Diet is acknowledged to be a leading behavioural risk factor for cardiometabolic diseases(Reference Forouzanfar, Alexander and Anderson1). Among dietary factors, dairy products are of particular interest due to their inverse association with cardiometabolic health(Reference Rice, Quann and Miller2), thought to be related to their high nutrient density and mineral content, but also due to the controversy arising from their blood cholesterol raising saturated fat content(Reference Ramsden, Zamora and Majchrzak-Hong3). Several meta-analyses of prospective cohort studies on the associations of dairy products with CVD(Reference Guo, Astrup and Lovegrove4, Reference Alexander, Bylsma and Vargas5) and type 2 diabetes(Reference Aune, Norat and Romundstad6–Reference Gao, Ning and Wang8) have suggested that the associations vary by dairy type. There is a general concordance of observational results suggesting an inverse association between yogurt consumption and type 2 diabetes(Reference Aune, Norat and Romundstad6–Reference Gao, Ning and Wang8). In contrast, evidence for associations between other dairy types and type 2 diabetes(Reference Gijsbers, Ding and Malik7, Reference Mozaffarian9) or CVD(Reference Guo, Astrup and Lovegrove4) is inconsistent with either null or inverse associations reported for the consumption of cheese and low-fat milk(Reference Guo, Astrup and Lovegrove4, Reference Gijsbers, Ding and Malik7, Reference Mozaffarian9).
Adiposity is one of the most well-described potential pathways which might link total dairy consumption to cardiometabolic disease pathogenesis. Evidence from randomised clinical trials shows that total dairy products decrease body weight(Reference Geng, Qi and Huang10) and body fat mass(Reference Geng, Qi and Huang10), and increase body lean mass(Reference Stonehouse, Wycherley and Luscombe-Marsh11) under energy restriction. However, evidence on associations between types of dairy products and markers of adiposity, especially markers of fat and lean mass distribution, is sparse. Visceral adipose tissue (VAT) and subcutaneous adipose tissue (SCAT) are compartments of abdominal fat. While abdominal fat is associated with cardiometabolic diseases(Reference Wajchenberg12), VAT has been more strongly associated with cardiometabolic risk than SCAT(Reference Fox, Massaro and Hoffmann13), which has been suggested to be potentially protective(Reference Porter, Massaro and Hoffmann14). Specifically the ratio of VAT to SCAT was reported to be strongly associated with cardiometabolic risk independent of BMI and VAT(Reference Kaess, Pedley and Massaro15). Due to the higher cost of the methods to estimate abdominal fat distribution, for example, dual-energy X-ray absorptiometry (DEXA), most large-scale population studies examine waist circumference as a proxy for abdominal fat, which cannot fully capture the distribution between VAT and SCAT. Only a few studies have investigated associations between dairy products (total(Reference Josse, Atkinson and Tarnopolsky16) and low-fat(Reference Pallister, Jackson and Martin17)) and VAT, but evidence is lacking on associations of dairy consumption with SCAT and VAT:SCAT.
We aimed to investigate the associations of total and different types of dairy products with body fat and lean mass and their distribution in a large population-based study in the UK.
Methods
Study design and population
The Fenland Study is a cohort study with baseline measurements conducted between 2005 and 2015 (n 12 434 with 27 % response rate). Eligible participants were born between 1950 and 1975 and were invited via their general practice to attend the clinical sites at Ely, Wisbech, or Cambridge, UK, where the clinical and dietary assessments were conducted. Exclusion criteria, assessed by general practitioners, were known history of diabetes, psychotic or terminal illness, inability to walk unaided and pregnancy or lactation. The study was approved by the Cambridge Local Ethics Committee. All participants gave written informed consent.
For the present analysis, we evaluated baseline data from 12 065 participants after exclusion of pregnant women (n 5), participants with missing dietary data (n 17), those in the bottom and top 1 % of total energy intake (n 250) and those in the top 1 % of total dairy consumption (n 97). Participants with missing outcome data were also excluded (n 1 to n 812, depending on the outcome). A study flow chart of participant inclusion is presented in Fig. 1.
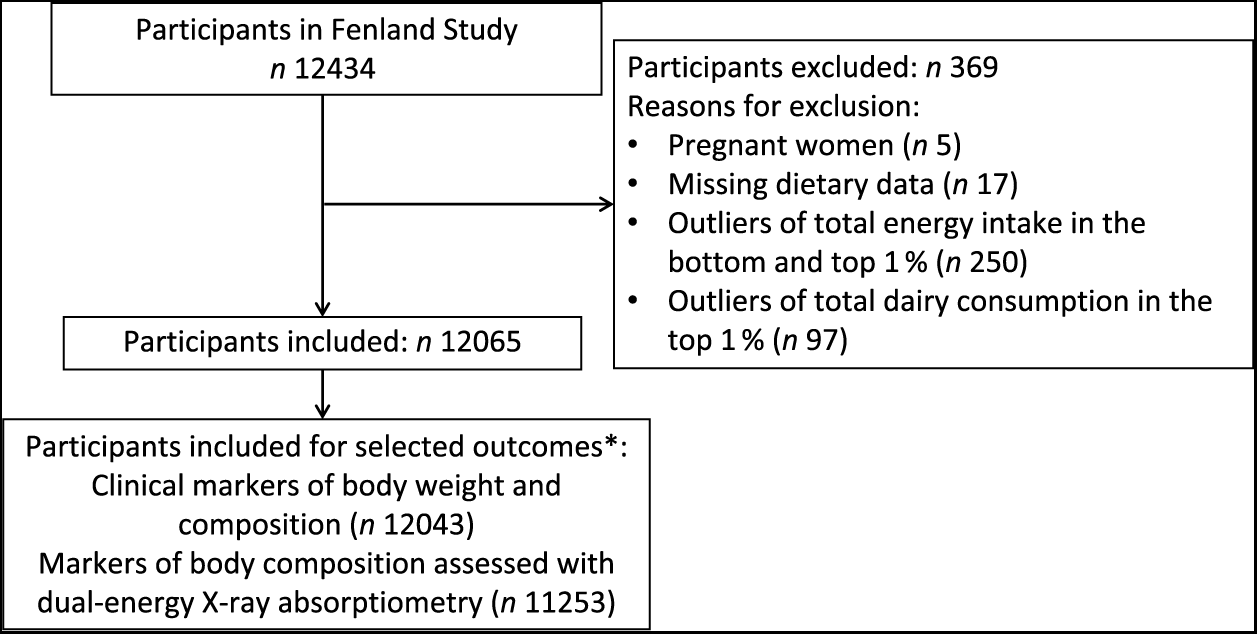
Fig. 1. Participant selection for analyses of associations of dairy consumption with cardiometabolic markers in over 12 000 adults of the Fenland Study, UK. * A minimal number of participants for each sub-group of the outcomes is presented. Numbers slightly varied depending on missing information of each outcome variable.
Dietary assessment
Participants’ diet over the previous year was assessed with a 130-item semi-quantitative FFQ(Reference Bingham, Welch and McTaggart18). The dietary data were processed using FETA software(Reference Mulligan, Luben and Bhaniani19). Dairy products were assessed in servings/d and were categorised as we previously described(Reference O’Connor, Lentjes and Luben20) (Table 1). Dairy servings were defined as one average glass (200 g) for milk, 125 g pot for yogurt, a medium serving of 40 g for cheese, one tablespoon (15 g) for single cream, one tablespoon (30 g) for double cream, one teaspoon (10 g) for butter and one average scoop/tub (60 g) for ice cream.
Table 1. Definitions of dairy groups (Fenland Study, UK)
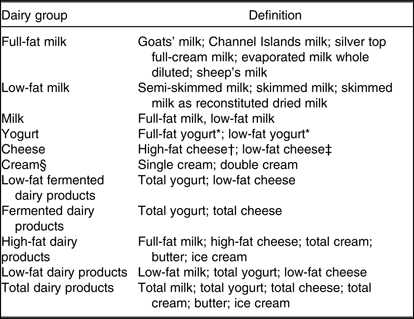
* The variables derived directly from the FFQ questions were used.
† The variable derived directly from the FFQ questions on hard cheese intake was used. The assumption made here is that high-fat cheese is equivalent to hard cheese.
‡ The variable derived directly from the FFQ questions on cottage and low-fat soft cheese intake was used. The assumption made here is that low-fat cheese is equivalent to cottage and low-fat, soft cheese.
§ Cream was used as a contributor to high-fat and total dairy products, but results separately for it and its types are not presented, as the very low intakes result in very unstable and imprecise estimates.
Adiposity measures
These measurements were conducted at each clinical site according to standardised procedures. BMI was calculated as weight divided by height squared (kg/m2). Waist and hip circumferences were averaged from two repeated measures with a non-stretch tape. Total body fat and its distribution (peripheral fat, VAT, SCAT) and lean mass and its distribution (appendicular lean mass) were estimated with a DEXA scan (Lunar Prodigy Advanced fan beam scanner, GE Healthcare; GE encore software, version 14.10.022 to 16, GE Medical Systems(Reference Clifton, Day and Rolfe21)). Specifically, the DEXA system demarcated the boundaries of the android region based on an established protocol. VAT was estimated as part of the android region from an inbuilt algorithm of the DEXA software. SCAT was calculated after subtracting VAT from the android fat mass. These DEXA estimates of abdominal VAT and SCAT have been validated against amounts of VAT and SCAT determined by computed tomography(Reference Kaul, Rothney and Peters22) and MRI(Reference Mohammad, De Lucia Rolfe and Sleigh23), the reference methods for adipose tissue quantification.
Assessment of sociodemographic and lifestyle factors
A general questionnaire was administered for information on ethnicity, occupation, education, income, marital status, smoking and medication use. Physical activity was objectively measured over 7 d using a combined heart rate and movement sensor (Actiheart, CamNtech)(Reference Brage, Brage and Franks24) and individually calibrated with a treadmill test to derive physical activity energy expenditure (kJ/kg per d)(Reference Brage, Ekelund and Brage25). This method was previously validated with doubly labelled water.
Statistical analyses
As primary exposures, we considered milk, yogurt and cheese. As primary outcome, we defined the ratio of VAT to SCAT (VAT:SCAT). Other outcomes included total and peripheral body fat mass, total and appendicular body lean mass and proxies of abdominal fat, that is, waist circumference and the waist:hip ratio. We presented results for VAT:SCAT as percentage change after back-transformation (exponentiation) of the log-transformed variable, because it was positively skewed.
Missing covariates were imputed by chained equations under the assumption of data missing at random(Reference White, Royston and Wood26). Multiple imputation involved linear, logistic and predictive mean matching models according to variable distribution, and five imputation data sets were derived. Further analyses accounted for variability due to imputation. To examine cross-sectional associations between different dairy types and adiposity markers, robust multiple linear regression was used, deriving multiple maximum likelihood estimators, which are robust against the influence of outliers(Reference Verardi and Croux27). The initial probability of false positive findings was set to 5 %. Because of the large number of tests, false discovery rate correction was applied accounting for correlations between tests(Reference Benjamini and Yekutieli28). Associations were considered significant if they passed this correction (P < 0·0005).
Associations were adjusted for potential confounders based on previous knowledge and biological plausibility. Specifically, we adjusted for sociodemographic, lifestyle and clinical factors including age, sex, test site, ethnicity, age at completion of full-time education, education level, occupation, household income, marital status, smoking status, pack-years of smoking, physical activity energy expenditure, hormone-replacement therapy (for women only), lipid-lowering medication, antihypertensive medication (categorical), plasma vitamin C (as a marker of diet quality, reflecting fruit and vegetable intake), dietary supplement use, total energy intake, consumption of dietary factors (fruit, vegetables not including potatoes, potatoes, legumes, processed cereals, whole-grain cereals, poultry and eggs, red meat, processed meat, fish, sauces, margarine, nuts, sweet snacks, sugar-sweetened beverages, artificially sweetened beverages, fruit juice, regular coffee, decaffeinated coffee, tea and alcoholic beverages) and BMI. Adjustment for BMI was performed to (1) examine associations independent of BMI (2) partly account for the possibility of dietary misreporting and (3) account for lifestyle confounding due to obesity status. Dairy types were mutually adjusted. When the outcome was lean mass, models were further adjusted for height.
Pre-specified tests for effect modification by sex and BMI for each association were investigated. As sensitivity analyses, we repeated regression analyses in the complete-case data set, to examine stability of results based on five imputed data sets in the primary analyses. To assess whether non-linear associations were present, restricted cubic spline regressions (three knots at the 10th, 50th and 90th percentiles) were fitted in the most adjusted model above. For the same purpose, categorical exposures were used with five categories including non-consumers and quartiles among consumers for types of dairy products. Quartiles were generated from the residuals of the regression of total energy intake against dairy products(Reference Willett, Howe and Kushi29). In addition to the analyses including BMI as a covariate, we also adjusted for the ratio of total energy intake to BMR as an indicator of dietary misreporting(Reference Livingstone and Black30). In post hoc analyses, we examined whether the identified significant associations can be explained by nutrients contained in dairy products including Ca, K, Mg, P, vitamin A, vitamin B12, lactose, monounsaturated fat and saturated fat from the dairy exposure one by one. All analyses were conducted using Stata 14.2 (StataCorp LP).
Results
Descriptive characteristics
We evaluated 12 065 adults (53·8 % women) with a mean age of 48·8 (sd 7·5) years (Table 2). Almost two-thirds of high yogurt consumers were women. Participants of non-White ethnic background were more frequently low dairy consumers compared with participants of White ethnic background. The ethnic difference was most marked for cheese intake: the percentage of non-White participants among non-consumers of cheese was 6·3 % higher than among cheese consumers. Yogurt and cheese consumption was positively associated with higher socio-economic status and negatively associated with likelihood of being current smokers (Supplementary Table S1). Among dairy consumption levels, overall, low-fat dairy consumption was approximately seven times higher than that of high-fat dairy products (Supplementary Table S1).
Table 2. Descriptive characteristics of sociodemographic, behavioural and clinical factors* for the bottom (non-consumers) and top categories of milk, yogurt and cheese consumption (g/d), as well as in the total sample of 12 065 adults of the Fenland Study, UK
(Medians and interquartile ranges (IQR); column percentages)
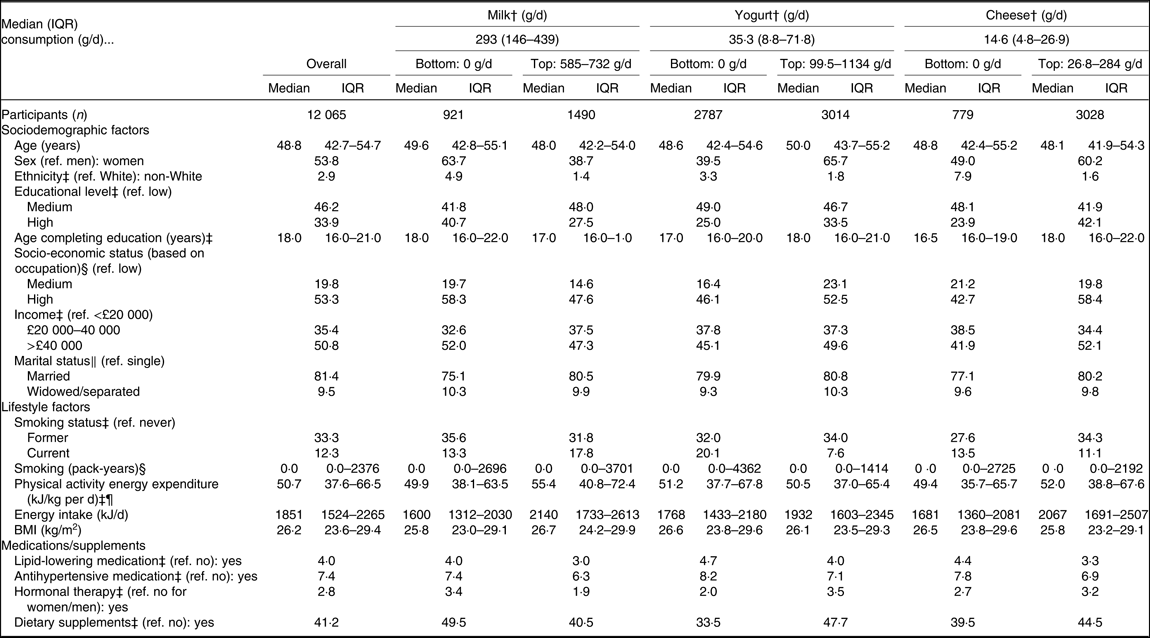
ref., Reference.
* Continuous variables are presented as medians and IQR and categorical variables are presented as column percentages.
† Five categories. Milk: non-consumers, 146, 293, 439, 585 or 732 g/d (categories presented: non-consumers and 585–732 g/d). Yogurt: non-consumers and quartiles within consumers (1st quartile: 8·8–8·8 g/d, 2nd quartile: 17·6–35·3 g/d, 3rd quartile: 54·2–71·8 g/d, 4th quartile: 99·5–1134 g/d; categories presented: non-consumers and 4th quartile within consumers). Cheese: non-consumers and quartiles within consumers (1st quartile: 2·4–4·8 g/d, 2nd quartile: 6·6–13·2 g/d, 3rd quartile: 14·6–25·8 g/d, 4th quartile: 26·8–284·6 g/d; categories presented: non-consumers and 4th quartile within consumers). Dairy consumption was assessed with an FFQ. Milk consumption was assessed with two questions. In the first question, participants could choose the type of milk that they consumed most frequently (options: ‘full cream, silver’, ‘semi-skimmed, red/white’, ‘skimmed/blue’, ‘Channel Islands, gold’, ‘dried milk’, ‘soya’, ‘other’, ‘none’). In the second question, participants could choose one of six categories for the daily amount of milk consumed (options: ‘none’, ‘quarter of a pint’ (146 g/d), ‘half a pint’ (293 g/d), ‘three quarters of a pint’ (439 g/d), ‘one pint’ (585 g/d), ‘more than one pint’ (732 g/d)). Full-fat yogurt, low-fat yogurt, high-fat cheese, low-fat cheese, butter, cream and ice-cream were assessed with questions including nine frequencies, which ranged from ‘never or less than once/month’ to ‘6+ per d’.
‡ Percentage of missing values <5 % with a total of 28·8 % of missing values when accounting for non-overlapping missing values across all the variables.
§ Percentage of missing values 5–15 % with a total of 28·8 % of missing values when accounting for non-overlapping missing values across all the variables.
‖ Percentage of missing values 15–25 % with a total of 28·8 % of missing values when accounting for non-overlapping missing values across all the variables.
¶ Physical activity was objectively measured with a combined heart rate and movement sensor.
Dairy products and body composition
Habitual milk consumption was significantly associated with higher BMI; each additional serving of milk/d was associated with 0·26 (95 % CI 0·16, 0·36) kg/m2 higher BMI (Supplementary Table S2). Similar associations were observed for low-fat milk and for low-fat and total dairy products. Low-fat dairy consumption was associated with 2·6 % lower (95 % CI –3·91, –1·23 %) VAT:SCAT. A similar association was observed for VAT (Supplementary Fig. S1).
Habitual milk consumption was significantly associated with 0·33 (95 % CI 0·19, 0·46) kg higher lean mass per serving/d (Fig. 2). Low-fat milk showed similar associations (Fig. 2). The association was partly attenuated when adjusted for height, but it was still significant (0·18 (95 % CI 0·10, 0·27) kg/serving of milk per d). Effect modification by BMI was suggested for the association between high-fat dairy products and appendicular lean mass (P-interaction=0·0001). Among adults with non-overweight BMI (<25 kg/m2), appendicular lean mass was lower by 0·11 (95 % CI –0·22, –0·001) kg per one serving of high-fat dairy product, but associations were not observed among overweight (0·05 (95 % CI –0·07, 0·18) kg) or obese adults (0·02 (95 % CI –0·16, 0·20) kg).

Fig. 2. Associations of types of dairy consumption (servings/d) with markers of body composition estimated with dual-energy X-ray absorptiometry (DEXA) among over 12 000 adults in the Fenland Study, UK. Forest plots represent regression coefficients with their 95 % CI adjusted for age (years), sex, test site (Cambridge, Ely, Wisbech), ethnicity (White, non-White), total energy intake (kJ/d), other dairy types, educational level (low, medium, high), age when full-time education finished (years), socio-economic status based on occupation (low, medium, high), income (<£20 000, £20 000–40 000, >£40 000), marital status (single, married, widowed/separated), smoking status (never, former, current smoker), pack-years of smoking, energy expenditure due to physical activity (kJ/kg per d), lipid-lowering medication (yes/no), antihypertensive medication (yes/no), hormone-replacement therapy (yes/no in women), intakes (g/d) of fruit, vegetables, potatoes, legumes, processed cereals, whole-grain cereals, poultry and eggs, red meat, processed meat, fish, sauces, margarine, nuts, sweet snacks, sugar-sweetened beverages, artificially sweetened beverages, fruit juice, regular coffee, decaffeinated coffee, tea and alcoholic beverages, plasma vitamin C levels (µmol/l), dietary supplement use (yes/no) and BMI (kg/m2). * Statistically significant associations after false discovery rate corrections. See categorisation of dairy types in Table 1. VAT, visceral adipose tissue; SCAT, subcutaneous adipose tissue.
Other dairy types were not significantly associated with BMI, nor any dairy products with any other anthropometric measure (Figs. 2 and 3, Supplementary Fig. S1).

Fig. 3. Associations of types of dairy consumption (servings/d) with waist circumference and the waist:hip ratio among over 12 000 adults in the Fenland Study, UK. Forest plots represent regression coefficients with their 95 % CI adjusted for age (years), sex, test-site (Cambridge, Ely, Wisbech), ethnicity (White, non-White), total energy intake (kJ/d), other dairy types, educational level (low, medium, high), age when full-time education finished (years), socio-economic status based on occupation (low, medium, high), income (<£20 000, £20 000–40 000, >£40 000), marital status (single, married, widowed/separated), smoking status (never, former, current smoker), pack-years of smoking, energy expenditure due to physical activity (kJ/kg per d), lipid-lowering medication (yes/no), antihypertensive medication (yes/no), hormone-replacement therapy (yes/no in women), intakes (g/d) of fruit, vegetables, potatoes, legumes, processed cereals, whole-grain cereals, poultry and eggs, red meat, processed meat, fish, sauces, margarine, nuts, sweet snacks, sugar-sweetened beverages, artificially sweetened beverages, fruit juice, regular coffee, decaffeinated coffee, tea and alcoholic beverages, plasma vitamin C levels (µmol/l), dietary supplement use (yes/no) and BMI (kg/m2). See categorisation of dairy types in Table 1.
Other analyses
Results were not altered when complete-case analyses were performed, and findings were similar when adjusting for the ratio of total energy intake to BMR rather than adjusting for BMI or after further adjustment for dairy nutrients (Supplementary Table S3). There was no indication of a non-linear association from analyses with restricted cubic splines or categorical exposures after correction for multiple testing.
Discussion
Our study reports two main findings. The first was that habitual daily consumption of one serving of low-fat dairy products was associated with a 3 % lower ratio of VAT to SCAT as a marker of fat mass distribution, a measure which is associated with diabetes risk independently of total fat mass(Reference Kaess, Pedley and Massaro15). Second, habitual daily consumption of one serving of milk was associated with a 0·33 kg higher body lean mass.
There are no previous studies on the association between dairy consumption and VAT:SCAT. A randomised controlled trial showed a reduction in VAT among those consuming six to seven servings of dairy products per d compared with those consuming less than four servings/d(Reference Josse, Atkinson and Tarnopolsky16), and a cross-sectional study of twins reported an inverse association between low-fat fermented dairy products and VAT(Reference Pallister, Jackson and Martin17), but we have not identified any studies of the association between dairy consumption and SCAT. Although total dairy consumption has been consistently associated with a lower body fat mass in randomised controlled trials(Reference Abargouei, Janghorbani and Salehi-Marzijarani31–Reference Chen, Pan and Malik33), the number of studies for dairy subtypes is limited. We found no significant associations between any dairy type and total or peripheral fat mass or waist circumference and waist:hip ratio as proxies for fat mass distribution. The direction of the associations we observed between low-fat dairy consumption and total body fat mass, and waist circumference was consistent with the findings of previous studies and with our findings for VAT:SCAT. The higher dairy amounts used in feeding trials compared with the consumption levels reported in observational studies could partly explain the lack of significance in certain associations. In addition, it is expected that the direction of the association of low-fat dairy products with VAT:SCAT is consistent with that of the association of low-fat dairy products with waist circumference and the waist:hip ratio, because waist circumference is a proxy measure of abdominal fat. VAT:SCAT was estimated with higher accuracy than waist and hip circumferences. This might partly explain as to why the association with VAT:SCAT was significant after multiple test correction, but not the association with waist circumference and the waist:hip ratio.
No mechanism has been reported for an inverse association between low-fat dairy consumption and VAT:SCAT. Nevertheless, a plausible explanation could be that VAT is more metabolically active with a more efficient glucose uptake than SCAT(Reference Christen, Sheikine and Rocha34), and therefore the effects of dairy nutrients on fat mass are more pronounced in the VAT than in SCAT. Among dairy nutrients, Ca may reduce the fat content of the adipose tissue because intracellular Ca promotes lipolysis and reduces lipogenesis(Reference Zemel35) and Ca supplementation was previously shown to decrease VAT without changing body weight or total abdominal fat(Reference Rosenblum, Castro and Moore36).
With our analyses and specifically the observed positive association between milk consumption and body lean mass, we extend the previous understanding on the positive association between total dairy consumption and total lean mass(Reference Abargouei, Janghorbani and Salehi-Marzijarani31) to include specific dairy subtypes. Dairy products and mainly milk have been consistently associated with bone health due to their nutrients content including Ca, P, vitamin D and protein, which might partly explain the positive association with lean mass(Reference Thorning, Raben and Tholstrup37). Another potential mechanism is the increasing effect of milk on growth hormone(Reference Rich-Edwards, Ganmaa and Pollak38, Reference Van Vught, Nieuwenhuizen and Veldhorst39), which has been associated with a higher lean mass through a higher bone mineral density and muscle mass(Reference Carroll and Bengtsson40).
Strengths and limitations
Our study has several strengths including its large sample size (n 12 065), and the inclusion of several dairy subtypes, which allowed the investigation of potentially different associations with adiposity for different dairy types. By employing DEXA, we were able to use more accurate methods to assess VAT and SCAT than previous studies that used waist circumference and waist:hip ratio as proxies of central adiposity(Reference Wajchenberg12). Our statistical approaches were thorough including the adjustment for important potential confounders including objectively measured physical activity, the derivation of estimates robust to outliers and the handling of missing data with multiple imputations.
Our study also has limitations. The cross-sectional design increases the risk of reverse causation and limits inference for causal association and our results can thus be used mainly for hypothesis generation. Although the FFQ used was assessed for validity in a similar population(Reference Bingham, Welch and McTaggart18) and we adjusted for BMI as an established factor of dietary misreporting(Reference Trijsburg, Geelen and Hollman41), we cannot exclude the possibility of error due to dietary misreporting caused by the use of self-reported methods of dietary assessment. Commensurate with this, the FFQ was limited in the detail on types of dairy products consumed, so we were not able to estimate the nutrient content accurately and thus made assumptions based on average estimates. The questionnaire also limited the assessment of various dairy definitions (e.g. sweetened yogurt) and the influences of such variations on study results. Although we adjusted for many potential confounders, residual confounding cannot be ruled out. We were also not able to disentangle whether BMI was a confounder, a mediator or both in our cross-sectional analysis, while we could examine the associations independent of BMI. If BMI is on the causal pathway, adjustment for BMI would have attenuated the findings towards the null. Future prospective analyses of change in weight (or BMI) in relation to dairy products will be better placed to investigate this further. The response rate of our study was moderate at 27 %. This should be placed in the context of the internal validity of our study with our use of objective assessments, that is, DEXA on the full cohort, but we acknowledge limited external validity (generalisability) of our findings for the UK general population. Moreover, our population was largely White European, who overall consume higher amounts of dairy products compared with other populations especially in South Asia, South America and Africa(42), so generalisability of our results to other ethnic groups with different consumption patterns might be limited. Specifically, consumption of different dairy groups has a limited range in our study, with low levels of high-fat dairy products and high levels of low-fat dairy products. This might compromise the power to detect associations across a broader range of dairy consumption, which might be seen in other populations.
Conclusion
We observed an inverse association between low-fat dairy products and the ratio of visceral to subcutaneous fat, which suggests that abdominal obesity may be a potential pathway for the association of dairy consumption with cardiometabolic disease. In addition, the observed association of higher milk consumption with a higher body lean mass could also be a potential explanation for the overall metabolic associations observed for dairy consumption. These findings are important for hypothesis generation and should stimulate further investigation in prospective studies, clinical trials and mechanistic studies of the link between dairy products and cardiometabolic disease.
Acknowledgements
The authors thank Abigail Britten, Vasileios Kaimakis, Steven Knighton, Richard Powell, Adam Dickinson, Susie Boatman and Inge Loudon for technical assistance as members of the Fenland Study team.
The Fenland Study was funded by the Medical Research Council and the Wellcome Trust. The present work was supported by the Medical Research Council (N. G. F., grant number MC_UU_12015/5) (N. J. W., grant number MC_UU_12015/1) (S. B., grant number MC_UU_12015/3); the National Institute of Health Research Cambridge (NIHR) Biomedical Research Centre (N. G. F., S. B. and N. J. W., grant number IS-BRC-1215-20014); and the Cambridge Trust (E. T.). The funders had no role in the design, analysis or writing of this article.
N. J. W., N. G. F., S. J. G. and S. B. designed research, and N. G. F., F. I. and E. T. designed the study question; E. D. L. R. provided specialist input to anthropometric measurements; E. T. and F. I. analysed data; E. T., F. I. and N. G. F. drafted the manuscript; E. T. had primary responsibility for final content. All authors read and approved the final version.
There were no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519001776








