The developmental origins of health and disease (DOHaD) or the fetal origins of adult disease (FOAD) models posit that environmental exposures early in development, and particularly during intrauterine life, have lasting implications for health and disease across the life span (Barker, Reference Barker1990, Reference Barker1994, Reference Barker1994, Reference Barker1995; Gluckman & Hanson, Reference Gluckman and Hanson2004). There is compelling support for the DOHaD/FOAD hypotheses in terms of adult physical health and mental health (Hanson & Gluckman, Reference Hanson and Gluckman2014). A large epidemiological literature provides support for the FOAD hypothesis by demonstrating that small size at birth is associated with increased risk for many pathologies throughout the life span, including heart disease, obesity, diabetes (Barker, Eriksson, Forsén, & Osmond, Reference Barker, Eriksson, Forsén and Osmond2002; Jornayvaz et al., Reference Jornayvaz, Vollenweider, Bochud, Mooser, Waeber and Marques-Vidal2016), and psychiatric illness (Class, Rickert, Larsson, Lichtenstein, & D'Onofrio, Reference Class, Rickert, Larsson, Lichtenstein and D'Onofrio2014; Sømhovd, Hansen, Brok, Esbjørn, & Greisen, Reference Sømhovd, Hansen, Brok, Esbjørn and Greisen2012; Thompson, Syddall, Rodin, Osmond, & Barker, Reference Thompson, Syddall, Rodin, Osmond and Barker2001). Small size at birth is also linked to neurological development (DiPietro et al., Reference DiPietro, Kivlighan, Costigan, Rubin, Shiffler, Henderson and Pillion2010). Small size at birth is not likely to be the cause of subsequent disease outcomes, but rather being born small reflects various prenatal perturbations. Prospective research therefore is needed to characterize the prenatal environment and investigate how it shapes developmental trajectories (Howland, Sandman, Davis, & Glynn, Reference Howland, Sandman, Davis and Glynn2020). Building on the findings that intrauterine experiences shape mental health outcomes (O'Donnell & Meaney, Reference O'Donnell and Meaney2017), research suggests that prenatal exposures can also have transformative neurobiological effects on fetal brain circuit maturation.
The prenatal period is a time of rapid growth and the beginning of neurologic development for the fetal brain (Huttenlocher & Dabholkar, Reference Huttenlocher and Dabholkar1997; Stiles & Jernigan, Reference Stiles and Jernigan2010). The extraordinary rate of brain maturation in utero means that both salutary and deleterious environmental signals have the potential to alter the trajectory of brain development. During the transformation from a zygote to a human newborn in the 9 months of full-term gestation, cell division and differentiation is both rapid and highly coordinated (Huttenlocher & Dabholkar, Reference Huttenlocher and Dabholkar1997; Stiles & Jernigan, Reference Stiles and Jernigan2010). White matter microstructure is fundamental to transmission of neural communication, and undergoes pronounced development during the prenatal period (Knickmeyer et al., Reference Knickmeyer, Gouttard, Kang, Evans, Wilber, Smith and Gilmore2008). White matter tracts begin emerging within the fetal brain between 13 and 19 gestational weeks, with evidence that major white matter tracts (e.g., the corpus callosum, superior and inferior fasciculi, and cingulum) are present by birth (Gilmore, Knickmeyer, & Gao, Reference Gilmore, Knickmeyer and Gao2018).
Maternal psychological distress during the prenatal period is one important environmental signal that shapes developmental trajectories in the offspring. Rates of psychological distress including elevated symptoms of anxiety and depression are seen in up to 25% of pregnant women (Muzik & Borovska, Reference Muzik and Borovska2010) with rates even higher among socioeconomically at-risk populations (Katz, Crean, Cerulli, & Poleshuck, Reference Katz, Crean, Cerulli and Poleshuck2018; Koleva, Stuart, O'Hara, & Bowman-Reif, Reference Koleva, Stuart, O'Hara and Bowman-Reif2011). The high prevalence of prenatal psychopathology is of critical public health importance, as it indicates impairment not only in maternal psychological wellbeing, but also has robust long-term consequences for child mental health (Capron et al., Reference Capron, Glover, Pearson, Evans, O'Connor, Stein and Ramchandani2015; Davis & Narayan, Reference Davis and Narayan2020; O'Donnell, Glover, Barker, & O'Connor, Reference O'Donnell, Glover, Barker and O'Connor2014; Plant, Pariante, Sharp, & Pawlby, Reference Plant, Pariante, Sharp and Pawlby2015). Prenatal maternal distress is associated with a range of neurodevelopmental outcomes, including behavioral problems, difficult temperament, negative emotionality, and internalizing problems (Davis & Sandman, Reference Davis and Sandman2012; Park et al., Reference Park, Kim, Kim, Shin, Yoo, Lee and Cho2014; Van den Bergh, Calster, Smits, Huffel, & Lagae, Reference Van den Bergh, Calster, Smits, Huffel and Lagae2008). Importantly, prenatal maternal symptoms of distress predict later infant and child psychopathology and risk mechanisms even when maternal symptoms of distress are subclinical and below diagnostic categorical thresholds (Glynn, Howland, & Fox, Reference Glynn, Howland and Fox2018; O'Connor, Monk, & Fitelson, Reference O'Connor, Monk and Fitelson2014; Sandman, Buss, Head, & Davis, Reference Sandman, Buss, Head and Davis2015). These findings highlight the importance of investigating the intergenerational consequences of maternal distress from a transdiagnostic perspective as supported by the National Institute of Mental Health's research domain criteria (RDoC) initiative (Gao et al., Reference Gao, Ostlund, Brown, Kaliush, Terrell, Vlisides-Henry and Conradt2021; Insel et al., Reference Insel, Cuthbert, Garvey, Heinssen, Pine, Quinn and Wang2010).
Accumulating evidence across experimental studies in rodents and observational studies in humans have demonstrated that postnatal stress exposure, particularly when experienced during early life, is associated with variability in the structure and function of frontolimbic and temporal circuitry (for review see Chen & Baram, Reference Chen and Baram2016; McLaughlin, Weissman, & Bitrán, Reference McLaughlin, Weissman and Bitrán2019). These neural circuits are important for affective processing, including the evaluation of social stimuli and emotion regulation (Dannlowski et al., Reference Dannlowski, Stuhrmann, Beutelmann, Zwanzger, Lenzen, Grotegerd and Kugel2012; Hartley & Phelps, Reference Hartley and Phelps2010). In experimental research with rodents and nonhuman primates, prenatal stress exposure causes changes in the basic neuroarchitecture of the amygdala, with evidence of stress-related increases in dendritic arborization in rodents (Mitra, Jadhav, McEwen, Vyas, & Chattarji, Reference Mitra, Jadhav, McEwen, Vyas and Chattarji2005) and alterations in neuronal cytoskeleton and synapse formation following glucocorticoid exposure in the fetal baboon (Antonow-Schlorke, Schwab, Li, & Nathanielsz, Reference Antonow-Schlorke, Schwab, Li and Nathanielsz2003). Human studies have focused primarily on prenatal influences on volume and thickness (Davis et al., Reference Davis, Hankin, Glynn, Head, Kim and Sandman2020; Moog et al., Reference Moog, Entringer, Rasmussen, Styner, Gilmore, Kathmann and Buss2018; Rifkin-Graboi et al., Reference Rifkin-Graboi, Bai, Chen, Hameed, Sim, Tint and Qiu2013; Sandman et al., Reference Sandman, Buss, Head and Davis2015).
There are few studies in humans linking prenatal maternal distress to white matter integrity (for review see Demers, Aran, Glynn, & Davis, Reference Demers, Aran, Glynn, Davis, Wazana, Székely and Oberlander2021). Microstructural integrity of white matter tracts can be assessed noninvasively through DTI, providing an important methodology to study maturation of neural circuits and to probe white matter changes over the life span. Common DTI metrics include fractional anisotropy (the fraction of diffusion that is directionally dependent, i.e., anisotropic), mean diffusivity (the total water mobility), axial diffusivity (the diffusivity along the main fiber orientation), and radial diffusivity (the diffusivity perpendicular to the main fiber). Changes in diffusion can arise from a variety of biological events, including alterations in axonal fiber integrity, membrane proliferation, axon density, organization, and myelination. As fractional anisotropy is sensitive to this variety of microstructural changes in the white matter, it can be difficult to interpret biologically. In contrast, studies have shown that changes in axial diffusivity largely reflect perturbation to axonal fiber organization and density, while decreases in radial diffusivity primarily indicate increases in axonal myelination (Winklewski et al., Reference Winklewski, Sabisz, Naumczyk, Jodzio, Szurowska and Szarmach2018) (see Table 1 for DTI metric definitions). Higher fractional anisotropy is generally thought to indicate enhanced white matter integrity (Soares, Marques, Alves, & Sousa, Reference Soares, Marques, Alves and Sousa2013). A recent longitudinal study of normative early childhood brain circuit maturation has reported widespread increases in fractional anisotropy, as well as decreases in radial diffusivity and axial diffusivity, between birth and 1 year of age for the major white matter tracts (Stephens et al., Reference Stephens, Langworthy, Short, Girault, Styner and Gilmore2020).
Table 1. Diffusion tensor imaging (DTI) metric definitions

A relatively small literature demonstrates links between prenatal maternal distress and persisting alterations in neural circuit development assessed with measures of white matter microstructure into childhood (Hay et al., Reference Hay, Reynolds, Grohs, Paniukov, Giesbrecht, Letourneau and Lebel2020; Sarkar et al., Reference Sarkar, Craig, Dell'Acqua, O'Connor, Catani, Deeley and Murphy2014) and young adulthood (Marečková, Klasnja, Andrýsková, Brázdil, & Paus, Reference Marečková, Klasnja, Andrýsková, Brázdil and Paus2019). Although these studies suggest that distress during pregnancy is associated with lasting influences on white matter integrity, the ability to reach clear conclusions is limited by the fact that the imaging outcomes are collected later in childhood at a time when the postnatal environment has had a significant impact. In an effort to isolate the unique effects of the distress during the prenatal period, several studies have investigated whether associations remain even when controlling for postnatal distress (El Marroun et al., Reference El Marroun, Zou, Muetzel, Jaddoe, Verhulst, White and Tiemeier2018; Lebel et al., Reference Lebel, Walton, Letourneau, Giesbrecht, Kaplan and Dewey2016; Wen et al., Reference Wen, Poh, Ni, Chong, Chen, Kwek and Qiu2017). Assessments of white matter integrity shortly after birth allow for a more rigorous test of the hypothesis that prenatal maternal distress affects brain circuit development before postnatal factors, including parental care (Glynn & Baram, Reference Glynn and Baram2019), can exert an influence (see Figure 1 for conceptual model).

Figure 1. Model of intergenerational transmission of risk. The prenatal period is a time when the fetal brain is highly susceptible to the effects of prenatal maternal depression and other signals of maternal psychological and physiological distress. However, the influence of prenatal depression exposure on neonatal neural circuit maturation remains poorly understood. Alterations in the neurodevelopment of white matter microstructure is one potential etiological mechanism through which prenatal stress influences child outcomes.
Several longitudinal studies have considered associations between prenatal distress exposure and development of white matter architecture in neonates. Within this small extant literature, there is emerging evidence that exposure to environmental signals in utero may influence the trajectory of white matter development as early as infancy. However, the literature to date is relatively limited, and existing studies have used inconsistent methodological approaches, both in terms of the assessment of prenatal distress and the brain regions investigated. Many of the studies assessing the role of prenatal distress on infant white matter integrity have focused specifically on amygdala–prefrontal circuits, given the importance of this circuitry in emotion regulation, and have found mixed results (Humphreys, Camacho, Roth, & Estes, Reference Humphreys, Camacho, Roth and Estes2020; Posner et al., Reference Posner, Cha, Roy, Peterson, Bansal, Gustafsson and Monk2016; Rifkin-Graboi et al., Reference Rifkin-Graboi, Bai, Chen, Hameed, Sim, Tint and Qiu2013). One study demonstrated associations between categorically high and low symptoms of prenatal depression exposure and decreased structural connectivity of the amygdala–ventromedial prefrontal cortex circuit in newborns (Posner et al., Reference Posner, Cha, Roy, Peterson, Bansal, Gustafsson and Monk2016). Rifkin-Graboi et al. (Reference Rifkin-Graboi, Bai, Chen, Hameed, Sim, Tint and Qiu2013) found evidence of decreased white matter integrity within the bilateral amygdala; this effect was only obtained when prenatal depressive symptoms were categorized into high versus low–normal groups; no significant associations were obtained when analyses were conducted with depressive symptoms assessed dimensionally. There is recent evidence that prenatal distress also may be associated with increases in white matter structural integrity of the amygdala–prefrontal tract. In a recent pilot study of 33 infants scanned approximately 5 weeks after birth, Humphreys et al. (Reference Humphreys, Camacho, Roth and Estes2020) found that prenatal stress was associated with increased structural connectivity between the amygdala and the medial prefrontal cortex. This finding held when covarying for preconception stress, highlighting the unique importance of the prenatal period independent from the effects of cumulative life stress across the mother's life span.
One limitation of these studies is the focus on amygdala–prefrontal circuitry without consideration of other circuits within the neonatal brain. Emerging evidence from studies using a whole brain approach suggests that prenatal distress also may influence the development of circuits implicated in affective (temporolimbic tracts) and sensory (occipitotemporal tracts) processing. For example, voxel-wise whole brain analyses comparing neonates of mothers reporting categorically high versus low prenatal anxiety identified decreases in fractional anisotropy in regions corresponding to the right insula and dorsolateral prefrontal cortex, inferior–frontal occipital fasciculus, uncinate fasciculus, posterior cingulate, and parahippocampus (Rifkin-Graboi et al., Reference Rifkin-Graboi, Meaney, Chen, Bai, Hameed, Tint and Qiu2015). Similarly, another study employing voxel-wise analyses found that elevations of a composite of prenatal anxiety and depressive symptoms was associated with increased radial and axial diffusivity within the corona radiata, external capsule, and dorsolateral prefrontal cortex. Despite finding differences in white matter diffusivity, no significant differences were found with fractional anisotropy, suggesting that different metrics of white matter integrity have varying sensitivities to maternal distress (Dean et al., Reference Dean, Planalp, Wooten, Kecskemeti, Adluru, Schmidt and Davidson2018). In an opposing finding, a recent study in 6-month-old infants demonstrated that prenatal maternal distress was associated with lower radial diffusivity and elevated fractional anisotropy within the corpus callosum, which in turn predicted later behavioral problems at 18 months (Borchers, Dennis, King, Humphreys, & Gotlib, Reference Borchers, Dennis, King, Humphreys and Gotlib2020).
The current study fills a significant gap in the literature by investigating the integrity of affective (i.e., temporolimbic) and sensory processing (i.e., occipitotemporal) circuits in addition to frontolimbic circuitry. Based on experimental and human studies of early life stress exposure, we aimed to investigate associations between prenatal distress and structural integrity within circuits involved in emotional regulation (i.e., uncinate fasciculus and cingulum), affective processing (i.e., fornix), and sensory processing (i.e., inferior fronto-occipital fasciculus). This study used a prospective and longitudinal design with a racially and ethnically diverse sample of mother–infant dyads recruited early in pregnancy to investigate the association between prenatal maternal distress and infant white matter development. The state-trait anxiety inventory (STAI), a measure that is consistent with putative measures and constructs within the negative valence system of the RDoC framework (Cuthbert & Insel, Reference Cuthbert and Insel2013), was used to assess prenatal maternal stress. The RDoC initiative emphasizes evaluating and validating dimensional constructs, integrating psychosocial and biological factors, and may therefore be an important framework to use in the investigation of intergenerational mechanisms of risk. Neonatal diffusion-weighted images were collected and analyzed using a robust atlas building approach (Verde et al., Reference Verde, Budin, Berger, Gupta, Farzinfar, Kaiser and Styner2014), which allows for the extraction of diffusion tensor metrics along respective white matter tracts of interest. This method facilitates both subject outlier detection and the specification of localized regions along a given tract for targeted hypothesis testing.
Materials and Method
Study overview
Pregnant women were assessed at 17 and 29 gestational weeks to measure prenatal maternal distress. Neonatal white matter microstructure was assessed during natural sleep via DTI at 42–45 weeks’ postconceptional age (~2–5 weeks after birth).
Study participants
Participants included 85 mother–infant dyads who were drawn from a longitudinal investigation of the impact of maternal mental health during pregnancy on offspring developmental outcomes (the Care Project) (Davis, Hankin, Swales, & Hoffman, Reference Davis, Hankin, Swales and Hoffman2018) and whose assessment was completed prior to the start of the COVID-19 pandemic being declared a state of emergency (March 10, 2020). Recruitment was primarily from obstetrics clinics at two major medical centers in Denver, Colorado. All study procedures were approved by the Institutional Board for the Protection of Human Subjects at the University of Denver and the University of Colorado Anschutz medical campus, and all mothers provided written informed consent for themselves and their infant.
Inclusion criteria for mothers’ enrollment in the study were (a) maternal age between 18 and 45 years, (b) singleton pregnancy, (c) gestational age (GA) less than 25 weeks, and (d) proficiency in English. Exclusion criteria included (a) current illicit drug or methadone use, (b) major health conditions requiring invasive treatments (e.g., dialysis, blood transfusions, chemotherapy), (c) current or past symptoms of psychosis or mania based on the structured clinical interview (SCID) for the Diagnostic and statistical manual of mental disorders, fifth edition, and (d) current participation in cognitive behavioral therapy or interpersonal therapy.
Additional exclusion criteria for the current study included (a) preterm birth <34 gestational weeks (n = 0), (b) major fetal or chromosomal anomalies (n = 1) and neonatal complications requiring a neonatal intensive care unit stay (e.g., mechanical ventilation; n = 0), and (c) any infant magnetic resonance imaging (MRI) contraindications (n = 2) (e.g., metal implant). Of the infants who attended the MRI scan, three were unable to be scanned (e.g., infant did not fall asleep during the scanning window), two DTI scans were not acquired because the infant woke up in the scanner, and three scans failed initial quality control procedures. Following additional quality control procedures for DTI image processing (see section on materials and methods for further details), 13 of the 85 subjects were removed for the bed nucleus of the stria terminalis amygdala (BNST)–amygdala tract and three were excluded for the right anterior cingulate tract.
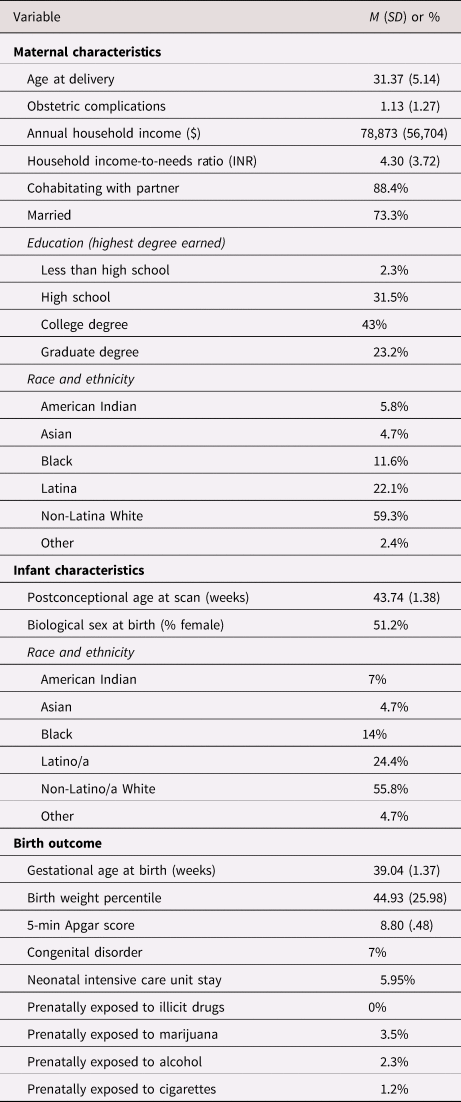
Mothers in the study were 21–41 years old (M = 31.37, SD = 5.14) at delivery (see Table 2 for sample characteristics). Median annual household income was $70,000, and 31% of participants were living at or near federal classification of poverty (less than 200% income-to-needs ratio [INR]). Infants (51% female) were 39 weeks’ gestation at birth on average and scanned at 43 weeks postconceptional age (range 41.6–49.4). Fifty-nine percent of participants were non-Hispanic white and 22% were Hispanic/Latina, with the remainder of the sample identifying as Black, Asian, or Multi-ethnic.
Table 2. Demographic and medical characteristics of the sample
Maternal distress symptoms
Pregnant women's levels of distress were assessed via the 20-item state anxiety subscale of the STAI (Spielberger, Reference Spielberger1983) at 17 and 29 weeks’ gestation. Factor analyses of the STAI indicate that items comprise a higher-order negative affectivity factor, and this factor structure suggests that the STAI is best conceptualized as a measure of general distress (Bados, Gómez-Benito, & Balaguer, Reference Bados, Gómez-Benito and Balaguer2010; Bieling, Antony, & Swinson, Reference Bieling, Antony and Swinson1998). Participants indicate how they have felt over the past week, including today. Items include: “I am tense” and “I am worried”. All items were rated on a 4-point Likert scale, with higher scores indicating greater distress. The STAI has been extensively used to measure distress during pregnancy (e.g., Davis & Sandman, Reference Davis and Sandman2010; Fischbein et al., Reference Fischbein, Nicholas, Kingsbury, Falletta, Baughman and VanGeest2019). Within the current sample, internal consistency was excellent (α = .96 at 17 weeks and α = .96 at 29 weeks).
Sociodemographic characteristics
Maternal birth date, socioeconomic status, cohabitation with child's father, marital status, educational attainment, and race and ethnicity were collected via maternal interview. A family INR was calculated by dividing the total reported household income by the poverty threshold corresponding to the number of persons living in the household at the time of study entry, specified by the U.S. Census Bureau (2020).
Pregnancy and birth outcomes
Prenatal obstetric complications, birth outcomes, infant biological sex at birth, birth weight, and 5-min Apgar score were abstracted from the medical record. In addition, birth weight percentile, which accounts for gestational age at birth (GAB) and infant biological sex, was determined. Estimated date of delivery was determined by early ultrasound measures and date of last menstrual period based on the American College of Obstetricians and Gynecologists guidelines and used to calculate GAB and postconceptional age at scan (Committee on Obstetric Practice, the American Institute of Ultrasound in Medicine, and the Society for Maternal-Fetal Medicine, 2017). An obstetric complications score was calculated, indicating the presence or absence of pregnancy-related complications, including prenatal infection, pregnancy-included hypertension, gestational diabetes, oligohydramnios, polyhydramnios, preterm labor, vaginal bleeding, placenta previa, or anemia (Hobel, Reference Hobel, Bolognese, Schwartz and Schneider1982). Seventy-one percent of the women had none or one of the obstetric complications on this index. Fetal exposures to illicit drugs, marijuana, cigarettes, and alcohol were assessed via maternal interview and presence of positive infant toxicology screens at birth.
Magnetic resonance imaging acquisition
Infants were scanned unsedated during natural sleep. Noise from the scanner was reduced by the use of malleable ear plugs and neonatal ear covers. Headphones played white noise during image acquisition. A Siemens Skyra 3 T MRI system equipped with a 20-channel head coil at the Brain Imaging Center at the University of Colorado Anschutz medical campus was used.
Diffusion tensor images were obtained using a simultaneous multislice sequence (repetition time, TR = 6,100 ms, echo time, TE = 60, field of view, FOV = 220, matrix size = 128 × 128; 50 axial slices with 2.0 mm thickness; phase-encoding [PE] direction = anterior–posterior, AP). Diffusion MRI data were acquired with three diffusion weightings (b-values) (b = 300, 800, 2,000 s/mm2), with 10, 30, and 64 unique gradient directions per respective shell (104 gradient directions total). In addition, 18 interspersed b = 0 s/mm2 images were acquired as a baseline. The total acquisition time was 7 min (multiband acceleration 3, TE/TR 92/3,600 ms).
Image processing
A study-specific quality control protocol was applied to all raw diffusion-weighted imaging (DWI) data using DTIPrep (www.nitrc.org/projects/dtiprep), which includes slice-wise and gradient-wise artifact detection, as well as eddy current and motion correction. For all analyses, the b = 300 and b = 800 shells (40 gradients total) were used to calculate the diffusion tensor images using the weighted least-squares algorithm (Salvador et al., Reference Salvador, Peña, Menon, Carpenter, Pickard and Bullmore2005). Lower b-values were employed for calculation of the diffusion tensor given the decreased signal-to-noise and the increased non-Gaussian contribution to the diffusion signal at higher b-value acquisitions (Jones & Basser, Reference Jones and Basser2004). As an additional quality control step, interactive tractography was performed in Slicer (http://www.slicer.org) and visually assessed for artifacts undetectable by voxel-wise inspection, such as any consistently observed directional biases. Skull and nonbrain tissue were masked using the brain extraction tool (BET) (Smith, Reference Smith2002) on the geometric mean of the DWI image, followed by manual correction, if necessary.
Two motion scores were calculated per subject: (a) The number of DWI gradients removed by the DTI Prep preprocessing pipeline, and (b) the number of DWI gradients with significant levels of corrected motion, defined as any gradient with a corrected rotation exceeding 1 degree or a translation exceeding 1 mm. These two scores were summed to create the single motion artifact covariate used in the association analyses.
Using the UNC−Utah National Alliance for Medical Image Computing DTI framework (Verde et al., Reference Verde, Budin, Berger, Gupta, Farzinfar, Kaiser and Styner2014), a study-specific DTI atlas was created from the sample data. Nonlinear, diffeomorphic pair-wise registration was performed to map individual subject DTIs into atlas space, and registration accuracy was visually inspected in DTI-AtlasBuilder to determine if the computed transforms were appropriate. Major fiber tracts were determined semi-automatically in this atlas space (Ngattai Lam et al., Reference Ngattai Lam, Belhomme, Ferrall, Patterson, Styner and Prieto2018). Resulting deformation fields were then used to map the atlas fibers into individual subject space, where diffusion tensor metrics were extracted at evenly spaced points (arc lengths) along each fiber tract. These metrics included, fractional anisotropy (FA, a measure of the directional coherence for the fiber tracts), mean diffusivity (MD, the average magnitude of molecular displacement by diffusion), axial diffusivity (AD, the length of the longest axis of diffusion tensor), and radial diffusivity (RD, the average length of two remaining axes of the diffusion tensor). As an additional quality control step, individuals were excluded from further association analyses for a given tract if their fractional anisotropy profile was weakly correlated with the population tract average profile (correlation <0.70). A low correlation typically flags poor alignment of the subject's DTI to the atlas across the respective fiber regions. For each subject, the profile of the respective diffusion tensor metric was then averaged along the respective fiber to yield robust tract metric averages for the association analyses.
Of note, the bed nucleus of the stria terminalis amygdala (BNST) and the cingulate gyrus both have a lower signal-to-noise ratio within the developing neonate brain compared to the other fiber tracts examined in this study. Regions along these two tracts exhibit fractional anisotropy values approaching the noise floor, leading to increased variability among subjects. To address this issue, a tract region of interest along each tract was selected for the average computation based on fractional anisotropy signal and tract anatomy (Supplementary Appendix Figure 1). As the signal-to-noise ratio of these two tracts remains lower than for the other tracts of interest, even after tract region selection, a lower correlation threshold of <0.50 was used to exclude those subjects that exhibit poor alignment with the atlas. Three subjects with a correlation threshold lower than 0.50 were removed for the BNST–amygdala tract and 13 were excluded for the right anterior cingulate tract.
Statistical analyses
Partial correlations were used to examine associations between self-reported distress at 17 and 29 weeks’ GA and white matter integrity in 13 tracts correcting for motion artifact level, postconceptional age and biological sex at birth. The following tracts were investigated: bilateral BNST–amygdala, cingulate anterior portion, cingulate–hippocampal, fornix, inferior occipital fasciculus, uncinate and corpus callosum (see Figure 2). For tracts associated with prenatal maternal distress, hierarchical linear regressions were then conducted to evaluate robustness of findings after including obstetric and sociodemographic covariates. Based on prior research demonstrating associations with either the predictor or outcome, we included the following covariates in these regression analyses: postconceptional age at scan, infant biological sex, INR, birth weight percentile, GAB, obstetric complications, and motion artifact level (Davis et al., Reference Davis, Buss, Muftuler, Head, Hasso, Wing and Sandman2011; Jha et al., Reference Jha, Meltzer-Brody, Steiner, Cornea, Woolson, Ahn and Knickmeyer2016; Kim et al., Reference Kim, Davis, Sandman, Sporns, O'Donnell, Buss and Hetrick2016a; Thompson et al., Reference Thompson, Kelly, Chen, Beare, Alexander, Seal and Cheong2019). Postconceptional age, biological sex, and motion artifact level were analyzed within the first block of the model; the remaining covariates were entered into the second block. Three subjects were missing self-report data for INR and STAI at 29 weeks’ GA. Little's (Reference Little1988) missing completely at random (MCAR) test was nonsignificant, χ2 (76) = 88.63, p = .152, suggesting that data were missing completely at random. Missing data were imputed using expectation maximization procedures in SPSS version 26.

Figure 2. Diffusion tensor metrics are calculated along select white matter tracts. (a) White matter fiber tracts analyzed in the current study. Top: red = uncinate (UNC); yellow = genu of corpus callosum (genu); light blue = inferior fronto-occipital fasciculus (IFOF). Bottom: purple = bed nucleus of the stria terminalis amygdala (BNST); blue = fornix (FNX); green = cingulum hippocampal part (CGH); orange = cingulum gyrus part (CGC) (i.e., anterior cingulum). (b) Sagittal (left) and axial (right) view of the fractional anisotropy (FA) (top) and axial diffusivity (AD) (bottom) calculated for a single subject.
For tracts that showed significant associations with distress symptoms, follow-up analyses were then conducted with mean diffusivity, radial diffusivity, and axial diffusivity to further investigate the nature of the white matter microstructure associations. Sensitivity analyses using identical statistical analyses were conducted excluding infants of mothers with regular substance (n = 4) or psychotropic medication (n = 8) use during pregnancy.
Results
The STAI scores at 17 and 29 weeks’ GA were 36.0 (SD = 12.9) and 33.9 (SD = 11.6), respectively. The STAI scores at the two time points were correlated (r = .675, p = <.001). Lower INR was associated with higher STAI at both 17 (r = −0.266, p = .014) and 29 weeks’ GA (r = −0.230, p = .034). Maternal STAI at 29 weeks’ GA was positively associated with postconceptional age at scan (r = .217, p = .046); maternal STAI was not associated with any other birth outcome or demographic characteristic.
White matter microstructure
Higher prenatal maternal STAI scores at 29 weeks’ GA were associated with increased fractional anisotropy within the right anterior cingulate tract (r = .313, p = .009), correcting for biological sex, motion artifact level, and postconceptional age. No other significant associations were found with prenatal distress exposure and tract fractional anisotropy at 29 weeks’ GA, or earlier in gestation (see Table 3). This association between maternal distress at 29 weeks’ GA and higher fractional anisotropy in the right anterior cingulate remained after considering GAB, BW percentile, and INR in the regression, b = .283, t (64) = 2.319, p = .024 (Figure 3a).

Figure 3. STAI at 29 weeks’ GA and right anterior cingulate tract. Maternal distress is associated with increased (a) fractional anisotropy (FA) and (b) increased axial diffusivity (AD). Residuals plotted after accounting for biological sex at birth, postconceptional age, and motion.
Table 3. Partial correlations of prenatal distress and tract fractional anisotropy (FA) controlling for biological sex at birth, postconceptional age, and motion

Note: STAI = state-trait anxiety inventory; FA = fractional anisotropy; PCA = postconceptional age at scan; GA = gestational age.
*p < .05, **p < .01, **p < .001 uncorrected p values.
Three follow-up regression analyses were then conducted with metrics of mean, radial, and axial diffusivity to further investigate the nature of the white matter microstructure associations within the right anterior cingulate tract. Higher prenatal maternal STAI at 29 weeks’ GA was associated with increased axial diffusivity within the right anterior cingulate, b = .254, t (64) = 2.067 p = .043 (Figure 3b). No other significant associations were found with mean diffusivity, b = .101, t (64) = .818, p = .417, or radial diffusivity, b = −.023, t (64) = −.184, p = .855, within the right anterior cingulate. Sensitivity analyses demonstrated that removal of the infants of mothers with prenatal substance use (n = 4) and medication exposure (n = 8) showed similar effect sizes for both fractional anisotropy and axial diffusivity. Partial correlations with biological sex, motion artifact level, and postconceptional age demonstrated a similar effect in the association between distress at 29 weeks’ GA and right cingulate fractional anisotropy (r = .290, p = .023) and axial diffusivity (r = .393, p = .002).
Discussion
The DOHaD/FOAD hypothesis highlights the importance of fetal experiences for shaping developmental trajectories with long-term consequences for health and well-being. Few studies, however, have prospectively examined the influence of prenatal exposures on neural circuit development. The current study evaluated the association between the STAI, an RDoC-informed indicator of prenatal maternal distress within the negative valence system and white matter integrity in neonates. Findings demonstrated that prenatal maternal distress during the third trimester was associated with alterations in neonatal white matter microstructure such that higher prenatal maternal distress at 29 weeks’ GA was associated with higher fractional anisotropy and axial diffusivity within the right anterior cingulate tract. Associations remained after considering biological sex at birth, postconceptional age, GAB, birth weight percentile, INR, and motion. Maternal distress was not associated with variability in the white matter microstructure of the other tracts under investigation, nor was maternal distress earlier in gestation. These findings provide evidence that variability in developing white matter microstructure may be an important ontogenetic vulnerability, although support for this hypothesis was limited to the right anterior cingulate tract.
Experimental work with rodents provides compelling evidence that prenatal exposure to stress shapes neural circuit development, particularly within circuits associated with threat–reactivity (for review see Bock, Rether, Gröger, Xie, & Braun, Reference Bock, Rether, Gröger, Xie and Braun2014; Chen & Baram, Reference Chen and Baram2016; van Bodegom, Homberg, & Henckens, Reference van Bodegom, Homberg and Henckens2017); however, the human literature is small and fairly inconsistent both in methods and findings. Within the emerging literature, three studies have focused specifically on amygdala–prefrontal circuitry and found evidence of both decreased (Posner et al., Reference Posner, Cha, Roy, Peterson, Bansal, Gustafsson and Monk2016; Rifkin-Graboi et al., Reference Rifkin-Graboi, Bai, Chen, Hameed, Sim, Tint and Qiu2013) and increased white matter integrity (Humphreys et al., Reference Humphreys, Camacho, Roth and Estes2020). Of note, the studies finding a negative association between prenatal stress and white matter integrity used a categorical approach in defining prenatal distress, whereas a more recent study assessing distress dimensionally found evidence of increased white matter structural integrity (Humphreys et al., Reference Humphreys, Camacho, Roth and Estes2020).
Building on emerging research from whole brain analyses (Dean et al., Reference Dean, Planalp, Wooten, Kecskemeti, Adluru, Schmidt and Davidson2018; Graham et al., Reference Graham, Jiang, McCorkle, Bellando, Sorensen, Glasier and Ou2020; Rifkin-Graboi et al., Reference Rifkin-Graboi, Meaney, Chen, Bai, Hameed, Tint and Qiu2015), our study sought to examine the role of prenatal distress exposure on circuits more broadly involved in processes of emotion regulation (temporolimbic) and perception (occipitotemporo) beyond a limited focus on amygdala–prefrontal circuitry. We found that prenatal distress in the third trimester of gestation was associated with increased fractional anisotropy and axial diffusivity within the right anterior cingulate tract. The cingulate plays an important role in appraisal, generation and regulation of emotion, with evidence that the anterior subregion is particularly involved in regulating emotional responses (Etkin, Egner, & Kalisch, Reference Etkin, Egner and Kalisch2011). This region may be susceptible to prenatal influences, as the development of the anterior cingulate has been linked to early life stress (Ansell, Rando, Tuit, Guarnaccia, & Sinha, Reference Ansell, Rando, Tuit, Guarnaccia and Sinha2012; Cohen et al., Reference Cohen, Grieve, Hoth, Paul, Sweet, Tate and Williams2006). In neonates, one previous whole brain study (Rifkin-Graboi et al., Reference Rifkin-Graboi, Meaney, Chen, Bai, Hameed, Tint and Qiu2015) identified white matter changes associated with prenatal maternal anxiety within the posterior cingulate, although results showed the opposite relation whereby elevated anxiety was associated with decreased fractional anisotropy. Further, prenatal maternal distress is associated with lower fractional anisotropy and higher diffusivity within the cingulate tract in 8-year-olds suggesting neurodevelopmental alterations in white matter integrity may persist into childhood (El Marroun et al., Reference El Marroun, Zou, Muetzel, Jaddoe, Verhulst, White and Tiemeier2018).
The implications of increased fractional anisotropy and axial diffusivity following prenatal distress are unclear. As fractional anisotropy typically increases across development, higher fractional anisotropy is thought to reflect improved anatomical connectivity and more advanced maturation (Dubois et al., Reference Dubois, Dehaene-Lambertz, Kulikova, Poupon, Hüppi and Hertz-Pannier2014; Soares et al., Reference Soares, Marques, Alves and Sousa2013). Prenatal stress is associated with acceleration of fetal maturation in preparation for survival outside the womb; for example, stressed fetuses tend to have greater lung maturity when born preterm (Glynn, Schetter, Hobel, & Sandman, Reference Glynn, Schetter, Hobel and Sandman2008; Schetter, Reference Schetter2009) and there is evidence that elevated levels of the stress hormone late in gestation are associated with benefits to brain development and cognitive function (Davis & Sandman, Reference Davis and Sandman2010; Davis, Head, Buss, & Sandman, Reference Davis, Head, Buss and Sandman2017). It is plausible that the observed association with higher fractional anisotropy reflects accelerated maturation of this brain region. However, the observed association of prenatal stress with higher axial diffusivity is contrary to the hypothesis of accelerated maturation given evidence that diffusivity normatively decreases over development (Geng et al., Reference Geng, Gouttard, Sharma, Gu, Styner, Lin and Gilmore2012). There is some support for the acceleration hypothesis from studies of postnatal adversity (Colich et al., Reference Colich, Williams, Ho, King, Humphreys, Price and Gotlib2017; Gee et al., Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013), although several others have found evidence for delays or more immature pattern of connectivity following early adversity (Cisler et al., Reference Cisler, James, Tripathi, Mletzko, Heim, Hu and Kilts2013; Silvers et al., Reference Silvers, Lumian, Gabard-Durnam, Gee, Goff, Fareri and Tottenham2016). Future research with replication and longitudinal follow-up is needed to determine whether the observation that prenatal adversity is associated with increased fractional anisotropy reflects accelerated maturation of neural circuits.
The biological mechanisms underlying the association between prenatal maternal distress and neural circuit maturation remain unknown. Dysregulation of the hypothalamic–pituitary–adrenal axis and immune system signaling are two promising pathways through which exposure to prenatal distress may influence white matter integrity. Fetal neural circuits are sensitive to stress hormone exposure. Experimental research in rodents demonstrates that exposure of immature neurons to corticotropin releasing hormone has a dose–response relation on dendritic branching and neuronal growth (Curran, Sandman, Davis, Glynn, & Baram, Reference Curran, Sandman, Davis, Glynn and Baram2017). Similarly, prenatal synthetic glucocorticoid treatment has been shown to have an impact on neuronal cell proliferation and neurogenesis within the fetal mouse brain (Noorlander et al., Reference Noorlander, Tijsseling, Hessel, de Vries, Derks, Visser and de Graan2014). Prefrontal and limbic regions (including the rostral anterior cingulate) are particularly affected by excess glucocorticoids because of the abundance of glucocorticoid receptors in these brain regions (Rodrigues, LeDoux, & Sapolsky, Reference Rodrigues, LeDoux and Sapolsky2009). Fetal exposure to glucocorticoids has been associated with neonatal white matter microstructure and structural connectivity (Stoye et al., Reference Stoye, Blesa, Sullivan, Galdi, Lamb, Black and Boardman2020), as well as persisting alterations into preadolescence in functional connectivity (Graham et al., Reference Graham, Rasmussen, Entringer, Ben Ward, Rudolph, Gilmore and Buss2019; Kim et al., Reference Kim, Davis, Sandman, Sporns, O'Donnell, Buss and Hetrick2016b) and brain structure (Buss et al., Reference Buss, Davis, Shahbaba, Pruessner, Head and Sandman2012; Davis et al., Reference Davis, Head, Buss and Sandman2017), including within the anterior cingulate (Davis, Sandman, Buss, Wing, & Head, Reference Davis, Sandman, Buss, Wing and Head2013). Further, pro-inflammatory cytokines are another promising mechanistic pathway. For example, elevated levels of cytokine IL-6, one of the most studied pro-inflammatory cytokines, is associated with reduced integrity of the uncinate fasciculus, a main frontolimbic fiber tract (Rasmussen et al., Reference Rasmussen, Graham, Entringer, Gilmore, Styner, Fair and Buss2019). In addition, prenatal maternal interleukin-6 concentration has also been linked with amygdala volume and amygdala connectivity regions involved in sensory processing, salience detection, and learning and memory (Graham et al., Reference Graham, Rasmussen, Rudolph, Heim, Gilmore, Styner and Buss2018).
Strengths and Limitations
A significant strength of this study is the investigation of neurodevelopmental differences in white matter microstructure within neonates prior to the intervening effects of the postnatal environment. Rigorous protocols were employed to acquire high-quality data in infants without sedation during natural sleep (Gilmore et al., Reference Gilmore, Lin, Corouge, Vetsa, Smith, Kang and Gerig2007; Howell et al., Reference Howell, Styner, Gao, Yap, Wang, Baluyot and Elison2019). These include alignment of the scan time with the infant nap schedule, a quiet room to feed and put the baby to sleep, swaddling and securing of the infant's head in a vacuum-fixation device to limit motion and allowing sufficient time between scan acquisitions to repeat scans if needed. Further, the majority of studies assess distress once during pregnancy and therefore cannot evaluate the importance of specific timing effects relative to the normative trajectory of white matter development. Consistent with evidence of the emergence of limbic and associative fiber tracks between 12 and 22 weeks’ GA (Dubois et al., Reference Dubois, Dehaene-Lambertz, Kulikova, Poupon, Hüppi and Hertz-Pannier2014), we found associations with prenatal maternal distress assessed at 29 weeks and not 17 weeks’ GA. Supporting this timing effect, prior research has found direct associations of prenatal maternal mood and fetal behavior from 27 to 28 weeks’ GA onwards (Van den Bergh, Mulder, Mennes, & Glover, Reference Van den Bergh, Mulder, Mennes and Glover2005), and a recent study showed that maternal self-reported distress during the third, but not second trimester, was associated with infant hippocampal connectivity (Scheinost, Spann, McDonough, Peterson, & Monk, Reference Scheinost, Spann, McDonough, Peterson and Monk2020). However, another study examining associations between prenatal maternal depression and child brain structure found associations that were limited to second trimester maternal stress (Lebel et al., Reference Lebel, Walton, Letourneau, Giesbrecht, Kaplan and Dewey2016). Myelination continues rapidly during infancy, with protracted microstructural maturation in childhood (for review see Lebel, Treit, & Beaulieu, Reference Lebel, Treit and Beaulieu2019); therefore, additional longitudinal research should be conducted to determine the role of prenatal maternal stress exposure on the developmental trajectory of white matter maturation.
There are several limitations to note. First, there is evidence that the role of prenatal distress exposure on neurodevelopment differs by sex (Clifton, Reference Clifton2010; Dean et al., Reference Dean, Planalp, Wooten, Kecskemeti, Adluru, Schmidt and Davidson2018; Sandman, Glynn, & Davis, Reference Sandman, Glynn and Davis2013; Wen et al., Reference Wen, Poh, Ni, Chong, Chen, Kwek and Qiu2017). Because of the limited sample size, we were underpowered to examine moderation by infants’ biological sex. Second, given that this study investigated naturally occurring variations in maternal distress, rather than experimental manipulations, it is also difficult to disentangle the effects of prenatal maternal distress exposure from other potential contributing factors such as genetic influences (O'Donnell & Meaney, Reference O'Donnell and Meaney2017). There is evidence that white matter microstructure such as fractional anisotropy is heritable (Kochunov et al., Reference Kochunov, Jahanshad, Marcus, Winkler, Sprooten, Nichols and Van Essen2015), and that genetic risk may serve as an important moderator between mothers’ depression and the neurodevelopment of offspring (Qiu et al., Reference Qiu, Shen, Buss, Chong, Kwek, Saw and Meaney2017). Findings for children conceived via in vitro fertilization who are not genetically related to their mothers replicate cross-fostering studies in rodents (Rice et al., Reference Rice, Harold, Boivin, van den Bree, Hay and Thapar2010) and demonstrate contributions of the prenatal environment to child development independent of genetic effects (Lewis, Rice, Harold, Collishaw, & Thapar, Reference Lewis, Rice, Harold, Collishaw and Thapar2011; for review see Natsuaki et al., Reference Natsuaki, Shaw, Neiderhiser, Ganiban, Harold, Reiss and Leve2014). Third, we used the STAI as one self-report measure intended to assess the RDoC-informed theoretical construct of potential threat (“anxiety”), as a narrow dimension assessment of a hypothesized construct located within the higher-order negative valence system. At the higher-order level, RDoC the negative valence system captures and reflects higher-order negative affectivity that cuts across traditional internalizing disorder categories (e.g., depression, social anxiety, panic disorder, generalized anxiety disorder) and may therefore be an important susceptibility factor to use in the investigation of intergenerational transmission of risk (Gao et al., Reference Gao, Ostlund, Brown, Kaliush, Terrell, Vlisides-Henry and Conradt2021). As with any measurement of a latent construct, our results are limited by the extent to which the STAI provides a valid indication of the hypothesized narrow-based construct of anxiety (Cronbach & Meehl, Reference Cronbach and Meehl1955) as conceptualized within the RDoC system. Future work would benefit from additional measures of this narrow-band construct of anxiety (e.g., Anxiety Sensitivity scale; Behavioral Inhibition scale) as well as expanded measurement of the higher-order negative valence system (e.g., loss, acute threat) as these other narrow-order dimensions within the negative valence system may show different patterns of associations with infant white matter. Fourth and finally, it is difficult to completely rule out the possibility that alternative factors such as obstetric or neonatal complications, exposure to psychiatric medications (Jha et al., Reference Jha, Meltzer-Brody, Steiner, Cornea, Woolson, Ahn and Knickmeyer2016), and substance use contribute to study findings (Donald et al., Reference Donald, Roos, Fouche, Koen, Howells, Woods and Stein2015; Gao et al., Reference Gao, Grewen, Knickmeyer, Qiu, Salzwedel, Lin and Gilmore2019; Walhovd, Watts, Amlien, & Woodward, Reference Walhovd, Watts, Amlien and Woodward2012). Sensitivity analyses, however, showed similar effects with removal of participants with medication and substance use, and covarying obstetric complications and birth outcomes did not impact study findings.
Implications
Findings from the present study provide added support for the DOHaD/FOAD hypothesis and the importance of the intrauterine environment by demonstrating that exposure to prenatal maternal distress is associated with some early alterations in portions of neonatal neural circuit maturation. Highlighting their predictive utility as a potential biomarker of vulnerability, there is preliminary evidence that white matter microstructural changes associated with prenatal distress are in turn associated with later behavioral problems (Borchers et al., Reference Borchers, Dennis, King, Humphreys and Gotlib2020), and internalizing symptoms (Rifkin-Graboi et al., Reference Rifkin-Graboi, Meaney, Chen, Bai, Hameed, Tint and Qiu2015). Future work should continue to investigate variability in white matter microstructure as an early marker of ontogenetic risk. The majority of studies in humans examining the programming influences of prenatal distress on infant neurodevelopment have been correlational in nature, limiting causal inferences. Experimental manipulation of prenatal depression is a promising avenue to resolve discrepancies in the literature to date examining child ontogenetic vulnerability to psychopathology. Therefore, our group is currently conducting a randomized controlled trial (Davis et al., Reference Davis, Hankin, Swales and Hoffman2018) to test whether treatment of maternal distress during pregnancy improves infant outcomes that are linked with subsequent development of psychopathology. Evaluation of the etiological mechanisms such as the development of white matter microstructure underlying intergenerational risk for psychopathology may help to inform targets for more effective intervention and prevention efforts.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579421000742
Acknowledgments
We gratefully acknowledge the support from the National Institute of Mental Health: P50MH096889 (EPD), R01MH109662 (MCH, EPD, BLH); R01MH111944 and R01HD053000 (JHG), P50 HD103573, U54 HD079124 (MAS) and T32 Training Fellowships (MH015442, CHD and MH106440, MMB).
Conflicts of Interest
None.









