Published online by Cambridge University Press: 30 September 2024
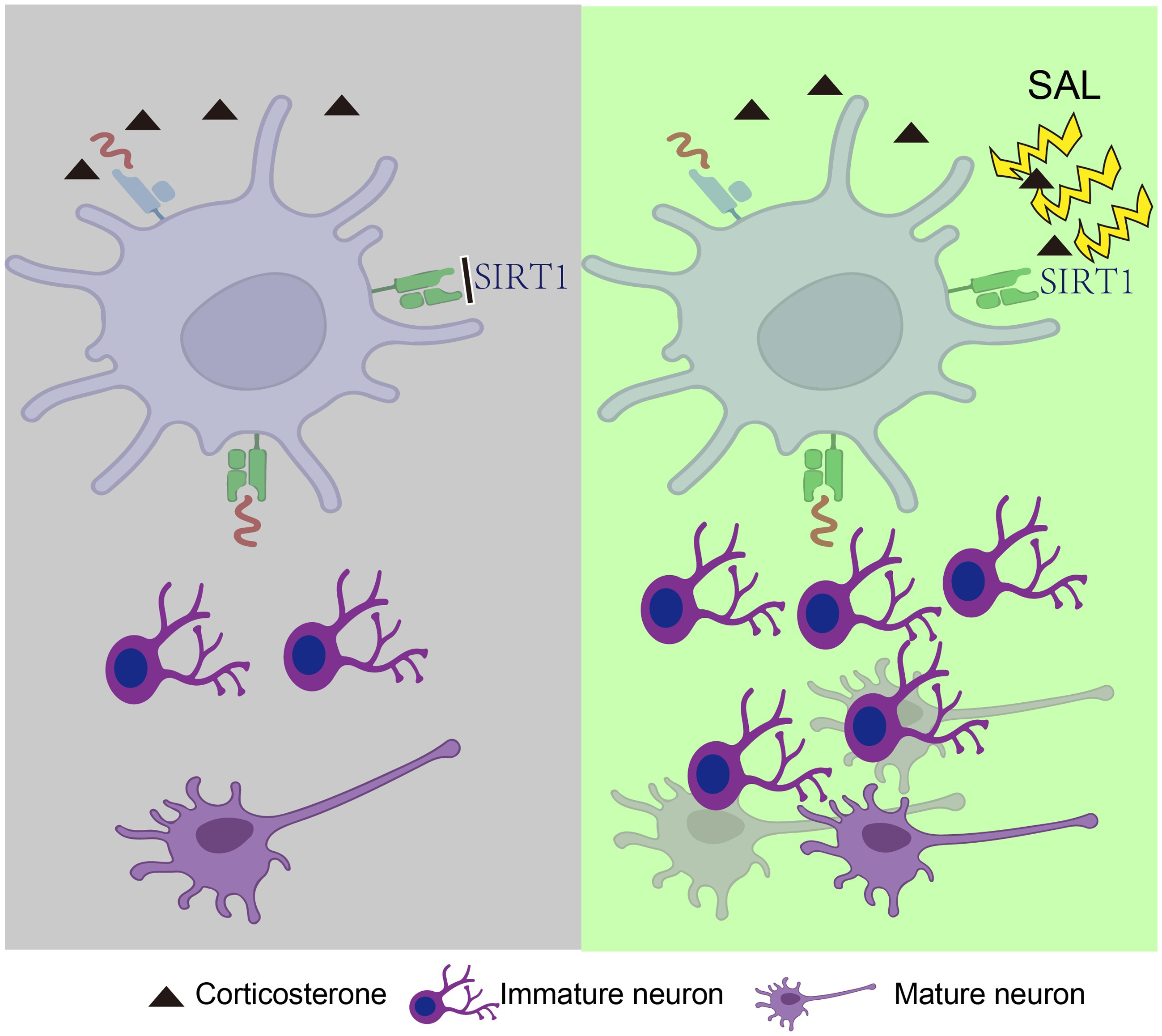
Depression is one of the major mental disorders, which seriously endangers human health, brings a serious burden to patients’ families. In this study, we intended to further explore the antidepressant-like effect and possible molecular mechanisms of Salidroside (SAL). We built corticosterone (CORT)-induced depressive mice model and used behavioural tests to evaluate depression behaviour. To explore the molecular mechanisms of SAL, we employed a variety of methods such as immunofluorescence, western blot, pharmacological interference, etc. The results demonstrated that SAL both at 25 mg/kg and 50 mg/kg can reduce immobility time in the tail suspension test (TST). At the same time, SAL treatment could restore the reduced sugar water intake preference in the sucrose preference test (SPT) in CORT-induced depressive mice and reduce the immobility time in TST and forced swimming experiments (FST). In addition, SAL treatment reversed the reduction in the number of Ki-67, BrdU, and NeuN in the hippocampus due to CORT treatment. SAL treatment also restored the expression of SIRT1, PGC-1α, brain-derived neurotrophic factor (BDNF) and other proteins in the hippocampus. In addition, after blocking SIRT1 signalling with EX527, we found that the treatment with SAL failed to reduce the immobility time in TST and FST, the level of SIRT1 and PGC-1α activity were correspondingly downregulated, and the expression of DCX and Ki-67 in the hippocampus failed to be activated. These findings suggested that SAL exerts antidepressant-like effects by promoting hippocampal neurogenesis through the SIRT1/PGC-1α signalling pathway.
Shan Xing, Shuyi Xu and Linjiao Wang contributed equally to this work.