Iodine is required for the production of thyroid hormones (thyroxine and tri-iodothyronine), which in turn are required for normal fetal brain and neurological development( Reference Zimmermann 1 ). A sufficient intake of iodine during pregnancy is needed to prevent the potential adverse effects of deficiency on the developing brain that can persist throughout life. Severe iodine deficiency during pregnancy is well known to result in cretinism, a disorder associated with mental retardation, deafness and motor dysfunction in children. Even mild-to-moderate iodine deficiency is associated with lower intelligent quotient and reading( Reference Bath, Steer and Golding 2 ) and spelling ability( Reference Hynes, Otahal and Hay 3 ) up to the age of 9 years.
Although it is important for pregnant women to have a sufficient intake of iodine, it is arguably more important that women of childbearing age, particularly those planning a pregnancy, should consume enough; emerging data suggest that pregnant women who have had a regular adequate intake of iodine have a better thyroid hormone profile than those who only begin iodine supplementation when they become pregnant( Reference Glinoer 4 – Reference Moleti, Lo Presti and Campolo 6 ). This is probably because the thyroid can store iodine that can be drawn on during the course of pregnancy( Reference Moleti, Di Bella and Giorgianni 5 ). As many pregnancies are unplanned and because pregnancy may not be confirmed until several weeks into the first trimester – a critical period for thyroid hormone need – it is essential that women of childbearing age consume an adequate amount of iodine on a regular basis and meet the RDA (US RDA) of 150 μg/d( 7 ).
For many years, the UK has been considered to be an iodine-sufficient country, despite reports of endemic goitre in the past( Reference Phillips 8 ). Iodine deficiency was eradicated in the UK through changes in the dairy-farming industry in the 1930s (i.e. through increased use of iodine-fortified cattle feed and iodine-containing disinfectants) and concurrent increase in milk consumption in the post-war years( Reference Phillips 8 ); from the 1960s, iodine sufficiency was assumed in the UK and there was a dearth of data on the status of the population. In fact, at the time that the present study was conducted, there were no national UK data on the iodine status of the population( Reference de Benoist, McLean and Andersson 9 ) and just two studies( Reference Kibirige, Hutchison and Owen 10 , Reference Barnett, Visser and Williams 11 ) on the iodine status of pregnant women. There are now UK-wide data that suggest that teenage schoolgirls are mildly iodine deficient( Reference Vanderpump, Lazarus and Smyth 12 ) and regional studies that report iodine deficiency in UK pregnant women( Reference Bath, Steer and Golding 2 , Reference Kibirige, Hutchison and Owen 10 , Reference Bath, Walter and Taylor 13 , Reference Pearce, Lazarus and Smyth 14 ). However, data on the iodine status of UK women of childbearing age are still lacking, and few studies have assessed measures of both dietary iodine intake and status. Assessment of urinary iodine excretion is a widely accepted method for measuring iodine status; approximately 90 % of ingested iodine is ultimately excreted in the urine( Reference Zimmermann 1 ). Total iodine excretion in a 24 h urine collection is considered to be preferable to iodine concentration measured in spot-urine samples for assessing iodine status in an individual( Reference Thomson, Colls and Conaglen 15 , Reference Vejbjerg, Knudsen and Perrild 16 ).
In the present study, we assessed iodine status from 24 h urine collections in UK women of childbearing age, i.e. in a cohort of women who could potentially become pregnant in the short-to-medium term. In addition, through the use of food diaries, we investigated which iodine-rich food groups had the most influence on iodine status. We also compared two methods for estimating iodine intake: (1) extrapolation from 24 h urinary iodine excretion and (2) estimation of intake by dietary assessment. This is the first study to be conducted on the iodine status of women of childbearing age in the UK using these methods and the first to report the comparison of two methods for estimating iodine intake.
Experimental methods
Recruitment and protocol
The study was conducted at the University of Surrey, Guildford, UK. Women of childbearing age (defined as still menstruating) were recruited during January and February 2007 and again in January and February 2008. Women were recruited by word of mouth in the University through friends and colleagues and through responses to an email advertisement. Exclusion criteria included current or recent pregnancy (in the last 6 months), breast-feeding, known thyroid disease and use of medication for thyroid disease – thyroxine, amiodarone, carbimazole or propylthiouracil.
The WHO recommends that the median iodine concentration estimated from spot-urine samples be compared with the published cut-off values for adequacy for assessing the iodine status of a population( 17 ). However, urinary iodine concentration (UIC) is not suitable for the assessment of individual iodine status; for this purpose, multiple spot-urine samples or 24 h urine collections (for the measurement of total iodine excretion) are required( Reference Konig, Andersson and Hotz 18 ). Though the 24 h iodine excretion estimated from a single 24 h urine collection does not account for the day-to-day variability in iodine intake, it does overcome the variability associated with urine volume that affects the interpretation of iodine concentration in a spot-urine sample and for that reason can be considered preferable to a single spot-urine sample( Reference Thomson, Colls and Conaglen 15 , Reference Vejbjerg, Knudsen and Perrild 16 ). The participants who volunteered to take part in the study were required to collect all the urine passed in a 24 h time period (completeness of the sample was self-reported). The participants were provided with clear instructions, a wide-necked jug and a leak-proof 5-litre container (both had been acid-washed and rinsed before use). They were advised not to wash the jug between urine collections and to ensure that the container lid was well sealed. The total urine volume of each participant was measured; 20 ml aliquots were taken and stored at − 20°C until analysis.
The participants completed a questionnaire to provide basic information on date of birth, use of nutritional supplements or medication and any dietary exclusion practised. They were required to keep a detailed food diary both for the 24 h before urine collection and during the 24 h of urine collection.
The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the University of Surrey Ethics Committee. Written informed consent was obtained from all participants.
Determination of urinary iodine concentration
Iodine concentration was measured on a Thermo Elemental X-Series ICP-MS system (Thermo Fisher Scientific) in the Trace Element Laboratory at the University of Surrey. To produce samples in the analytical range for inductively coupled plasma MS, the samples were first diluted with an alkaline diluent. The diluent used was prepared by dissolving 3·32 g of NH4H2PO4 and 1·16 g of (NH4)2H2EDTA (Analar grade; Sigma-Aldrich) in deionised water, adding 10·0 ml of NH3 solution (specific gravity 0·88), and making up to 1000 ml with deionised water. The final diluent solution contained 0·14 m-NH3, 0·003 m-(NH4)2H2EDTA and 0·029 m-NH4H2PO4. Using the alkaline diluent, 400 μl of each participant's urine sample were made up to 10 ml. Standard iodine solutions were prepared with KI (Analar grade; Fisher Scientific) for the construction of calibration curves. To obtain matrix-matched standards, 400 μl of control urine (taken from laboratory stock with median UIC of 80·6 and 46·5 μg/l in 2007 and 2008, respectively) were added to 400 μl of each standard. An internal standard was added to all samples: rhodium (103Rh) and iridium (192Ir) (both obtained from SPEX CertiPrep Limited) were made up in a working standard solution of 1 mg/l (parts per million) in 1 % v/v HNO3 (Trace analysis grade; Fisher Scientific). This was made up 1:10 with the diluent and 150 μl was added to each tube.
To evaluate the accuracy of the method, a number of certified reference materials were used (Seronorm urine (Bio-Stat House) in 2007 and EQUIP (ensuring the quality of urinary iodine procedures)( Reference Caldwell, Makhmudov and Jones 19 ) samples in 2008). The mean values obtained for the EQUIP-certified reference materials (U02, U05, U09, U10) were as follows: 28·9 (sd 0·1) μg/l (n 2) for U02 (certified mean 28·7 μg/l, range 20·1–37·3 μg/l); 45·9 (sd 0·0) μg/l (n 2) for U05 (certified mean 45·0 μg/l, range 31·5–58·5 μg/l); 298·0 (sd 0·0) μg/l (n 2) for U09 (certified mean 296·3 μg/l, range 251·9–340·7 μg/l); 11·4 (sd 0·6) μg/l (n 2) for U10 (certified mean 12·2 μg/l, range 8·5–15·9 μg/l) and 264 (sd 1·0) μg/l (n 3) for Seronorm urine (certified mean 282 μg/l, range 264–300 μg/l).
Derivation of total urinary iodine excretion and extrapolation to give estimated iodine intake
Total 24 h urinary iodine excretion was calculated for each participant by multiplying iodine concentration (μg/l) by total urine volume (in litres) collected. It has been estimated that approximately 90 %( Reference Zimmermann 1 ) of ingested iodine is eventually excreted in the urine, and this assumption allows dietary iodine intake to be estimated by dividing total urinary iodine excretion (μg/24 h) by 0·90.
Analysis of dietary iodine intake
The information recorded in the 48 h food diaries was entered into the WinDiets Research programme (version 2005; Robert Gordon University) to estimate iodine intake both on the day before and on the day of urine collection. Where portion weights were not recorded in the diaries, medium portion sizes were entered( 20 ). The iodine content of reported multivitamin and mineral supplements was added to the total iodine intake estimated from the food diaries on each day and an average iodine intake across the 48 h was calculated. The quantity of iodine-rich foods (milk, meat, fish and eggs) and soya drinks (as a potential replacement for iodine-rich cows’ milk) consumed was extracted from the participants’ diaries from each 24 h period and an average was calculated; the average value for each food item was then used to examine the relationship with urinary iodine excretion.
Classification of iodine status and estimation of the prevalence of iodine deficiency
The median UIC of the study group was compared with the WHO criteria for the risk of iodine deficiency (Table 1). To calculate the risk in individuals, total urinary iodine excretion in 24 h was compared with values reported in other studies( Reference Thomson, Colls and Conaglen 15 , Reference Als, Minder and Willems 21 ) and with the thresholds (i.e. μg/d) proposed in population-based studies (Table 1)( Reference Zimmermann and Andersson 22 ).
Table 1 Classification of the risk of iodine deficiency using measures of urinary iodine concentration and total iodine excretion in a 24 h period

* WHO criteria for adult populations( 17 ).
† Criteria for iodine deficiency in individuals for 24 h urinary iodine excretion, derived from Thomson et al. ( Reference Thomson, Colls and Conaglen 15 ) and Als et al. ( Reference Als, Minder and Willems 21 ).
The Dietary Reference Intake values published by the Institute of Medicine( 7 ) were used for evaluating iodine intake (either extrapolated from urinary iodine excretion or estimated from food diaries); neither the UK Dietary Reference Values( 23 ) nor the WHO recommendations( 17 ) provide a value for the estimated average requirement (EAR), which is required for prevalence estimates of nutrient deficiencies in a population( Reference Beaton 24 ). The percentage of women with an iodine intake below the adult EAR (95 μg/d)( 7 ) was used to describe the prevalence of deficiency in the cohort.
Statistical analysis
As variables were not normally distributed, median and interquartile ranges are reported; variables were transformed using the natural logarithm to allow parametric testing where possible. Data on the intake of food groups (e.g. fish and milk) were not normally distributed, even after transformation, and therefore non-parametric tests were used.
Independent t tests or one-way ANOVA were used to compare (log-transformed) 24 h urinary iodine excretion values between the groups. A paired t test was used to compare intake over the 48 h of dietary records and the two methods for iodine intake estimation. Analysis of the correlation between two continuous variables was conducted using Pearson's correlation when both variables were normally distributed or Spearman's rank when variables were not normally distributed. Forward stepwise linear regression (using log-transformed 24 h urinary iodine excretion values) was performed to evaluate the most important dietary predictors of iodine status; all dietary variables and dose of iodine in a multivitamin and mineral supplement were entered as independent variables.
Bland–Altman plots were used to compare the two methods for iodine intake estimation (i.e. extrapolation from 24 h urinary iodine excretion or from estimation from food diaries). The mean difference between the two methods was plotted against the mean of the methods.
Statistical significance was set at P< 0·05, and analysis was performed with the Statistical Package for Social Sciences (version 21.0; SPSS, Inc.).
Results
A total of twenty-six women volunteered to participate in 2007 and thirty-one in 2008, giving a total of fifty-seven women of childbearing age. Approximately 90 % of the participants were studying for a degree in nutrition or nutrition/dietetics. The median age of the participants was 23 (range 19–45) years. Among these participants, five (8·8 %) were lacto-ovo vegetarians and three (5·3 %) were pescatarians (excluded meat and poultry but included fish); there were no vegans in the study.
Iodine excretion and estimated iodine intake
A summary of UIC, 24 h urinary iodine excretion and estimated iodine intake (extrapolated from urinary iodine excretion) is given in Table 2. The median UIC value (63·1 μg/l) and the fact that 31·6 % (n 18) of the participants had a UIC < 50 μg/l indicated the group to be mildly iodine deficient by the WHO criteria( 17 ). However, the median 24 h urinary iodine excretion value (149·8 μg/24 h) indicated the same group to have an adequate iodine status according to the criteria listed in Table 1 ( Reference Thomson, Colls and Conaglen 15 , Reference Als, Minder and Willems 21 ).
Table 2 Summary of urinary iodine concentration, 24 h urine volume, 24 h urinary iodine excretion and extrapolated daily iodine intake for all the fifty-seven participants (Median values and 25th–75th percentiles)
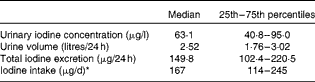
* Estimated by extrapolation from urinary excretion (dividing by 0·90).
When 24 h urinary iodine excretion was extrapolated to estimate daily intake (on the basis that 90 % is excreted), the median (167 μg/d) was above the adult RDA of 150 μg/d( 17 ). The estimated iodine intake for the fifty-seven participants is shown in Fig. 1; the dotted lines denote the RDA and EAR for adults( 7 ) and the EAR for pregnant women( 7 ). Among the participants, 14 % (n 8) had an estimated intake below the adult EAR of 95 μg/d( 7 ) and 40·3 % (n 23) had an estimated iodine intake below the adult RDA (150 μg/d). Furthermore, 42 % (n 24) had an iodine intake below the EAR for pregnancy (160 μg/d)( 7 ), none of whom was taking a multivitamin and mineral supplement containing iodine.

Fig. 1 Participants in ascending order of iodine intake extrapolated from 24 h urinary iodine excretion. ■ represents participants who took a supplement containing iodine. — (middle) represents the RDA for adults (150 μg/d)( 7 ). - - - represents the Institute of Medicine's estimated average requirement (EAR) values: - - - (lower) represents the EAR for adults (95 μg/d) and - - - (upper) represents the EAR for pregnant women (160 μg/d)( 7 ).
The median iodine intake estimated from the food diaries was above the adult EAR (95 μg/d), but below the RDA (150 μg/d) for both 24 h periods of dietary records as was the average (Table 3). A total of sixteen participants (28·1 %) had an iodine intake (averaged over the two 24 h periods) that was below the EAR, a value higher than that estimated from the extrapolation of 24 h urinary iodine excretion (n 8, 14 %). A total of thirty-four participants (59·6 %) had an average iodine intake below the EAR for pregnancy.
Table 3 Iodine intake estimated from food diaries plus supplements (Median values and 25th–75th percentiles)
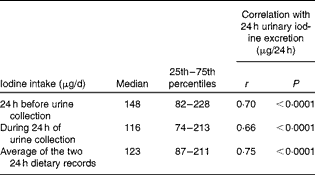
The values estimated from the 48 h food diaries showed that there was no significant difference in iodine intake between the two 24 h periods (paired t test: P= 0·23). This suggests little variation in iodine intake over two consecutive days in this population.
Relationship between the two methods for estimating iodine intake
Iodine intake estimated from the food diaries was strongly correlated with 24 h urinary iodine excretion (Table 3; Fig. 2(a)). Fig. 2 shows the correlation and agreement between the two methods for estimating iodine intake - i.e., estimation from the 48 h food diaries and supplements or from extrapolation of the 24 h urinary iodine excretion. There was a significant difference in the iodine intake estimated using the two methods (paired t test: P= 0·001). The Bland–Altman plot showed that there was a considerable lack of agreement between the methods as, on average, iodine intake estimated from the food diaries was lower than that estimated from urinary excretion (mean difference − 18·8 μg/d) and, on an individual basis, the difference ranged from − 144·4 to 106·8 μg/d (Fig. 2(b)); this suggests that the methods cannot be used interchangeably.

Fig. 2 (a) Correlation between iodine intake estimated from the food diaries and supplements (average of two 24 h periods) and that extrapolated from 24 h urinary iodine excretion; r 0·71 (r 2 0·50). (b) Bland–Altman plot showing differences between the two methods; — represents the mean difference ( − 18·8 μg/d) between the two methods and - - - represent the limits of agreement corresponding to ± 2 sd (upper limit: 106·8 μg/d; lower limit: − 144·4 μg/d).
Dietary exclusions, use of iodine-containing supplements and intake of iodine-rich food items
There was no significant difference in the 24 h urinary iodine excretion values among omnivores, vegetarians and pescatarians (P= 0·17). Use of iodine-containing multivitamin and mineral supplements in which the dose of iodine ranged from 75 to 200 μg/d was reported by six participants (10·5 %). Participants who used an iodine-containing supplement excreted significantly more iodine in 24 h than non-supplement users (240 v. 144 μg/24 h; P= 0·01).
In the 24 h before urine collection and/or during the 24 h of urine collection, cows’ milk was consumed by 84·3 % of the participants (n 48), fish was consumed by 28·1 % (n 16) and eggs by 21·1 % (n 12). Soya drinks were consumed by five participants (8·8 %), and there was a negative correlation between the intake of soya drinks and that of cows’ milk (r − 0·43, P= 0·001), suggesting that the participants were using soya drinks as an alternative to cows’ milk.
The intake of milk, dairy products, fish and eggs was positively correlated with 24 h urinary iodine excretion, whereas the intake of soya drinks and meat was negatively correlated (Table 4). The strongest correlation was observed for milk, followed by eggs (Table 4). When the variables listed in Table 4 were entered into a linear regression model along with the dose of iodine in any supplement used (with log-transformed 24 h urinary iodine excretion as the dependent variable), milk intake (P< 0·0005), egg consumption (P= 0·004) and intake of other dairy products (P= 0·013) were all found to be significant predictors of iodine status; the intake of soya drinks, fish and meat was not a significant predictor in the final model, which explained 49·3 % of the variation in 24 h urinary iodine excretion (r 2 0·493).
Table 4 Correlation between dietary components and 24 h urinary iodine excretion
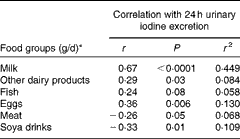
* Average from the 48 h food diaries (24 h before and during the 24 h of urine collection).
Discussion
Iodine intake and status
The median UIC (63·1 μg/l) is suggestive of mild iodine deficiency when using the current WHO cut-off values for adequacy( 17 ), echoing the finding of mild iodine deficiency in UK schoolgirls( Reference Vanderpump, Lazarus and Smyth 12 ). However, if using the more recently proposed cut-off values of 60–70 μg/l for adults( Reference Zimmermann and Andersson 22 ), these women would be classified as having an adequate iodine status. Indeed, based on the 24 h urinary iodine excretion, the risk of deficiency within the group was low, i.e. after accounting for total urine volume. The median intake based on urinary iodine excretion was above both the EAR and RDA, whereas the value estimated from the food diaries was above the EAR but below the RDA. The proportion with iodine intake below the EAR (14 and 28 % for intake extrapolated from urine and food diary estimates, respectively) suggested a degree of deficiency within the cohort. However, it is important to acknowledge that because of day-to-day variability in iodine intake, this does not necessarily mean that these individuals were iodine deficient.
The results of the present study highlight the fact that the degree of iodine deficiency in the cohort varies according to the method used for classification. It is important to highlight the fact that the WHO cut-off value for iodine adequacy in adults is based on the fact that goitre risk is low when the median urinary iodine excretion is above 100 μg/d, a value that was later used as the cut-off value for a spot-urine sample on the basis that the units (i.e. μg/d and μg/l) are interchangeable( Reference Zimmermann and Andersson 22 ). If the average urine volume is 1 litre/d, as it is likely to be in children, the units can be used interchangeably, but this is probably not appropriate for adults. Indeed, a lower cut-off value for iodine adequacy in adults has recently been proposed on the basis that the average urine volume in adults is more likely to be 1·5 litres/d and thus the cut-off value should be lowered to 60–70 μg/l( Reference Zimmermann and Andersson 22 ). In fact, in the present study, the mean urine volume was close to 2·5 litres, and this explains why the risk of deficiency is overestimated when using the UIC values rather than the 24 h urinary iodine excretion values. The food diaries indicated that the participants (mostly nutrition students) drank water throughout the day, and this accounted for the high urine volume observed in the present study. The results of the present study support the need for method-specific (24 h v. spot collection) and age-specific (adults v. schoolchildren/teenagers) criteria for iodine deficiency( Reference Als, Minder and Willems 21 , Reference Zimmermann and Andersson 22 ).
On balance, it is likely that this group had a minimal risk of iodine deficiency. Though the median UIC was suggestive of mild deficiency, this is likely to be a result of the high urine volume in the group and therefore dilute urine samples. It is important to point out that these women are by no means representative of UK women of childbearing age as they were highly educated (mostly science degree students/graduates) in an affluent area of the UK (Surrey). Furthermore, as over 90 % were studying for a degree in nutrition or nutrition/dietetics, it is likely that their knowledge of good nutrition may have skewed the results (see limitations for further explanation).
Although this was a study in women of reproductive age and not in those who were pregnant, there are implications for the pregnant state as iodine intake recommendations are higher for pregnant adults than for non-pregnant adults( 7 ). When intake is extrapolated from the measured 24 h urinary iodine excretion, 42 % of the participants (or 60 % if using data from the food diaries) did not achieve the EAR for pregnancy (i.e. 160 μg/d)( 7 ), suggesting that UK women may be unable to meet the increased iodine needs during pregnancy, as previously found in other UK studies( Reference Kibirige, Hutchison and Owen 10 , Reference Bath, Walter and Taylor 13 , Reference Pearce, Lazarus and Smyth 14 ) and in recent European studies( Reference Moleti, Di Bella and Giorgianni 5 , Reference Vandevijvere, Amsalkhir and Mourri 25 – Reference Raverot, Bournaud and Sassolas 27 ). Bearing in mind the fact that pregnant women are not given advice on iodine intake( 28 ), they are unlikely to modify their diet to increase the intake of iodine-rich foods when they become pregnant. Indeed, results from the Southampton Women's Survey, where dietary intake was estimated before and after pregnancy in the same women, show that dietary patterns do not change considerably when women become pregnant( Reference Crozier, Robinson and Godfrey 29 ); in terms of iodine-rich foods, the intake of fish and milk does not appear to change in early pregnancy, a time point that is critical for iodine supply for brain development( Reference Zimmermann 1 , Reference Bath, Steer and Golding 2 ). The results of the present study suggest that advice to women planning a pregnancy and those who are pregnant should include specific mention of iodine.
Relationship between the two methods used for estimating iodine intake
The present study provides the first opportunity to evaluate how iodine intake estimated from food diaries compares with iodine intake estimated by extrapolation from 24 h urinary iodine excretion; the results show a strong correlation between the two methods, both for intake in the 24 h before urine collection and for intake during the 24 h of urine collection. This suggests that intake over at least 48 h contributes to iodine excreted in the 24 h urine sample, a finding that echoes that of an earlier study carried out in Denmark( Reference Rasmussen, Ovesen and Christiansen 30 ). However, this finding may also be a result of the fact that there was no significant difference in iodine intake between the two 24 h periods. Despite strong correlations, the Bland–Altman plot showed that iodine intake estimated from the food diaries is lower than that extrapolated from 24 h urinary iodine excretion (Fig. 2(b)) by approximately 19 μg/d, on average; this explains why a higher percentage of participants had iodine intake below the EAR when using the food diaries than when estimating intake from 24 h urinary iodine excretion (28·1 v. 14 %). This finding is in contrast to data from Denmark, where 24 h urinary iodine excretion is lower than the intake estimated from either a FFQ or a weighed food diary( Reference Rasmussen, Ovesen and Bulow 31 ). Food diary analysis has been suggested to be an inaccurate method for estimating iodine intake, in part attributed to the fact that it is difficult to capture the amount of iodine ingested from iodised salt( Reference Zimmermann and Andersson 22 , Reference Rasmussen, Ovesen and Bulow 32 ); however, this criticism is less relevant in the UK where use of iodised salt is low( Reference Bath, Button and Rayman 33 , Reference Lazarus and Smyth 34 ). The lower iodine intake estimated from food diary analysis in the present study may at least partly be explained by under-reporting – a known problem when using this methodology( Reference Livingstone, Prentice and Strain 35 ). Furthermore, the food table values for iodine in the WinDiets programme may be inaccurate (as a result of poor-quality or out-of-date iodine data in food composition tables( Reference Zimmermann and Andersson 22 )) and values are missing for certain foods, which may result in an estimate that is lower than the actual intake.
Effect of food consumption on iodine intake and status
The intake of milk, eggs and dairy products was found to be positively associated with iodine status in the regression analysis. Iodine excretion correlated more strongly with the intake of milk than with that of other dietary components, reflecting the importance of milk and milk products as sources of dietary iodine in the UK( Reference Henderson, Irving and Gregory 36 ) and supporting previous associations between milk intake and urinary iodine status in UK women( Reference Vanderpump, Lazarus and Smyth 12 , Reference Bath, Walter and Taylor 13 ). Milk has also been found to be an important source of iodine for adults in other European countries( Reference Gunnarsdottir, Gustavsdottir and Steingrimsdottir 37 – Reference Soriguer, Garcia-Fuentes and Gutierrez-Repiso 39 ). Interestingly, there was a negative correlation between soya-drink intake and iodine excretion, but intake of soya drink was not a significant predictor of iodine status in a regression model when other dietary sources of iodine were included. This probably reflects the negative correlation between soya drinks and cows’ milk intake, suggesting that the negative correlation in univariate analyses was a result of the displacement of iodine-rich cows’ milk from the diet. Although the number of soya-drink consumers was relatively low in the present study, our findings warrant further investigation in view of the increasing use of alternatives to cows’ milk by UK women; for example, the volume of soya drinks sold in the UK increased by 10·1 % between January 2009 and January 2013( Reference Datum 40 ). Very few of these milk alternatives are fortified with iodine and therefore women who rely on these products are likely to be considerably more at risk of iodine deficiency than those who regularly consume cows’ milk.
Fish intake was positively correlated with iodine excretion, but the correlation failed to reach significance and was not a significant predictor of iodine status in the regression analysis, perhaps because of the relatively small number of fish consumers in the study. Other UK( Reference Vanderpump, Lazarus and Smyth 12 , Reference Bath, Walter and Taylor 13 ) and European studies( Reference Brantsaeter, Haugen and Thomassen 41 , Reference Johner, Thamm and Nothlings 42 ) have failed to find significant associations between iodine status and fish consumption. Egg consumption was positively associated with iodine status as has been found in previous studies in children( Reference Remer, Fonteyn and Alexy 43 ), though not in the study of UK teenage girls( Reference Vanderpump, Lazarus and Smyth 12 ). The results of the latter study were derived from ambiguous questions on egg consumption, which probably explains the disparity( Reference Bath and Rayman 44 ).
Study limitations
The present study is limited by the small number of participants involved; caution should therefore be exercised when interpreting the results of the subgroup analysis of dietary intake (e.g. of soya drinks). Although we had detailed information on the study participants (e.g. 48 h recorded dietary intake), accuracy would have been improved if we had had a repeated urine collection, even if only for a subsample of the cohort( Reference Zimmermann and Andersson 22 , Reference Charlton, Batterham and Buchanan 45 ); we could then have corrected for intra-individual variation in iodine intake. This might have resulted in an improved estimate of intake for individuals falling below the EAR; our estimate of individual intake on the basis of urinary iodine excretion may have resulted in misclassification of the percentage with estimated intake below the EAR. However, at the time that the present study was designed (2006), the use of multiple 24 h urine collections (as opposed to a single 24 h collection) was not considered as important as it is now. Completeness of the 24 h urine sample was self-reported and thus incomplete samples may have been measured; we consider that this is fairly unlikely as the participants were motivated individuals who understood the implications of incomplete urine collections. Finally, the food-diary analysis is limited by the inherent limitations of dietary-intake assessment, including under-reporting of intake and use of inaccurate food table values for iodine( Reference Rasmussen, Ovesen and Bulow 31 , Reference Livingstone, Prentice and Strain 35 ). We tried to reduce inaccuracies as far as possible, for example, by using the same researcher to code all diaries and enter the data into WinDiets.
Other limiting factors are that the study was carried out under circumstances likely to have maximised iodine intake and status. First, the sampling was conducted during the winter months and it is known that winter milk has a higher iodine concentration than summer milk due to an increased use of supplemented cattle feed( Reference Phillips, Nelson and Barker 46 – 49 ). Were the study to be repeated in the summer months, the percentage of women classified as iodine deficient would likely be higher. Second, the majority of the participants were students on a nutrition/dietetics degree programme and likely to have had a greater understanding of a healthy diet; indeed, the food diaries demonstrated that the group ate regular meals, with healthy food choices (such as fruit and vegetables) and were perhaps not typical of a population of young UK women. This may have resulted in a relatively high intake of iodine-rich foods such as fish and milk; indeed the average milk intake was higher than that of adult women reported in the recent National Diet and Nutrition Survey (NDNS) (150 v. 124 g/d)( Reference Bates, Lennox and Prentice 50 ).
Conclusion
For many years, the UK has been assumed to be iodine sufficient, but the present study adds to the growing evidence base that this may not be the case in women of childbearing age. Women entering pregnancy need to have an adequate iodine status to ensure optimal fetal neurological development and pregnancy outcome. The results of the present study suggest that a proportion of UK women may be entering pregnancy with low iodine stores, particularly in view of the fact that the study design probably resulted in a best-case scenario. Further study in UK women of childbearing age is required; from 2015, results will be available on iodine concentration measured in spot-urine samples in the NDNS, which will provide important data on these women. On the basis of the results of the present study, we suggest that urine samples should be corrected for urine dilution (i.e. by measurement of urinary creatinine concentrations). Finally, the present study highlights the need for revised cut-off values for iodine adequacy in adults, given that we found that the classification of iodine status differed depending on whether UIC values or 24 h urinary iodine excretion measures were used.
Acknowledgements
The authors are grateful to all study participants and to Dr Christine Sieniawska, at the Trace Element Laboratory in Southampton Hospital, for advice on urinary iodine analysis.
The costs of laboratory analysis and consumables were covered by the University of Surrey. S. C. B. is in receipt of an MRC Population Health Scientist Fellowship, which supported the data analysis and the writing of the manuscript.
The authors contributions’ are as follows: S. C. B., M. L. S. and M. P. R. designed the study; S. C. B. and M. L. S. recruited the subjects and participated in the laboratory analysis; S. C. B. conducted the statistical analysis and wrote the manuscript; M. M. analysed the food diaries with supervision from S. C. B.; A. W. and A. T. developed the laboratory analysis method and conducted the urine sample analysis; M. P. R. had primary responsibility for the final content. All authors prepared and reviewed the manuscript and approved the final content.
None of the authors has any conflicts of interest to declare.








