Blunt snout bream (Megalobrama amblycephala) is a major cultured freshwater fish species in China, also introduced in North America (northern Canada to southern Mexico), Africa and Euro-Asia( Reference Li, Liu and Jiang 1 , Reference Habte-Tsion, Liu and Ge 2 ). Blunt snout bream has great consumer demand in China, and its production has been rapidly increased and reached approximately 0·70 million tons in 2012( 3 ). This fish species has a bright future in aquaculture worldwide because of its adaptability to the local environment, compatibility with native species, being a good candidate for freshwater intensive culture, fast growth, high larval survival rate, tender flesh, and disease resistance( Reference Zhou, Ren and Zeng 4 ). However, few nutritional studies have reported about this species including three amino acids, such as dietary threonine (Thr)( Reference Habte-Tsion, Liu and Ren 5 , Reference Habte-Tsion, Ge and Liu 6 ), arginine( Reference Ren, Liao and Xie 7 ) and methionine( Reference Liao, Ren and Liu 8 ).
Thr is the third essential amino acid for growing fish fed with low-protein diets, and involved in many physiological and biochemical processes, including growth, feed efficiency, immune function, and maintenance of adequate feed intake( Reference Habte-Tsion, Liu and Ren 5 , Reference Habte-Tsion, Ge and Liu 6 , Reference Abidi and Khan 9 – Reference Gao, Yang and Liu 11 ). In our previous study, the dietary Thr requirement for juvenile blunt snout has been estimated to be 1·57 % of the diet, corresponding to 4·62 % of dietary protein( Reference Habte-Tsion, Liu and Ren 5 ). The same study demonstrated that juvenile blunt snout bream fed with a Thr-deficient diet exhibited poor growth performance and feed utilisation, while these parameters improved in response to the graded levels of dietary Thr, which confirmed the essentiality of Thr for this fish( Reference Habte-Tsion, Liu and Ren 5 ). A deficiency of dietary Thr also resulted in a reduction in growth performance and feed utilisation efficiency that caused an increase in the oxidation of other essential and non-essential amino acids present at normal levels in a diet of Senegal sole (Solea senegalensis)( Reference Ronnestad, Conceicao and Aragao 12 ) and African catfish (Clarias gariepinus)( Reference Ozorio, Booms and Huisman 13 ). In our previous study, the deficiency (0·58 %) and excess (2·58 %) of dietary Thr led to adverse effects on the growth and immunity of blunt snout bream( Reference Habte-Tsion, Ge and Liu 6 ). An anorectic status followed by weight loss also occurred in Indian major carp( Reference Abidi and Khan 9 ), grass carp( Reference Gao, Yang and Liu 11 ), common carp( Reference Nose, Halver and Tiews 14 ), Catla catla ( Reference Ravi and Devaraj 15 ), Japanese flounder( Reference Alam, Teshima and Koshio 16 ) and Indian catfish( Reference Ahmed 17 ) in response to diets lacking or low in Thr, but containing otherwise adequate levels of all nutrients. An excess level of dietary Thr in a diet also appeared to have adverse effects on the growth performance of Indian major carps (Labeo rohita ( Reference Abidi and Khan 9 ) and Cirrhinus mrigala ( Reference Ahmed, Khan and Jafri 18 )).
Fish growth is dependent on digestion ability, which has been found to correlate with the activity of digestive enzymes( Reference Hakim, Uni and Hulata 19 ). The activities of digestive enzymes in fish appear to be strongly correlated with the composition of diet( Reference Tibaldi, Hakim and Uni 20 , Reference Yu, Ai and Mai 21 ). Digestion functions in fish rely on the growth and development of digestive organs and activities of digestive enzymes( Reference García-Gasca, Galaviz and Gutiérrez 22 , Reference Perez-Casanova, Murray and Gallant 23 ). Among the essential amino acids, Thr is absorbed in greater proportion by the small intestine in mammals, suggesting that Thr is involved in intestinal functionality and maintenance( Reference Le Floc'h and Sève 24 , Reference van der Schoor, Wattimena and Huijmans 25 ). In piglets, Thr is used to the greatest extent by the portal-drained viscera (including the intestines and pancreas), and 60–80 % of dietary Thr is extracted by the portal-drained viscera in the first pass( Reference Schaart, Schierbeek and van der Schoor 26 ). Meanwhile, intestinal mucins are particularly enriched in Thr (up to 30 % of the amino acid composition)( Reference Faure, Moennoz and Montigon 27 ). Thr supplementation enhanced the activities of digestive enzymes in Jian carp( Reference Feng, Peng and Wu 28 ). Nevertheless, there is no report regarding the effect of dietary Thr on the activities of digestive enzymes in blunt snout bream, which needs to be investigated.
Protein synthesis is a key component of the processes involved in growth response( Reference Anthony, Reiter and Anthony 29 ). It is well known that the target of rapamycin (TOR) signalling pathway plays an important role in regulating protein synthesis in fish( Reference Feng, Peng and Wu 28 ) and mammals( Reference Holz, Ballif and Gygi 30 ). The eukaryotic translation initiation factor 4E-binding protein (4E-BP) family is one of the major downstream targets of TOR protein( Reference Schmelzle and Hall 31 ). The increased enzyme activity results from an increased synthesis of enzyme protein( Reference Thoenen, Kettlee and Burkard 32 ), which relates to gene expression( Reference Chang, Arsenijevic and Vladoianu 33 ). Gene expression levels of digestive enzymes have been shown to be related to nutritional factors in fish( Reference Wang, Xie and Zhu 34 , Reference Zhao, Liu and Jiang 35 ). The beneficial effect of Thr on the activities of digestive enzymes may be partly related to the synthesis and secretion of enzymes. Nevertheless, there is no report about the relationship between Thr and gene expression levels of digestive enzymes in the hepatopancreas of fish, which is worthy of investigation. Indeed, the present study hypothesised that Thr may play an important role in the growth and development of digestive organs, and that Thr may regulate the mRNA expression levels of digestive enzymes, TOR, 4E-BP2 and IGF-I in the hepatopancreas of blunt snout bream, which may go further to explain the effects of Thr in this fish species. Therefore, the present study was conducted to test the aforementioned hypothesis.
Materials and methods
Diet preparation and fish rearing
Diet preparation was carried out as described in our previous study( Reference Habte-Tsion, Liu and Ren 5 ). Briefly, five isonitrogenous (34 % crude protein in DM) and isoenergetic semi-purified diets were formulated to contain graded levels of dietary Thr (Table 1). Thr concentrations in five experimental diets were calculated to be 0·58 (unsupplemented control), 1·08, 1·58, 2·08 or 2·58 % of the diet. The diets were supplemented with 0, 0·5, 1·0, 1·5 or 2·0 % l-crystalline Thr. The diets were made isonitrogenous by adjusting the level of l-glutamic acid. A mixture of l-crystalline amino acids was prepared taking into account the amount of amino acids contributed by fishmeal, casein and gelatin. The mixture was supplemented to simulate the whole-body amino acid pattern of blunt snout bream, except for Thr. The mixture of l-crystalline amino acids was pre-coated with 3 g cooked carboxymethyl cellulose in water at 60°C, and then the carboxymethyl cellulose-bound crystalline amino acid mixture was blended with the other thoroughly mixed dry ingredients followed by the addition of oils and water, until well homogenised( Reference Millamena, Bautista-Teruel and Reyes 36 , Reference Alam, Teshima and Koshio 37 ). Pellets (1·5 and 2·0 mm in diameter) were produced using laboratory twin-screw extruder (Science and Technology Industrial Factory of South China University of Technology), air-dried to approximately 10 % moisture, sealed in vacuum-packed bags, and stored frozen ( − 15°C) before use in the feeding trial. Table 2 shows the amino acid composition of ingredients (g/100 g DM). The composition of amino acids in the experimental diets (Table 3, g/100 g of diet) was analysed according to the method described by Ren et al. ( Reference Ren, Liao and Xie 7 ).
Table 1 Ingredients and composition of the basal diet*

* Adopted from our previous study( Reference Habte-Tsion, Liu and Ren 5 ).
† Crude protein 67·4 % and crude lipid 9·3 %, provided by Tongwei Feed Group Company, Limited (origin Copeinca).
‡ Crude protein 90·2 %, purchased from Huaan Biological Products Company, Limited.
§ Crude protein 91·3 %, purchased from Shanghai Zhan Yun Chemical Company, Limited.
∥ Provided by Cargill.
¶ Amino acid premix (g/100 g of diet): l-histidine 0·31; l-isoleucine 0·68; l-leucine 0·87; l-lysine 1·09; l-methionine 0·43; l-phenylalanine 0·66; l-threonine 0·71; l-valine 0·56; l-aspartic acid 1·46; l-serine 0·55; glycine 1·37; l-alanine 1·25; l-cystine 0·14; l-tyrosine 0·27; l-tryptophan 0·12; l-glutamic acid 1·11; l-proline 0·12. Amino acids were obtained from Feeer Company, Limited.
** 35 % ascorbic acid equivalent, provided by Tongwei Feed Group Company Limited.
†† Premixes of vitamins (IU or mg/kg of premix) and minerals (g/kg of premix): vitamin A 900 000 IU; vitamin D 250 000 IU; vitamin C 10 000 mg; vitamin E 4500 mg; vitamin K3 220 mg; vitamin B1 320 mg; vitamin B2 1090 mg; vitamin B6 5000 mg; vitamin B12 116 mg; biotin 50 mg; pantothenate 1000 mg; folic acid 165 mg; choline 60 000 mg; inositol 15 000 mg; niacin acid 2500 mg; CuSO4.5H2O 2·5 g; FeSO4.7H2O 28 g; ZnSO4.7H2O 22 g; MnSO4.4H2O 9 g; Na2SeO3 0·045 g; KI 0·026 g; CoCl2.6H2O 0·1 g. The premixes were provided by Wuxi Hanove Animal Health Products Company Limited.
‡‡ Provided by Guangzhou Hinter Biotechnology Company, Limited.
§§ Values for the proximate composition of the test diets are means of triplicate analyses.
Table 2 Amino acid composition of ingredients (g/100 g DM)*

CAAP, crystalline amino acid premix; WBP, whole-body protein.
* Calculated according to the method described by Habte-Tsion et al. ( Reference Habte-Tsion, Liu and Ren 5 ) and Ren et al. ( Reference Ren, Liao and Xie 7 ). Tryptophan could not be measured because of its degradation during acid hydrolysis.
Table 3 Analysis of amino acid composition in the experimental diets (g/100 g of diet)*
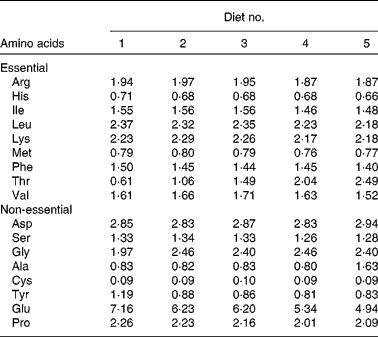
* Adopted from our previous study( Reference Habte-Tsion, Liu and Ren 5 ), and data are means of three replicates. Tryptophan could not be measured because of its degradation during acid hydrolysis.
The use of the experimental fish was according to the scientific research protocols of the Chinese Academy of Fishery Sciences (CAFS) and the Ministry of Agriculture, PR China, and complied with all relevant local and/or international animal welfare laws, guidelines and policies( Reference Spreij 38 ). Fish rearing was conducted as described in our previous study( Reference Habte-Tsion, Liu and Ren 5 ). Briefly, juvenile blunt snout bream were obtained from Freshwater Fisheries Research Center (FFRC) and acclimatised with experimental facilities for 2 weeks. During the acclimatisation period, fish were fed with the basal diet (0·58 % Thr) up to satiation. At the start of the feeding trial, similar size of fish (initial weight 3·01 (sem 0·01) g) was selected and restocked at a stocking density of thirty fish per tank. Overall, three tanks (300 litres/tank) were randomly arranged and assigned to each experimental diet. Fish were hand-fed with their respective diet four times daily (at 08.00, 11.00, 14.00 and 17.00 hours) to apparent satiation on the basis of visual observation. The uneaten diet was siphoned from each tank before feeding on daily basis. The following parameters were maintained during the experimental period: water temperature, 26–28°C; pH, 7·0–7·5; NH3 N, < 0·05 mg/l; dissolved oxygen, ≥ 6·0 mg/l; photoperiod, natural (light–dark cycle).
Sample collection
At the end of the 9-week feeding trial, fish were starved for 24 h to evacuate the contents of the alimentary tract before the sampling period. Fish were sampled (from each tank) and anaesthetised with 100 mg/l of tricaine methanesulfonate (MS-222). For each group, fifteen fish (five fish per tank) were weighed and dissected, and samples of their hepatopancreas and intestine were removed and weighed to calculate hepatopancreas weight (HW), hepatosomatic index (HSI), intestinal weight (IW) and intestosomatic index (ISI). Among the fifteen fish, nine were randomly selected and their hepatopancreas and intestine were used to measure the hepatopancreatic protein content (HPC) and intestinal protein content (IPC). Meanwhile, from same individual samples (nine fish), the hepatopancreas and intestinal segments (divided into the proximal intestine (PI), mid-intestine (MI) and distal intestine (DI)) were used to assay the activities of digestive enzymes. Another three fish from each tank (nine per group) were dissected, and samples of their hepatopancreas were quickly removed for gene expression assay. All samples were frozen in liquid N2 and then stored at − 80°C for the subsequent analysis of the following: activities of digestive enzymes (chymotrypsin, trypsin, amylase and lipase) in the hepatopancreas and three intestinal segments; gene expression levels of chymotrypsin, trypsin, amylase, lipase, TOR, 4E-BP2 and IGF-I in the hepatopancreas.
Enzyme activity assay
The hepatopancreas and intestinal samples were homogenised in ten volumes (w/v) of ice-cold physiological saline and centrifuged at 6000 g for 20 min at 4°C, and the supernatant was conserved at − 80°C for the analysis of enzyme activities. The IPC and HPC were measured using the method of Bradford( Reference Bradford 39 ). Trypsin and chymotrypsin activities were determined by the method described by Hummel( Reference Hummel 40 ). Lipase and amylase activities were assayed as described by Furne et al. ( Reference Furne, Hidalgo and Lopez 41 ).
Real-time PCR analysis
Real-time PCR analysis was carried out according to our previous studies( Reference Habte-Tsion, Liu and Ren 5 , Reference Habte-Tsion, Ge and Liu 6 ). Briefly, total RNA was extracted from the hepatopancreas of juvenile blunt snout bream using an RNAiso Plus Kit (Takara). Agarose gel electrophoresis at 1 % and spectrophotometric analysis (A260:280 nm ratio) were used to assess RNA quality and quantity. Subsequently, complementary DNA (cDNA) was synthesised using a PrimeScript™ RT reagent kit (Takara), according to the manufacturer's instructions. Briefly, oligo dT primers (50 μm) were used to reverse transcribe respective RNA in the presence of PrimeScript™ RT enzyme mix I, 5 × PrimeScript™ buffer, and RNase-free distilled water at 37°C for 15 min followed by inactivation at 85°C for 5 s. Specific primers for most of the target genes were designed according to the partial cDNA sequences of the target genes using the M. amblycephala transcriptome analysis( Reference Habte-Tsion, Liu and Ren 5 , Reference Habte-Tsion, Ge and Liu 6 , Reference Gao, Luo and Liu 42 ), and primers for lipase and β-actin were designed using the published sequences of blunt snout bream (GeneBank no. KF114279.1 and AY170122.2, respectively; Table 4). All primers were synthesised by Shanghai Biocolor, BioScience & Technology Company.
Table 4 Real-time PCR primer sequences

TOR, target of rapamycin; 4E-BP2, eukaryotic translation initiation factor 4E-binding protein 2; IGF-I, insulin-like growth factor-I.
Real-time PCR was used to determine mRNA levels using a PrimerScript™ Reagent Kit (Takara). Real-time PCR for the target genes were performed according to standard protocols. Briefly, cDNA (2·0 μl) was reacted with 10·0 μl SYBR® Premix Ex Taq II (2 × ), 0·8 μl forward primer (10 μM), 0·8 μl reverse primer (10 μM), 0·4 μl ROX™ reference dye or dye II (50 × ), and 6·0 μl RNase-free distilled water in a final reaction volume of 20 μl. The real-time PCR was carried out in a 7500 Real-Time PCR System (Applied Biosystems). The thermocycling conditions for the target genes were as follows: initial denaturation step at 95°C for 30 s, followed by forty cycles at 95°C for 5 s, 60°C for 34 s and 95°C for 30 s, 95°C for 3 s, 60°C for 30 s, respectively. The melting curve analysis was performed over a range of 50–95°C to verify that a single PCR product was generated. The concentration of the target genes was based on the threshold cycle number (C
T), and C
T for each sample was determined using 7500 Software version 2.0.4 (Applied Biosystems). The expression levels of the target genes were normalised to those of a housekeeping gene (β-actin) of blunt snout bream. The expression results were analysed using the
![]() $$2^{ - \Delta \Delta C _{T}} $$
method after verifying that the primers were amplified with an efficiency of approximately 100 %(
Reference Livak and Schmittgen
43
). Besides, cDNA concentration in each sample was determined according to gene-specific standard curves. Standard curves were generated for both target and endogenous control genes based on 10-fold serial dilutions. All standard curves exhibited correlation coefficients >0·99.
$$2^{ - \Delta \Delta C _{T}} $$
method after verifying that the primers were amplified with an efficiency of approximately 100 %(
Reference Livak and Schmittgen
43
). Besides, cDNA concentration in each sample was determined according to gene-specific standard curves. Standard curves were generated for both target and endogenous control genes based on 10-fold serial dilutions. All standard curves exhibited correlation coefficients >0·99.
Calculations and statistical analysis
The growth and development of digestive organs in juvenile blunt snout bream fed with the graded levels of dietary Thr for 9 weeks were calculated using the following formulas:
Statistical analysis was performed using SPSS version 19 (SPSS, Inc.). All data were subjected to one-way ANOVA followed by least significant difference multiple comparisons. Significant differences among group means as well as between intestinal segment (PI, MI and DI) means were further compared using Duncan's multiple range tests. P< 0·05 was considered statistically significant. Results are expressed as means with their standard errors. The relationship between dietary Thr and the growth and development of digestive organs and activities of digestive enzymes, as well as gene expression levels of digestive enzymes, TOR, 4E-BP2 and IGF-I in the hepatopancreas, respectively, were subjected to a second-degree polynomial regression analysis( Reference Zeitoun, Ullrey and Magee 44 ) and the quadratic regression model using SPSS version 19.
Results
Growth and development of digestive organs
Table 5 shows the growth and development of digestive organs in juvenile blunt snout bream fed with the graded levels of dietary Thr for 9 weeks. The HW, HSI, HPC, IW, ISI and IPC were significantly (P< 0·05) influenced by dietary Thr levels. The HW and IW increased as dietary Thr levels increased up to 1·58 % (P< 0·05), remained plateau up to 2·08 % and then declined. The highest HSI was found in the group fed with 1·58 % dietary Thr (P< 0·05). The HPC increased as dietary Thr levels increased, and a maximum value was found in the group fed with 1·58 % dietary Thr followed by those fed with 1·08 % dietary Thr (P< 0·05). The ISI increased with increasing dietary Thr levels up to 1·58 % and thereafter declined (P< 0·05). The highest IPC was obtained in the group fed with 1·58 % dietary Thr, while the lowest value was obtained in that fed with the basal diet (P< 0·05). Significant differences in the IPC were found between 1·58 and 2·08 % dietary Thr, and between 2·08 and 2·58 % dietary Thr (P< 0·05). The HW, HSI, HPC, IW, ISI and IPC of the group fed with the basal diet (0·58 % Thr) and the 2·58 % Thr diet were significantly (P< 0·05) lower than those of the group fed with the 1·58 % Thr diet. The HW, HSI, HPC, IW, ISI and IPC quadratically responded to the dietary Thr levels. Based on the quadratic equations in Table 5, the optimum Thr levels for each parameter were 1·87, 1·66, 1·59, 1·68, 1·61 and 1·68 %, respectively.
Table 5 Growth and development of digestive organs in juvenile blunt snout bream fed with the graded levels of dietary Thr for 9 weeks* (Mean values with their standard errors)

HW, hepatopancreas weight; HSI, hepatosomatic index; HPC, hepatopancreatic protein content; IW, intestinal weight; ISI, intestosomatic index; IPC, intestinal protein content.
a,b,c,dMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* n 15.
† n 9.
Activities of digestive enzymes in the hepatopancreas
Table 6 shows the activities of digestive enzymes in the hepatopancreas of juvenile blunt snout bream fed with the graded levels of dietary Thr for 9 weeks. The highest chymotrypsin activity was found in the group fed with 1·58 and 2·08 % dietary Thr (P< 0·05). Trypsin activity in the hepatopancreas increased as dietary Thr levels increased up to 1·58 %, remained plateau up to 2·08 % and thereafter declined (P< 0·05). Amylase and lipase activities in the hepatopancreas increased with increasing dietary Thr levels, and the highest activities were observed in fish fed with 1·58 % dietary Thr (P< 0·05). The activities of digestive enzymes in the group fed with 0·58 and 2·58 % dietary Thr were significantly lower than those in the group fed with 1·58 % dietary Thr (P< 0·05). Digestive enzyme activities in the hepatopancreas quadratically responded to the dietary Thr levels. Based on the quadratic equations in Table 6, the optimum Thr levels for chymotrypsin, trypsin, amylase and lipase, were 1·72, 1·72, 1·77 and 1·66 %, respectively.
Table 6 Activities of digestive enzymes in the hepatopancreas of juvenile blunt snout bream fed with the graded levels of dietary threonine (Thr) for 9 weeks (Mean values with their standard errors; n 9)
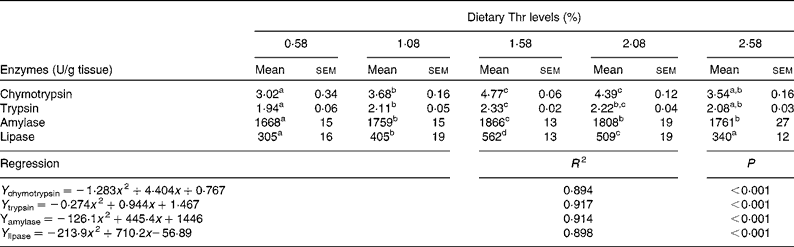
a,b,c,dMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Activities of digestive enzymes in intestinal segments
Digestive enzyme activities in the PI, MI and DI of blunt snout bream fed with the graded levels of dietary Thr for 9 weeks are presented in Table 7. The activities of chymotrypsin in the PI, MI and DI first increased up to 1·58 % dietary Thr and thereafter declined (P< 0·05). Thr supplementation significantly increased chymotrypsin activities in the PI, MI and DI, compared with the unsupplemented diet (P< 0·05). Trypsin activities in the PI, MI and DI increased with increasing dietary Thr levels up to 1·58 % and thereafter decreased (P< 0·05). Trypsin activities in the PI, MI and DI significantly responded to the addition of Thr in comparison with the basal diet (P< 0·05). Amylase activities in the PI, MI and DI increased as dietary Thr levels increased, and maximum activities were observed in the group fed the diet containing 1·58 % Thr (P< 0·05). The highest lipase activities in the PI, MI and DI were found in the group fed with 1·58 % dietary Thr (P< 0·05). Significantly lower digestive enzyme activities in the PI, MI and DI were found in the group fed with 0·58 and 2·58 % dietary Thr compared with those in the group fed with 1·58 % dietary Thr (P< 0·05). Significantly different levels of chymotrypsin, trypsin, amylase and lipase activities were found between the three intestinal segments (PI, MI and DI), with the highest activities in the PI followed by the MI (P< 0·01). Digestive enzyme activities in the three intestinal segments quadratically responded to the graded levels of dietary Thr. Based on the quadratic equations in Table 7, the optimum Thr levels were 1·73, 1·63 and 1·68 % for chymotrypsin in the PI, MI and DI, respectively; 1·78, 1·67 and 1·66 % for trypsin in the PI, MI and DI, respectively; 1·67, 1·62 and 1·61 % for amylase in the PI, MI and DI, respectively; 1·62, 1·61 and 1·62 % for lipase in the PI, MI and DI, respectively.
Table 7 Activities of digestive enzymes in the proximal intestine (PI), mid-intestine (MI) and distal intestine (DI) of juvenile blunt snout bream fed with the graded levels of dietary threonine (Thr) for 9 weeks (Mean values with their standard errors; n 9)

a,b,c,dMean values within a row with unlike superscript letters were significantly different (P< 0·05).
A,B,CMean values within a column with unlike superscript letters were significantly different (P< 0·05).
Gene expression of digestive enzymes in the hepatopancreas
Fig. 1 shows the gene expression levels of digestive enzymes, chymotrypsin, trypsin, amylase and lipase in the hepatopancreas of juvenile blunt snout bream fed with the graded levels of dietary Thr for 9 weeks. The relative mRNA expression levels of chymotrypsin increased as dietary Thr levels increased up to 1·58 % and thereafter fluctuated (Fig. 1(A)). The relative mRNA expression levels of trypsin (Fig. 1(B)) and lipase (Fig. 1(D)) increased with increasing dietary Thr levels up to 1·58 % and remained plateau thereafter. The highest relative mRNA expression level of amylase (Fig. 1(C)) was found in the group fed with 1·58 % dietary Thr (P< 0·05). The relative gene expression levels of digestive enzymes in the hepatopancreas quadratically responded to the dietary Thr levels (Y chemotrypsin= − 0·701x 2+2·870x− 0·918, R 2 0·479, P= 0·079; Y trypsin= − 1·02x 2+3·667x− 0·959, R 2 0·891, P= 0·015; Y amylase= − 1·009x 2+3·276x− 1·288, R 2 0·432, P= 0·098; Y lipase= − 0·731x 2+2·650x− 0·365, R 2 0·840, P= 0·030). Based on the quadratic equations, the optimum Thr levels for the relative mRNA expression levels of chymotrypsin, trypsin, amylase and lipase were 2·05, 1·80, 1·62 and 1·81 %, respectively.

Fig. 1 Relative mRNA expression levels of (A) chymotrypsin, (B) trypsin, (C) amylase and (D) lipase in the hepatopancreas of juvenile blunt snout bream fed with the graded levels of dietary threonine (Thr) for 9 weeks. Values are means, with standard errors represented by vertical bars (n 9). Mean values with unlike letters are significantly different (P< 0·05).
Gene expression of target of rapamycin, eukaryotic translation initiation factor 4E-binding protein 2 and insulin-like growth factor-I in the hepatopancreas
The relative mRNA expression levels of TOR, 4E-BP2 and IGF-I in the hepatopancreas of blunt snout bream fed with the graded levels of dietary Thr are shown in Fig. 2. The relative mRNA expression levels of TOR (Fig. 2(A)) and IGF-I (Fig. 2(C)) first increased up to 1·58 % dietary Thr, and the values were significantly decreased thereafter (P< 0·05). In contrast, the relative mRNA expression levels of 4E-BP2 (Fig. 2(B)) gradually decreased (P< 0·10) as dietary Thr levels increased up to 1·58 % and significantly increased thereafter (P< 0·05). The relative mRNA expression levels of TOR, 4E-BP2 and IGF-I in the hepatopancreas quadratically responded to the dietary Thr levels (Y TOR= − 1·355x 2+4·501x− 1·905, R 2 0·672, P= 0·005; Y 4E-BP2= 1·079x 2− 2·733x+2·485, R 2 0·895, P= 0·015; YIGF-I= − 1·146x 2+3·793x− 1·439, R 2 0·755, P= 0·007). Based on the quadratic equations, the optimum Thr levels for the relative mRNA expression levels of TOR, 4E-BP2 and IGF-I were 1·66, 1·27 and 1·65 %, respectively.

Fig. 2 Relative mRNA expression levels of (A) target of rapamycin (TOR), (B) eukaryotic translation initiation factor 4E-binding protein 2 (4E-BP2) and (C) insulin-like growth factor-I (IGF-I) in the hepatopancreas of juvenile blunt snout bream fed with the graded levels of dietary threonine (Thr) for 9 weeks. Values are means, with standard errors represented by vertical bars (n 9). Mean values with unlike letters are significantly different (P< 0·05).
Discussion
Animal growth depends on nutrient digestion and absorption, which is governed by the activities of digestive enzymes and absorptive mechanisms( Reference Klein, Cohn, Alpers, Shils, Olson, Shike and Ross 45 ). Moreover, it depends on intestinal development. In our previous study, dietary Thr supplementation improved the growth, feed utilisation efficiency and protein retention of juvenile blunt snout bream, and the requirement for juvenile blunt snout was estimated to be 1·57 % of the diet, corresponding to 4·62 % of dietary protein( Reference Habte-Tsion, Liu and Ren 5 ). In the present study, the HW, HSI and HPC were enhanced with increasing Thr levels up to 1·58 %, which suggested that Thr supplementation improved the growth and development of the hepatopancreas in juvenile blunt snout bream. This is consistent with the trends of growth performances demonstrated in our previous study( Reference Habte-Tsion, Liu and Ren 5 ). Similar results were also reported in the hepatopancreas of Jian carp fed with graded levels of dietary Thr( Reference Feng, Peng and Wu 28 ). However, further studies are needed to evaluate the specific mechanisms by which Thr improved the growth of the hepatopancreas in fish. Moreover, in our previous study, the deficiency (0·58 %) and excess (2·58 %) of dietary Thr had a negative impact on the weight gain of blunt snout bream( Reference Habte-Tsion, Ge and Liu 6 ). Similarly in the present study, imbalanced Thr levels (both 0·58 and 2·58 %) significantly reduced the HW, HSI and HPC in juvenile blunt snout bream, suggesting that an unbalanced Thr level could have adverse effects on the growth and development of the hepatopancreas. Nevertheless, specific mechanisms underlying the adverse effects of Thr-imbalanced diets on the growth and development of the hepatopancreas in fish need to be elucidated.
A low-Thr diet reduced the gut weight of rats( Reference Faure, Moennoz and Montigon 27 ) and the intestinal mass of piglets( Reference Stoll 46 ). In the present study, the IW and ISI increased with increasing Thr levels up to 1·58 %, which could be related to the fact that Thr supplementation improved the intestinal growth and development of blunt snout bream. This is in agreement with the trends of growth performances demonstrated in our previous study( Reference Habte-Tsion, Liu and Ren 5 ). Similar studies have considered the effects of Thr( Reference Feng, Peng and Wu 28 ), isoleucine( Reference Zhao, Liu and Jiang 35 ), lysine( Reference Zhou, Zhao and Jiang 47 ), methionine( Reference Tang, Wang and Jiang 48 ) and arginine( Reference Chen, Feng and Kuang 49 ) on the growth and development of digestive organs in fish. Intestinal development is related to protein concentration in the intestinal tissue( Reference Goldspink, Lewis and Kelly 50 ). The present study showed that the IPC was improved by the supplementation of dietary Thr. Similarly, Thr increased the protein content of the intestine in Jian carp( Reference Feng, Peng and Wu 28 ) and the protein content of the jejunum in rats( Reference Faure, Moennoz and Montigon 27 ). In our previous study, dietary Thr regulated the mRNA expression levels of the TOR pathway in the three intestinal segments of blunt snout bream( Reference Habte-Tsion, Liu and Ren 5 ). An in vitro study has also indicated that the protein synthesis rate of enterocytes in the intestine was enhanced by Thr supplementation( Reference Feng, Peng and Wu 28 ). These studies suggested that Thr may enhance protein synthesis ability in fish as it does in terrestrial animals, and this important role of Thr in protein synthesis could be one of the primary causes of the improved growth and development of digestive organs. Moreover, in the present study, unbalanced Thr levels (both 0·58 and 2·58 %) significantly decreased the IW, ISI and IPC of juvenile blunt snout bream, suggesting that an imbalanced Thr level could have adverse effects on the growth and development of the intestine. Similarly in our previous study, a deficiency and an excess of Thr levels had a negative impact on the weight gain of blunt snout bream( Reference Habte-Tsion, Ge and Liu 6 ).
In fish, digestive enzymes such as chymotrypsin, trypsin, amylase and lipase are synthesised in the exocrine pancreas and are secreted into the lumen of the intestine( Reference Zambonino-Infante and Cahu 51 ). Digestive enzyme activities can directly reflect digestive ability( Reference Wen, Zhou and Feng 52 ). In the present study, chymotrypsin, trypsin, amylase and lipase activities were significantly improved by the addition of Thr at an adequate (1·58 %) level in the hepatopancreas and intestinal segments, suggesting that dietary Thr at an adequate level could enhance the digestive ability of fish. The improved activities of digestive enzymes could be due to the improved growth of the hepatopancreas and the intestine by Thr in blunt snout bream. This is comparable with our previous study reporting that an optimum dietary Thr level improved the growth of blunt snout bream( Reference Habte-Tsion, Liu and Ren 5 ). An adequate level of Thr also improved the digestive capacity and growth of Jian carp( Reference Feng, Peng and Wu 28 ). In the present study, different levels of chymotrypsin, trypsin, amylase and lipase activities were found between the three intestinal segments (PI, MI and DI), with the highest activities being observed in the PI followed by the MI. Similarly, the level of digestive enzyme activities differed between the gut sections, with all activities found to be significantly higher in the pyloric caecum compared with the foregut/midgut and hindgut sections of yellowtail kingfish( Reference Bowyer, Qin and Adams 53 ). Nevertheless, this is the first report regarding the different activity levels of digestive enzymes (chymotrypsin, trypsin, amylase and lipase) between the PI, MI and DI in fish fed with dietary Thr, which needs to be elucidated.
Fish exocrine pancreas is the main site for the synthesis and secretion of digestive enzymes( Reference Zambonino-Infante and Cahu 51 ). Digestive enzyme activity is known to be related to the synthesis and secretion of enzymes in fish( Reference Beccaria, Diaz and Connes 54 ). The increased enzyme activity results from an increased synthesis of enzyme protein( Reference Thoenen, Kettlee and Burkard 32 ), which is associated with gene transcription and translation( Reference Chang, Arsenijevic and Vladoianu 33 ). In terrestrial animals, Thr participated in the amino acid composition of chymotrypsinogen, achymotrypsin and trypsinogen( Reference Smith 55 ), and was necessary for amylase synthesis in pigeon pancreas( Reference Hokin 56 ). In the present study, Thr supplementation up-regulated the gene expression levels of digestive enzymes, such as chymotrypsin, trypsin, amylase and lipase in the hepatopancreas of blunt snout bream, which suggested that the beneficial effect of Thr on the activities of digestive enzymes may be partly related to the synthesis of enzymes in fish as it does in terrestrial animals. However, this is the first report about the effect of Thr on the gene expression levels of digestive enzymes in fish. Whether Thr supplementation improved the activities of digestive enzymes via regulating enzyme synthesis in fish needs to be elucidated.
The limiting step in protein synthesis is translation initiation, which is regulated by the TOR signalling pathway( Reference Holz, Ballif and Gygi 30 , Reference Wang and Proud 57 ). 4E-BP, a family of translational repressors, is a well-known target of TOR kinase and regulation of protein synthesis( Reference Anand and Gruppuso 58 ). In the present study, dietary Thr modified the mRNA expression levels of TOR and 4E-BP2 in the hepatopancreas of juvenile blunt snout bream, which may go further to support the results of protein accretion improvement in this digestive organ. This is comparable with our previous study showing that dietary Thr modified the mRNA expression levels of TOR and 4E-BP2 in the three intestinal segments of blunt snout bream( Reference Habte-Tsion, Liu and Ren 5 , Reference Habte-Tsion, Ge and Liu 6 ), and with another study conducted in the muscle, hepatopancreas and intestine of Jian carp( Reference Feng, Peng and Wu 28 ). Furthermore, the mRNA expression levels of TOR in the hepatopancreas of blunt snout bream increased with increasing dietary Thr levels up to 1·58 % and then decreased, while a reverse pattern was found in the mRNA expression levels of 4E-BP2 in the present study, which is consistent with the trends of our previous study( Reference Habte-Tsion, Liu and Ren 5 ).
Growth in fish and other vertebrates is under endocrine control, particularly through the growth hormone–IGF axis( Reference Picha, Turano and Beckman 59 ). Insulin plays an important role in the regulation of protein synthesis( Reference Schmelzle and Hall 31 ). In rats, IGF-I increased enterocyte proliferation( Reference Dahly, Guo and Ney 60 ). Thr participates in the amino acid composition of IGF-I, and plays an important role in maintaining the structure of IGF-I( Reference Rinderknecht and Humbel 61 ). In the present study, 1·58 % dietary Thr up-regulated the mRNA expression levels of IGF-I in the hepatopancreas of blunt snout bream, suggesting that an adequate level of Thr may regulate protein synthesis ability via regulating the IGF-I level in fish. However, this is the first study in fish and a specific mechanism remains to be elucidated. Meanwhile, a similar expression pattern was found between the mRNA expression levels of IGF-I and TOR in the present study.
In conclusion, the present study showed that 1·58 % dietary Thr improved the growth and development of the hepatopancreas and the intestine, and enhanced the activities of digestive enzymes in blunt snout bream. Dietary Thr regulated the mRNA expression levels of digestive enzymes (chymotrypsin, trypsin, amylase and lipase), TOR, 4E-BP2 and IGF-I in the hepatopancreas of juvenile blunt snout bream, which could explain further the positive effects of Thr supplementation on the digestion capacity, and the growth and development of digestive organs of this fish. The present study could provide a new molecular tool for studies on fish nutrition, and shed light on the regulatory mechanisms that dietary Thr enhanced digestive function in fish.
Acknowledgements
The authors gratefully acknowledge the postgraduate students of Fish Disease and Nutrition Department, FFRC, CAFS, Wuxi City, PR China for their help during the sampling period.
The present study was funded by grant from the Modern Agro-industry Technology Research System, PR China (grant no. CARS-46), the Special Fund for Agro-Scientific Research in the Public Interest (grant no. 201003020), and the National Nonprofit Institute Research Grant of FFRC, CAFS, PR China (grant no. 2014A08XK02). All funders had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: H.-M. H.-T., B. L., M. R., J. X. and X. G. designed the study; H.-M. H.-T., M. R., R. C., Q. Z. and L. P. performed the feeding trial and collected the sample; H.-M. H.-T., M. R. and B. L. analysed the data; H.-M. H.-T. wrote the manuscript; M. R., B. L. and X. G. provided advice and critical review of the manuscript. All authors read and approved the final version of the manuscript.
There are no conflicts of interest.












