005: 432).
Material
South-east bay: USNM 442385 (1).
Gomphosus varius Lacépède, 1801
Material
South-east bay: USNM 442392 (1); USNM 442393 (1).
Halichoeres ornatissimus (Garrett, 1863)
Material
South-east bay: USNM 442448 (1).
Novaculichthys taeniourus (Lacépède, 1801)
Remarks
We observed, but did not collect, a single individual on 10 May 2017 while snorkelling in the outer reef slope of the Bay near Kapapa Island.
Oxycheilinus unifasciatus (Streets, 1877)
Remarks
We sequenced the mtCOI DNA barcode of two specimens (USNM 442374 and USNM 442462) and compared them to DNA barcodes of specimens from French Polynesia and Futuna (see below).
Material
South-east bay: USNM 442374 (1); USNM 442462 (1).
Thalassoma ballieui (Vaillant & Sauvage, 1875)
Material
South-east bay: USNM 442366 (1).
Thalassoma duperrey (Quoy & Gaimard, 1824)
Material
South-east bay: USNM 442216 (1); USNM 442373 (1); USNM 442441 (1).
Family Lutjanidae Gill, 1861
Lutjanus fulvus (Forster in Bloch & Schneider, 1801)
Remarks
The three species of Lutjanus reported from Kāne‘ohe Bay were introduced to Hawaii in the mid to late 1950s to enhance fisheries (Appendix 1; Mundy, Reference Mundy2005: 43). We collected specimens of one of these species in the south-east portion of the Bay.
Material
South-east bay: USNM 442213 (1); USNM 442244 (1); USNM 442246 (1).
Family Mullidae Rafinesque, 1815
Mulloidichthys flavolineatus (Lacépède, 1801)
Material
South-east bay: USNM 442214 (1); USNM 442365 (1); USNM 442375 (1); USNM 442405 (1); USNM 442380 (1); USNM 442390 (1); USNM 442395 (1); USNM 442477 (1); USNM 442484 (1).
Parupeneus multifasciatus (Quoy & Gaimard, 1825)
Material
South-east bay: USNM 442371 (1); USNM 442461 (1).
Parupeneus porphyreus (Jenkins, 1903)
Material
South-east bay: USNM 442476 (1).
Family Polynemidae Rafinesque, 1815
Polydactylus sexfilis (Valenciennes, 1831)
Figure 10

Fig. 10. Polydactylus sexfilis (Valenciennes, 1831), family Polynemidae. USNM 442250. Photo by D.E. Pitassy.
Remarks
This is the only species of polynemid that lives in the Hawaiian Islands. It has long been cultured and remains a sought-after food fish. Our four specimens were collected using a castnet just outside the wall of the He‘eia fishpond; one is illustrated in Figure 10.
Material
South-east bay: USNM 442250 (1); USNM 442251 (1); USNM 442252 (1); USNM 442253 (1).
Family Pomacanthidae Jordan & Evermann, 1898
Centropyge potteri (Jordan & Metz, 1912)
Material
South-east bay: USNM 442445 (1).
Family Pomacentridae Bonaparte, 1831
Abudefduf sp.
Material
Central bay: USNM 442429 (1).
Abudefduf abdominalis (Quoy & Gaimard, 1825)
Material
South-east bay: USNM 442215 (1); USNM 442486 (1); USNM 442488 (1).
Abudefduf sordidus (Forsskål, 1775)
Material
South-east bay: USNM 441932 (2); USNM 442211 (1); USNM 442262 (1); USNM 442270 (1). North-west bay: USNM 442147 (4).
Abudefduf vaigiensis (Quoy & Gaimard, 1825)
Material
South-east bay: USNM 441982 (7); USNM 442263 (1); USNM 442265 (1); USNM 442269 (1); USNM 442271 (1).
Remarks
The widespread Indo-Pacific Abudefduf vaigiensis was first reported in Hawaiian waters in 1991 (Severns & Fiene-Severns, Reference Severns and Fiene-Severns1993; Randall, Reference Randall2007). It is hypothesized to have been carried to the archipelago via rafting, perhaps on abandoned fishing nets or by other indirect human activities (Mundy, Reference Mundy2005). It has been reported to hybridize with the endemic Abudefduf abdominalis (B. Mundy personal communication; Coleman et al., Reference Coleman, Gaither, Kimokeo, Stanton, Bowen and Toonen2014).
Chromis sp.
Material
Central bay: USNM 442169 (3).
Chromis agilis Smith, 1960
Material
South-east bay: USNM 442447 (1).
Chromis hanui Randall & Swerdloff, 1973
Material
South-east bay: USNM 442442 (1).
Dascyllus albisella Gill, 1862
Material
South-east bay: USNM 442398 (1); USNM 442369 (1).
Plectroglyphidodon imparipennis (Vaillant & Sauvage, 1875)
Material
North-west bay: USNM 442480 (1).
Stegastes marginatus (Jenkins, 1901)
Material
South-east bay: USNM 442451 (1); USNM 442454 (1)
Family Priacanthidae Günther, 1859
Heteropriacanthus carolinus (Cuvier, 1829)
Remarks
We follow the recent revision of the circumtropical Heteropriacanthus cruentatus by Fernandez-Silva & Ho (Reference Fernandez-Silva and Ho2017) and recognize our specimens as H. carolinus.
Material
Central bay: USNM 442408 (1).
Family Scaridae Rafinesque, 1810
Chlorurus spilurus (Valenciennes, 1840)
Remarks
Mundy (Reference Mundy2005) reported this species from Hawaii as Chlorurus sordidus (Forsskål, 1775). Subsequently, specimens of the widespread C. sordidus have been recognized as C. sordidus in the Red Sea and Indian Ocean, and C. spilurus in the Pacific and Eastern Indian oceans (see Choat et al., Reference Choat, Carpenter, Clements, Rocha, Russell, Myers, Lazuardi, Muljadi, Pardede and Rahardjo2012).
Material
South-east bay: USNM 442268 (1); USNM 442276 (1); USNM 442367 (1); USNM 442401 (1); USNM 442487 (1); USNM 442489 (1); USNM 442490 (1).
Scarus psittacus Forsskål, 1775
Material
South-east bay: USNM 442274 (1).
Central bay: USNM 442328 (1); USNM 442332 (1).
Family Sphyraenidae Rafinesque, 1815
Sphyraena barracuda (Edwards, 1771)
Material
South-east bay: USNM 442241 (1); USNM 442248 (1); USNM 442272 (1).
Family Zanclidae Bleeker, 1876
Zanclus cornutus (Linnaeus, 1758)
Material
South-east bay: USNM 442210 (1); USNM 442368 (1).
Order Tetraodontiformes Berg, 1940
Family Balistidae Rafinesque, 1810
Rhinecanthus rectangulus (Bloch & Schneider, 1801)
Figure 11

Fig. 11. Rhinecanthus rectangulus (Bloch & Schneider, 1801), family Balistidae. USNM 442410. Photo by D.E. Pitassy.
Remarks
This species is the state fish of Hawaii (see Hoover, Reference Hoover2016: 317). Its Hawaiian name is humu humu nuku nuku ā pua`a. Our two specimens were taken on spear. A specimen from the Central bay is illustrated in Figure 11.
Material
Central bay: USNM 442410 (1); South-east bay: USNM 442458 (1).
Sufflamen bursa (Bloch & Schneider, 1801)
Remarks
We collected specimens of this triggerfish while spearfishing.
Material
South-east bay: USNM 442438 (1); USNM 442444 (1).
Family Diodontidae Billberg, 1833
Diodon hystrix Linnaeus, 1758
Figure 12

Fig. 12. Diodon hystrix Linnaeus, 1758, family Diodontidae. USNM 442206. Photo by L.R. Parenti.
Remarks
We found this specimen, illustrated in Figure 12, discarded on Laenani Beach in the central portion of the Bay; it had the remnants of a fishing hook in its mouth.
Material
Central bay: USNM 442206 (1).
Family Monacanthidae Nardo, 1843
Cantherhines sandwichiensis (Quoy & Gaimard, 1824)
Material
Central bay: USNM 442413 (1); USNM 442450 (1).
Family Ostraciidae Rafinesque, 1810
Ostracion meleagris Shaw in Shaw & Nodder, 1796
Remarks
We follow Randall (Reference Randall2007) and recognize this taxon at the species level. It is recognized at the subspecies level by Mundy (Reference Mundy2005) who lists Ostracion meleagris camurum Jenkins, 1901 as a Johnston Atoll and Hawaiian Islands endemic. The other subspecies, Ostracion meleagris meleagris Shaw in Shaw & Nodder, 1796, ranges broadly throughout the Indo-Pacific, from east Africa to southern Japan, the Ogasawara Islands, Australia, Micronesia and French Polynesia (Mundy, Reference Mundy2005).
Material
South-east bay: USNM 442455 (1). Central bay: USNM 442418 (1).
Family Tetraodontidae Bonaparte, 1831
Arothron hispidus (Linnaeus, 1758)
Material
South-east bay: USNM 442222 (1); USNM 442223 (1). Central bay: USNM 442327 (1); USNM 442330 (1).
Canthigaster amboinensis (Bleeker, 1864)
Material
South-east bay: USNM 442446 (1). Central bay: USNM 442417 (1).
Canthigaster jactator (Jenkins, 1901)
Material
Central bay: USNM 442315 (1); USNM 442320 (1).
Discussion
The DNA barcode
The mitochondrial cytochrome oxidase-c subunit I (mtCOI) gene, the common DNA barcode region for animals (Hebert et al., Reference Hebert, Cywinska, Ball and Dewaard2003), was sequenced from a tissue sample of at least one specimen of 94 of the species that we collected. Other species in our report were seen but not taken or were fixed without a tissue sample. These DNA barcode sequences were used to confirm species identification if they matched barcodes of other specimens identified as that species from French Polynesia or elsewhere in the Indo-Pacific.
We compared our data to barcode sequences from individuals collected principally in French Polynesia because the voucher specimens for that material are deposited in museum collections (e.g. Delrieu-Trottin et al., Reference Delrieu-Trottin, Williams, Bacchet, Kulbicki, Mourier, Galzin, de Loma, Mou-Tham, Siu and Planes2015) and, therefore, may be used for morphological comparison to confirm identification. Sequence data that are not linked to museum vouchers are less reliable because identification of the taxon used to generate the data cannot be confirmed (e.g. Dillman et al., Reference Dillman, Zhuang, Zhang, Zhang, Mugue and Hilton2014). Additionally, we used mtCOI sequences to verify our hypothesis that the populations of some species of fishes from Kāne‘ohe Bay are genetically distinct from other populations within the Indo-Pacific. Documenting DNA barcodes in the current study also allows future monitoring of fish species composition at Kāne‘ohe Bay by analysing environmental DNA (eDNA) in water samples (Ficetola et al., Reference Ficetola, Miaud, Pompanon and Taberlet2008).
Here we discuss the results of DNA barcoding as case studies for six taxa. Data on barcoded specimens, including their USNM catalogue numbers, GenBank and BOLD database accession IDs, are in the Supplementary Material (Table S1).
Case 1: Saurida
We collected two species of Saurida, S. gracilis and S. nebulosa, that we recognized by morphology (as above). BOLD data for mtCOI, as summarized in a Neighbour-joining (NJ) tree, suggest there are at least three lineages within S. gracilis and two separate lineages of S. nebulosa as seen in Figure S1.
Saurida gracilis
We identify three lineages of S. gracilis: Lineage 1, Kāne‘ohe Bay – This lineage includes all of our samples identified as S. gracilis (Table S1). There is less than 0.50% difference in mtCOI between our samples and two other samples in this lineage from French Polynesia. We examined these French Polynesian specimens; they also key out to S. gracilis; Lineage 2, General Indo-Pacific – This lineage includes sequences from specimens from French Polynesia, the western Pacific and the western Indian Ocean. There is a 9.5–9.7% difference in mtCOI between these and our Kāne‘ohe Bay lineage; and Lineage 3, SW Indian Ocean – This lineage includes samples from off the southern African coast and nearby islands. There is a 13.8–14.1% difference in mtCOI between these and our Kāne‘ohe Bay lineage. We hypothesize that these lineages are part of a widespread S. gracilis species complex in need of revision. Further, the sequences from Lineage 3, SW Indian Ocean, are from GenBank; they need to be verified with vouchered specimens and tissue samples.
Saurida nebulosa
We identify two, distinct lineages of S. nebulosa within Kāne‘ohe Bay: Lineage 1 includes two of our samples and sequences of specimens from Australia, Indonesia and Mauritius; Lineage 2 includes two more of our samples and sequences of specimens from French Polynesia. Variation within each lineage is less than 1%, whereas the two lineages differ by 4.5–4.9% mtCOI sequence divergence. We infer that these lineages are part of what may be an S. nebulosa species complex. Sequence divergence among the lineages is not as deep as that of lineages of S. gracilis. Yet, our specimens that key out to S. nebulosa are in two distinct lineages in the BOLD NJ tree, as in Figure S1. Further work is needed to test hypotheses of species limits of the Kāne‘ohe Bay species of Saurida.
Case 2: Sebastapistes coniorta Jenkins, 1903
Once regarded as endemic to the Hawaiian and Johnston islands, Sebastapistes coniorta has been reported recently from New Caledonia and Wake Atoll (Fricke et al., Reference Fricke, Kulbicki and Wantiez2011). Randall (Reference Randall2007) considered that reports of this species living outside of Hawai‘i and throughout Oceania could be of the morphologically similar S. tinkhami. We sequenced the mtCOI DNA barcode of one specimen of S. coniorta from Kāne‘ohe Bay and compared it with the barcodes of three specimens of S. tinkhami from French Polynesia. The mtCOI DNA barcodes of the three French Polynesian specimens, MNHN 2008-360, USNM 423383 and USNM 400555, differ from each other by just 0.2%. Our Kāne‘ohe Bay specimen (USNM 442298) differs from these three by 3.1–3.5%. Our Hawaiian S. coniorta are genetically distinct from the French Polynesian S. tinkhami, confirming the hypothesis that these are distinct species. Voucher specimens and tissue samples of specimens identified as S. coniorta from New Caledonian and Wake Atoll should be examined to confirm their species identification.
Case 3: Acanthurus triostegus (Linnaeus, 1758)
A genetic analysis of populations of A. triostegus throughout its broad range suggested that populations from Hawaii (including O‘ahu) and the Marquesas, French Polynesia, are distinct from other Pacific populations (Planes & Fauvelot, Reference Planes and Fauvelot2002, Figure 2). The mtCOI DNA barcode of our Kāne‘ohe Bay specimen is just 1.56–1.76% different from that of Gambier, Austral, and Manua'e (Scilly) Islands, French Polynesia, populations identified as A. triostegus, which supports the observation that there is little genetic difference among populations of this species in these areas.
Case 4: Asterropteryx semipunctata Rüppell, 1830
This goby is one of the most common species throughout Kāne‘ohe Bay. It lives in a variety of shallow marine habitats and is relatively easy to collect and, therefore, is well-studied in the Bay (e.g. Privitera, Reference Privitera2002; Hagedorn et al., Reference Hagedorn, Daly, Carter, Cole, Jaafar, Lager and Parenti2018). The species is broadly distributed and ranges from the western Indian Ocean to the central Pacific. Its type locality is the Red Sea. We tested the hypothesis that Hawaiian populations of this broadly distributed Indo-Pacific species may be genetically distinct. Four specimens of A. semipunctata from the Gambier Islands, French Polynesia (USNM 401104, USNM 399599, USNM 399547, USNM 399551) have nearly identical barcodes, differing from each other only by 0.6% or less. Our specimen from Kāne‘ohe Bay (USNM 442231) differs from these four by 6.3–7.1% sequence divergence. This difference between French Polynesian and Hawaiian populations suggests that this widespread species needs revision and may represent a species complex.
Case 5: Kuhlia xenura (Jordan & Gilbert, 1882)
There are two valid species of the genus Kuhlia in Hawaii, following Randall & Randall (Reference Randall and Randall2001): the widespread Kuhlia sandvicensis (Steindachner, 1876) and K. xenura (Jordan & Gilbert, 1882), a Hawaiian endemic. Kuhlia xenura is distinguished from K. sandvicensis by a larger eye, among other morphological characters. We sequenced the mtCOI barcodes of three specimens we identified as K. xenura from Kāne‘ohe Bay and compared it with the barcodes of six specimens of K. sandvicensis from French Polynesia. Two of our specimens (USNM 442289, USNM 442361) differ from the French Polynesian specimens (MNHN 2008–686, MNHN 2008-687, USNM 424191, USNM 399546, USNM 399548, USNM 399602) by 6.2–6.4% mtCOI sequence divergence. A third specimen of K. xenura from Kāne‘ohe Bay (USNM 442430) differed from the French Polynesian specimens by only 1.4–1.6%. This specimen has a relatively large eye as seen in Figure 9, a character of K. xenura, according to Randall & Randall (Reference Randall and Randall2001). We conclude that our specimens from Kāne‘ohe Bay represent K. xenura, but that additional study may reveal potential hybridization between these two Kuhlia species in Hawaii.
Case 6: Oxycheilinus unifasciatus (Streets, 1877)
This species of Oxycheilinus has a distinctive white band that circumvents the body just anterior to the caudal peduncle. We sequenced the mtCOI barcode of two specimens we identified as O. unifasciatus from Kāne‘ohe Bay and compared it with the barcodes of three specimens identified as O. unifasciatus from the Gambier and Austral islands, French Polynesia (USNM 404650, USNM 423436, USNM 423374) and two specimens from Futuna (USNM 446422, USNM 446380). The mtCOI sequences of the five Polynesian specimens differ from each other by 0.2–0.5%. Our Hawaiian specimens (USNM 442374, USNM 442462) differ from these by 2.3–2.8% sequence divergence. Our specimens of Oxycheilinus unifasciatus have a relatively large mouth that extends posteriorly beyond the anterior rim of the orbit and represent unrecognized morphological and genetic diversity.
Fish diversity of Kāne‘ohe Bay
To develop a working list of the fish species that live in Kāne‘ohe Bay, we compiled the taxa reported in the surveys of Watson & Leis (Reference Watson and Leis1974), Coles et al. (Reference Coles, DeFelice and Eldredge2002), Greenfield (Reference Greenfield2003) and the current study (Appendix 1). This list of some 388 taxa is not complete, nor have all the species on it been confirmed to live in the Bay. Further we do not expect many of the taxa that Watson & Leis (Reference Watson and Leis1974) reported only as larvae and eggs to be found in the Bay as adults (Appendix 1). The identification and distribution of each species should be validated with a vouchered specimen and, ideally, a tissue sample.
Nearly all of our 109 species are represented by a formalin-fixed voucher specimen and one or more tissue samples, a colour photograph and collection data. Greenfield (Reference Greenfield2003) reported 202 species classified in 49 families; specimens of some 190 species were fixed in formalin and catalogued in the collections of the California Academy of Sciences (CAS), San Francisco. The formalin-fixed specimens collected by Watson & Leis (Reference Watson and Leis1974) representing some 80 listed taxa representing over 175 different types of eggs or larvae were ultimately distributed among various museum and university collections. Coles et al. (Reference Coles, DeFelice and Eldredge2002) developed their list of some 263 species with a combination of literature reports and museum collections. Watson & Leis (Reference Watson and Leis1974), Coles et al. (Reference Coles, DeFelice and Eldredge2002) and Greenfield (Reference Greenfield2003) did not collect tissue samples for genetic analysis as that was not yet the custom.
Different fish collecting methods yield different species. Only 10 species appear on all four lists: nine native species, synodontid Saurida gracilis; the atherinid Atherinomorus insularum; the gobiids Asterropteryx semipunctata, Eviota epiphanes and Psilogobius mainlandi; the apogonid Foa brachygramma; the kuhliid Kuhlia xenura; the pomacentrids Abudefduf abdominalis and Stegastes marginatus; and one introduced species, the blenniid Omobranchus obliquus. We did not obtain many of the small, cryptic fish species reported from Kāne‘ohe Bay by Greenfield (Reference Greenfield2003) who collected using ichthyocides with dipnets on scuba. For example, Greenfield (Reference Greenfield2003) collected 22 species of gobiids, whereas we collected just 11 (Appendix 1). Moray eels, family Muraenidae, are particularly difficult to capture with our methods; we recorded just three species compared with the 13 of Greenfield (Reference Greenfield2003). Likewise, Watson & Leis (Reference Watson and Leis1974) reported just two of some nearly 40 species of eels known for their extensive leptocephalus larval phases. In contrast, our focused effort on the larger, more conspicuous reef fishes using spears yielded 11 species of acanthurids, whereas Greenfield (Reference Greenfield2003) collected just six acanthurids. Our study was also unique among the four in documenting the occurrence of the balistid Rhinecanthus rectangulus, the state fish of Hawaii, in the Bay.
Using plankton tows, Watson & Leis (Reference Watson and Leis1974) collected larvae and eggs over a period of one year. The cryptic gobioids Schindleria pietschmani and S. praematura, not reported in the three other studies, were abundant in their collections. Further, both Greenfield (Reference Greenfield2003) and our study did not report the Bay's endemic anchovy Enchrasicholina purpurea.
Also of interest is the report by Watson & Leis (Reference Watson and Leis1974) from the Sampan Channel of larvae or eggs of some 20 species in oceanic families including Gonostomatidae, Myctophidae, Stomiidae and Chlorophthalmidae (Appendix 1). Although the adults of these species are not expected to be found in the Bay, they may be important components of environmental DNA (eDNA) analyses. Collection of tissue samples along with voucher specimens is critical for future eDNA analyses of water and aquatic sediment samples of the Bay. Also, the eggs and larvae of these taxa may provide an important trophic component to other post-metamorphic fishes that visit, or reside in, Kāne‘ohe Bay (Brandl et al., Reference Brandl, Tornabene, Goatley, Casey, Morais, Côté, Baldwin, Parravicini, Schiettekatte and Bellwood2019).
Conclusions
We collected 109 species of fishes in Kāne‘ohe Bay during the 2017 MarineGEO survey. Nearly all the species we report are represented by one or more tissue samples in addition to a formalin-fixed voucher specimen, a colour photograph, plus other data. We were successful in collecting the larger, more conspicuous reef fishes that live throughout the Bay, but were unable to collect many of the smaller, cryptic fishes. To fill in these gaps, we will continue to explore methods to record a higher diversity of fish species in the Bay and maintain voucher specimens and tissue samples.
The mtCOI DNA barcode provides evidence to confirm the identifications of fish species in Kāne‘ohe Bay. It also provides evidence to differentiate these populations from those of the same nominal species in French Polynesia and elsewhere throughout the Indo-Pacific. Despite the observed genetic distances, we do not propose species validity/synonymy from barcode sequences alone because these data are insufficient to make such conclusions (see Ebach & Holdrege, Reference Ebach and Holdrege2005). Genetic lineages may represent valid species, but we do not recognize them as such without description of concordant morphological differences (e.g. Baldwin et al., Reference Baldwin, Castillo, Weigt and Victor2011). Even with these caveats, we find the mtCOI DNA barcode sequences to be a useful tool to assess diversity and to provide preliminary data for eventual environmental DNA (eDNA) surveys of Kāne‘ohe Bay.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315420000417.
Acknowledgements
We thank the chairs of the MarineGEO Hawaii Team, M. Hagedorn, L.R. Parenti, C. Lager and M.X. Weber and the Hawai‘i Institute of Marine Biology and its Director, the late Ruth Gates, for facilitating this research. Extraordinary fishers joined us in the field, including Archana Anand, Felipe Carvalho, Jon Daly, Daniel Gross, Mike Henley, Eileen Nalley, Molly Timmers, Leon Weaver, Charley Westbrook and Julie Zill. Hi‘ilei Kawelo, Executive Director of Paepae o He‘eia, and staff graciously invited us to collect with them in the He‘eia fishpond. High school student Haven Bashaw participated in many of our field and laboratory activities. Tina Ramoy and Ned Busch graciously volunteered their time. Dan Mulcahy, Kenan Matterson and Mike Trizna assisted with the DNA barcoding at the Laboratory of Analytical Biology. Jeff Clayton provided additional valuable technical assistance. We are grateful to Bruce Mundy, Rich Pyle, Jeff Leis, Jack Randall, Arnold Suzumoto and Bill Watson for their help with various aspects of this project including generous sharing of their extensive, expert knowledge of Hawaiian fishes. David G. Smith provided information on names of eels. Freya Goetz provided information on collection methods. Bruce Mundy graciously reviewed a draft of the manuscript and provided extensive, detailed comments for which we are most grateful. This is contribution 51 from the Smithsonian's MarineGEO network.
Financial support
We thank the following for their generous financial support of the MarineGEO survey: the Tennenbaum Marine Observatory Network, the Smithsonian Institution (SI) Global Genome Initiative, the SI DNA Barcode Network, the Leonard P. Schultz Fund and the Herbert R. and Evelyn Axelrod Endowment, Division of Fishes, National Museum of Natural History, the Smithsonian Tropical Research Institute, the Smithsonian Conservation Biology Institute, the Castle Foundation, the Matthew Frank Foundation and the Zilber Family Foundation.
Appendix 1. Working list of fishes in Kāne‘ohe Bay with a comparison of previous studies