Introduction
Natural steroidal chemosignals active in human nasal receptors
In the early 1990s, we reported that naturally occurring steroidal molecules in humans androsta-4,16-dien-3-one (ER670, PH56 or androstadienone (ADO)) and estra-1,3,5,(10,16-tetraen-3-ol (ER830, PH78 or estratetraenol (ETE)), administered in concentrations below olfactory threshold can induce depolarization of the local electrogram recorded from the nasal chemosensory mucosa in human subjects.Reference Monti and Grosser1 We called these naturally occurring molecules “putative pheromones.” In pharmacology in vitro studies using isolated living human nasal chemosensory cells, ADO and ETE induced robust transient calcium (Ca++) membrane currents supporting a membrane (nongenomic) effect of these steroidal compounds.Reference Monti2
In subsequent studies, using an experimental miniprobe that is the extension of a computerized olfactometer for local and topical administration of volatile substances while simultaneously recording the local electrogram from receptors (EGNR) in the nasal chemosensory mucosa,Reference Monti and Grosser1, Reference Monti3 we reported a rapid depolarizing effect of odorless steroids ADO and ETE on the nasal electrogram of human volunteers. This rapid nongenomic effect was followed by rapid activation of autonomic nervous system (ANS) reflexes and subtle behavioral changes that were distinct for ADO and ETE.Reference Monti and Grosser1, Reference Monti, Jennings-White, Dolberg and Berliner4, Reference Grosser, Monti, Jennings-White and Berliner5
ADO and ETE are inactive when administered systemically. In a pharmacokinetic study in human volunteers, ADO administered intranasally at 1-hour intervals during 12 consecutive hours was not detected in plasma samples collected at hourly intervals during dosing (HPLC [high-performance liquid chromatography]-mass-mass, assay sensitivity = 2.857 ng/mL).Reference Bustillos-Ventura and Mancera6 Furthermore, intranasal and systemic administration of ADO and ETE to laboratory animals (rodent, lagomorph, canid, swine) in doses 100-fold higher than the dose to use in clinical studies did not induce any behavioral or ANS effects. It was concluded that odorless ADO and ETE induced species-specific pharmacological effects through activation of nasal chemosensory cells.Reference Grosser, Monti, Jennings-White and Berliner5, Reference Monti, Jennings-White and Berliner7
Later independent contributions to this field confirmed similar ANS changes, subtle psychological effects, and distinct activation of the hypothalamus (HYP) measured with PET, after intranasal administration of ADO and ETE to human volunteers.8–19
Other reports showed non–sex-specific effects of putative pheromones ADO and ETE influencing the perception of emotional stimuli in human volunteers.20–22 The non–sex-dimorphic effects of ADO and ETE were recently questioned in work using these steroidal molecules at high concentrations,Reference Ye, Zhuang, Smeets and Zhou19 but this is not supported by previous publications using ADO and ETE in concentrations below the olfactory threshold.Reference Monti3, Reference Grosser, Monti, Jennings-White and Berliner5 In a recent article,Reference Banner, Gabay and Shamay-Tsoory23 men with “high social anxiety” (mean LSAS [Liebowitz Social Anxiety Scale] score of 53.2) reported increased sensitivity to threat and avoidance after nasal delivery of ADO. The adverse effects of ADO could be explained by the high concentration used (above olfactory threshold), although the authors masked ADO odor with eugenol. These results are not consistent with the positive effects of low doses of odorless ADO (below olfactory threshold and without using odor masking) reported in women volunteers.Reference Monti3, Reference Grosser, Monti, Jennings-White and Berliner5 Also, the “high social anxiety” subjects had lower baseline LSAS scores than those required (LSAS ≥ 60) in studies of the therapeutic effect of intranasally administered pherines in subjects with social anxiety disorder (see next section: mean LSAS on entrance was 97.9).Reference Liebowitz, Salman, Nicolini, Rosenthal, Hanover and Monti24, Reference Liebowitz, Hanover, Draine, Lemming, Careri and Monti25
Pherine molecules
Our preliminary findings led to the development of a new class of more potent neuroactive steroids, focusing on the following advantages: (a) 100% availability of ligand at the peripheral receptor sites immediately after intranasal administration, (b) ultralow dose needed to induce pharmacological effects due to direct administration to the receptor sites, and (c) fast oligosynaptic neural paths from nasal receptors to basal forebrain areas contributing to rapid onset of effect.
The new synthetic neuroactive steroidsFootnote a were odorless and specifically designed and formulated to engage human nasal chemosensory receptors (nongenomic effect), looking for rapid and more potent behavioral and ANS effects than their naturally occurring predecessors. The substances were screened in vitro and in vivo, and those without toxicity in laboratory animal studies and lacking binding affinity to steroidal hormone receptors were studied in human volunteers for profiling their pharmacological effects on nasal receptors, ANS reflexes, and psychological effects and to gain information about their possible therapeutic indication. Neuroactive steroids meeting the above profile were included in a new family of therapeutic pharmaceuticals that we called pherines. Reference Monti, Jennings-White, Dolberg and Berliner4
Pherines are formulated in a water-based excipient for intranasal administration in spray form using a metered spray pump. Low microgram quantities of pherine administered locally and topically to the surface of the nasal chemosensory mucosal lining produce robust, dose-dependent depolarization of the electrogram (mass receptor potential) ENRG (Figure 1) followed by selective and dose-dependent brain activation of a behavioral and ANS response.Reference Monti, Jennings-White, Dolberg and Berliner4, Reference Monti, Jennings-White and Berliner7, Reference Liebowitz, Salman, Nicolini, Rosenthal, Hanover and Monti24, Reference Liebowitz, Hanover, Draine, Lemming, Careri and Monti25, 27–29
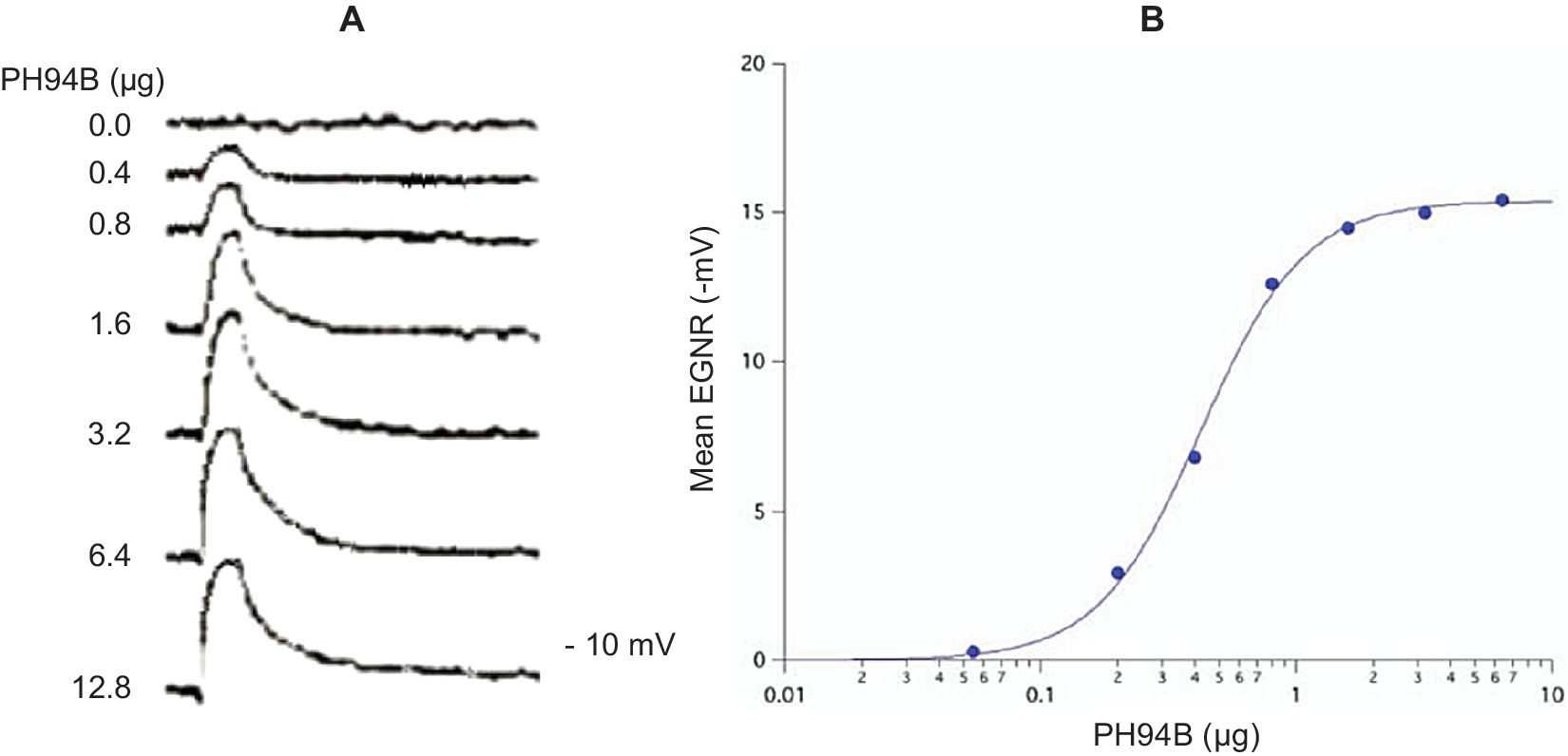
Figure 1. (A) Representative electrogram (EGNR) traces recorded from the surface of the dorsomedial chemosensory mucosa of the nasal septum in a young adult male volunteer during local administration of control (0.0) and different doses of PH94B. (B) Dose-dependent relationships of PB94B nasal spray on the amplitude of the EGNR in clinically healthy male and female subjects (n = 20). ED50 = .4 μΜ; Hill coefficient = 1. The EGNR was recorded using the Multifunctional Miniprobe (MM®), which is an extension of a computer-driven olfactometer. The therapeutic dose range to use in clinical studies was obtained from the dose–response relationships of PH94B.
Pharmacokinetic studies in human volunteers, administering to the nasal receptor area different doses of androsta-4,16-dien-3β-ol (PH94B or Aloradine), a neuroactive steroid from the androstane family of pherines, produced dose-dependent and reversible activation of the EGNR (ED50 = .4 μM; Hill Coefficient = 1). The dose–response relationships were used to find the effective dose range to administer in clinical studies (Figure 1).
An in vitro pharmacology studyReference Winegar and Monti-Bloch30 in primary cultures of isolated human nasal chemosensory cells using the radiometric Ca++ indicator Fura-2 showed significantly increased intracellular Ca++ in response to PH94B, and this effect was dose dependent (ED50 = 1.0 μM) and similar to that reported for other neuroactive steroidal compounds acting on nasal receptors.Reference Zhang, De La Cruz, Pinto, Nicolae, Firenstein and Gilad31
Activation of nasal receptors by PH94B was followed by decreased sympathetic tone (assessed using physiologic sinus arrhythmia), decreased cardiac and respiratory rate, decreased frequency of electrodermal activity events and electromyogram, and increased body core temperature.Reference Monti, Jennings-White, Dolberg and Berliner4 In a separate study, intranasal PH94B also induced specific and dose-dependent activation of brain areas that was different from control and from the effect of primary odors (Figure 2).

Figure 2. Selective and dose-dependent brain activation induced by odorless pherine PH94B (A) is different from control (SHAM) and brain activation induced by primary odors shown in (B). The results are averaged functional MRI images from human healthy volunteers (n = 8). Warmer colors on the color bars correspond to increased brain activation.
In a pharmacokinetic study in healthy volunteers, with PH94B administered intranasally in spray form at 1-hour intervals during 12 consecutive hours, the concentration of the pherine in plasma samples collected at hourly intervals during dosing was below the detection level of the analytical method (HPLC-M-M; assay sensitivity = 2.857 ng/mL).Reference Bustillos, Mancera, Jagannath and Sanchez32
The behavioral effects of PH94B were assessed in a double-blind placebo-controlled study involving 90 subjects meeting DSM-IV criteria for social anxiety disorder. Subjects underwent two sets of laboratory-based challenges involving public speaking and social interaction.Reference Liebowitz, Salman, Nicolini, Rosenthal, Hanover and Monti24 During the first set (visit 2), all subjects were pretreated with placebo in a single-blind fashion 15 minutes before each challenge. Those demonstrating significant symptoms were brought back a week later (visit 3) and randomized to PH94B or placebo pretreatment, each followed 15 minutes later by second rounds of performance and social challenges. PH94B was significantly more effective than placebo in reducing both performance and social anxiety as rated by subject self-reports and investigator ratings.Reference Liebowitz, Salman, Nicolini, Rosenthal, Hanover and Monti24
A subsequent 4-week double-blind crossover studyReference Liebowitz, Hanover, Draine, Lemming, Careri and Monti25 was conducted to obtain a preliminary estimate of the efficacy of PH94B when used in real-world situations for a longer period of time. Subjects meeting DSM-IV criteria for social anxiety disorder (n = 22) were randomized to use 1.6 to 3.2 microgram (μg) intranasal PH94B or placebo on an as-needed basis 15 minutes before confronting stressful performance or social situations in their daily life for 2 weeks, after which they were crossed over to the opposite treatment for an additional 2 weeks. Despite the small sample, PH94B demonstrated significant treatment efficacy in several ways. On the primary outcome measure, subjects with marked social anxiety disorder experienced significantly less peak anxiety during social and performance events in their daily lives when using PH94B than when pretreated with placebo (Effect Size = .658, r = .832).Reference Liebowitz, Hanover, Draine, Lemming, Careri and Monti25 Also, between-groups comparisons for the first 2 weeks of treatment showed effect sizes in favor of PH94B on the LSAS total score (effect size = .812) and LSAS avoidance subtotal score (effect size = 1.078), and significantly more subjects rated themselves as treatment responders on the Patient Global Improvement evaluation. What was particular noteworthy is that the degree of improvement seen with PH94B compared to placebo on the LSAS seen in this trial after 2 weeks was comparable in magnitude to that seen after 12 weeks with Food and Drug Administration (FDA) approved medications for Social Anxiety Disorder such as paroxetineReference Stein, Liebowitz, Lydiard, Pitts, Bushnell and Gergel33 and sertraline.Reference Liebowitz, DeMartinis and Weihs34 These findings extend those of the study that was based on laboratory challenges and shows intranasal PH94B efficacy in real-life situations.
In vitro pharmacology studies using isolated living human nasal chemosensory cells show that PH10 (pregn-4-en-20-yn-3-one), a neuroactive steroid pherine molecule from the pregnane family, induces significant dose-dependent inward membrane currents (ED50 = .2 μM, Hill Coefficient = 1),Reference Monti, Garity and Murray35 and significant dose-dependent depolarization of the electrogram (ENRG) recorded from the nasal chemosensory mucosa in men and women volunteers. The ERG response is followed 15 minutes after intranasal administration of 3.2 μg PH10 by increased plasma NE, 5-HT, and DA, increased sympathetic nervous system tone and frequency of electrodermal activity events.Reference Monti, Diaz Sanchez and Schapper27
In a more recent placebo-controlled, parallel group dose ranging trial in 30 adults with major depressive disorder intranasal PH10 (low dose: 3.2 μg/day and high dose: 6.4 μg/day) reduced Hamilton Depression scores substantially more than did placebo.Reference Monti, Nicolini, Liebowitz and Hanover28 The effect size at the end of the 8-weeks trial was: Effect SizeHigh Dose vs Placebo = .95 and Effect Size Low Dose vs Placebo = .74), suggesting rapid antidepressant activity. Drug–placebo separation appeared during the first week of treatment (Effect SizeLow Dose vs Placebo = .72, and Effect SizeHigh Dose vs Placebo = 1.01).
Body
Olfactory neural circuits
Traditionally, it is accepted that in most mammals, olfaction is accomplished by two subsystems: main olfactory system (MOS) broadcasting sensory inputs from odor chemosignals to the main olfactory bulbs (OB) projecting to the olfactory tubercle, piriform cortex, medial amygdala (MeA), and cortical amygdala (CA)Reference Sosulki, Bloom, Cutforth, Axel and Datta36, Reference Kevetter and Winans37; and the accessory olfactory system (AOS) conveying pheromone chemosignals to the accessory olfactory bulbs (AOB), which in turn reach basolateral amygdala (BLA) neurons that project to the anterior and ventromedial HYP, bed nucleus of the stria terminalis (BNST), medial preoptic area (MPA), striatum (ST), locus coeruleus (LC), parabrachial nucleus (PN), and prefrontal cortex (PFC).Reference Kevetter38, Reference Halpern and Martinez-Marcos39 Activation of these neural circuits is involved in the modulation of different social behaviors.
More recently, it was reported that, in mammals, there are subsets of OB neurons that share synaptic connections in the same limbic amygdala nuclei as the AOS, and olfactory activity behaviors originally assigned to the AOS are mediated through the main olfactory epithelium and the MOS.40–48 Therefore, since humans do not have an identifiable AOB, we hypothesize that this could be the neural path by which chemosensory inputs triggered by pherines could reach the amygdala nuclei.
Numerous studies show that the MeA, a key structure in the control of social behavior, projects to GABAergic neurons in the centrolateral amygdala (CeL) that trigger the forward inhibitory GABAergic circuits directly mediating fear and anxiety and also to the BNST and the HYP involved in the regulation of innate defense responses49–51 (Figure 3).

Figure 3. Schematic diagram showing the olfactory connections to the limbic amygdala and related areas. The olfactory bulb (OB) connections to the limbic amygdala are shorter and bypass the thalamus thus being a fast (shorter latency) neural input to the basal forebrain compared to other sensory afferent systems.
Abbreviations: BLA, basolateral amygdala; BNST, bed nucleus of the stria terminalis; CA, cortical amygdala; CeA, central amygdala; CeL, centrolateral amygdala; CeM, centromedial amygdala; HYP, hypothalamus; LC, locus ceruleus; MeA, medial amygdala; OB, olfactory bulb; PFC, prefrontal cortex; RN, raphe nucleus; THAL, thalamus; VTA, ventral tegmental area.
It has been shown that olfactory projections to the cortical amygdala can also trigger BLA neuronsReference Keshavarzi, Power, Albers, Sullivan and Sah52 which synapse with the important contingent of GABAergic forward inhibitory neurons in the lateral (CeL) and medial (CeM) division of the central amygdala involved in the modulation of fear and anxiety.Reference Pape, Jüngling, Seidenbecher, Lesting and Reinscheid42, Reference Mucignat-Caretta, Redaelli and Caretta44, Reference Thompson, Salcedo, Restrepo and Finger48, Reference Ciocchi, Herry and Grenier49, 53–56 More recent evidence shows that GABAergic-PKCδ-positive OFF-neurons in the CeL facilitate the release of neuropeptide S (NPS) in the LC and GABA from anterolateral BNST through forward inhibition of GABAergic neurons in the CeM, and there is concurrent inhibition of NE, DA, and 5-HT release from the midbrain and decreased sympathetic system tone through inhibition of neurons in the posterior HYP.Reference Pape, Jüngling, Seidenbecher, Lesting and Reinscheid42, Reference Guthman and Vera51, 53–61
Furthermore, neural inputs from the BLA reach GABAergic–PKCδ-negative ON-neurons in the CeL. It has been reported that CeL outputs via an intercalated feed-forward series of GABAergic interneurons and also through CRH neurons can stimulate glutamatergic neurons in the BNST oval area and in the prefrontal cortex, with concurrent stimulation of NE, DA, and serotonin release from the midbrain (LC, VTA, and RN), (Figure 3). Activation of these circuits leads to simultaneous inhibition of GABAergic neurons in the BNST and NPS-releasing neurons in the LC, increased sympathetic system tone, and a neuroendocrine response,Reference Jüngling, Lange and Szkudlarek53, 60–62 all in agreement with the neurocircuits of mood disorders.Reference Price and Drevets63
Proposed mechanisms of pherine activity
We hypothesize that the PH94B-induced rapid decrease (latency ≤ 400 ms) of sympathetic toneReference Monti, Nicolini and Longgi64 and rapid improvement (latency = 10-15 minutes) in performance anxiety and social interaction anxietyReference Liebowitz, Salman, Nicolini, Rosenthal, Hanover and Monti24, Reference Liebowitz, Hanover, Draine, Lemming, Careri and Monti25 are triggered by sensory inputs originating in nasal chemosensory neurons that stimulate subset of OB neurons projecting to the MeA and BLA. There is evidence that MeA and BLA neurons trigger the forward inhibitory GABAergic–PKCδ-positive OFF-neurons in the CeL and CeM amygdala, which downstream effects mediating behavioral actions that directly mediate social behavior, fear, and anxietyReference Guthman and Vera51, Reference Keshavarzi, Power, Albers, Sullivan and Sah52, Reference Forster, Novick, Scholl and Watt56, Reference Price and Drevets63, Reference Monti, Nicolini and Longgi64 (Figure 3). The modulation of neural circuits involved in the pathogenesis of social anxiety disorder55–57, Reference Adhikari59, 65–68 appears to be consistent with the PH94B-induced acute anxiolytic effects and autonomic nervous system changes reported in our clinical studies in patients diagnosed with social anxiety disorder.Reference Liebowitz, Salman, Nicolini, Rosenthal, Hanover and Monti24, Reference Liebowitz, Hanover, Draine, Lemming, Careri and Monti25
We also hypothesize that the rapid change in sympathetic tone and dose-dependent improvement in Hamilton Depression scores (HAM-D) scores induced after intranasal administration of PH10Reference Monti, Nicolini, Liebowitz and Hanover28 are the result of activation of glutamatergic neurons in the BLA that in turn trigger the GABAergic–PKCδ-negative ON-neurons in the CeL. The antidepressant effect of PH10 through activation of nasal chemosensory receptors is supported by other independent studies showing an important association of the olfactory system and mood disorders.Reference Price and Drevets63, 68–71
Conclusions
There is extensive evidence showing the human olfactory system’s role in social behavior, food ingestion, appetite regulation, awareness of the surrounding environment, and detection of hazards.Reference Stevenson72, Reference Krusemark, Novak, Gitelman and Li73 Unlike other sensory systems, olfactory inputs do not have a synaptic relay in the thalamus to be routed to the cortex. Rather, they are wired to the limbic amygdala, HYP, and hippocampus, which provides olfaction with a unique and potent power to influence mood, acquisition of new information, and its use in many different contexts including social interaction, fear, emotions, and the memory components of behavior.74–80
Thus, it is reasonable to assume that in humans, there are functional neural circuits reporting afferent information from olfactory chemosensory receptors, via the OB, to the basal forebrain areas (Figure 3) that influence behavior, mood, and emotions, and that the neuroanatomical areas involved are the same as the dysfunctional areas described in laboratory animals with bilateral olfactory bulbectomy81–83 and in subjects with congenital absence of OB, who develop anxiety and depression in early life.84–88 The documented olfactory bulb-amygdala connectionsReference Sobel and Prabhakaran29, Reference Hagino-Yamagishi40, Reference Kang, Baum and Cherry41, 43–52, Reference Tovote, Fadok and Lüthi54, Reference Forster, Novick, Scholl and Watt56, Reference Calhoon and Tye66, Reference Nuss67, Reference Redmond, Kelly and Leonard69, Reference Sokolowski and Corbin89 seem to be compatible with the neural circuits involved in the pathophysiology of social anxiety disorder, specific phobias, generalized anxiety disorder, and depression.57–59, Reference Miller, Marcolutti, Shen and Zweifel61, Reference Price and Drevets63, 65–67, Reference Krusemark, Novak, Gitelman and Li73, Reference Negoias, Croy and Gerber84
Therefore, neural circuits from nasal chemosensory neurons to OB cells that project axons to the MeA and BLA and to GABAergic PKCδ-OFF-neurons in the CeL and the CeM could explain the anxiolytic effect of pherine PH94BReference Liebowitz, Salman, Nicolini, Rosenthal, Hanover and Monti24, Reference Liebowitz, Hanover, Draine, Lemming, Careri and Monti25 through the modulation of GABA and NPS and the decreased sympathetic tone. Also, the rapid antidepressant effect of PH10Reference Monti, Nicolini, Liebowitz and Hanover28 could be explained by the activation of GABAergic–PKCδ-negative ON-neurons and CRH-releasing neurons in the CeL that induce release of glutamate from the BLA and the oval neurons in the BNST and catecholamines from the midbrain and concurrent increase of sympathetic tone. Since pherine molecules are species specific, animal testing would not be helpful in elucidating the relevant brain pathways. Therefore, further studies using functional magnetic resonance imaging (fMRI) and spectroscopy magnetic resonance imaging (sMRI) during nasal administration neuroactive pherine molecules to human subjects are needed to confirm the functional connectivity and changes in central nervous system (CNS) neurotransmitters proposed here.
Specific chemosensory inputs triggered by pherines could be an important portal for the modulation of neurotransmitter release in the telencephalon and ANS function and behavior. The effects of pherines in the CNS, bypassing the hurdles of systemic administration and the brain–blood barrier, and their safety and tolerability demonstrated in clinical studies provide a novel approach to address the interrelationships between the dysfunctional neuronal circuits in patients with anxiety disorders and mood disorders. Pherines also open a new approach for administration of therapeutic pharmaceuticals, with the advantage of rapid onset of efficacy and more accurate and safe therapeutic effect for the management of neuropsychiatric conditions that require acute intervention.
Acknowledgments
Our thanks to Rita Hanover for her valuable help with the analysis and presentation of figures. Also, our thanks to Ann Draine for her help reviewing the manuscript and for her valuable suggestions.
Funding
The original basic and clinical research studies done using pherine molecules shown in this review article were funded by Pherin Pharmaceuticals Inc., Los Altos, California, USA.
Disclosures
Dr. Monti reports financial support from Pherin Pharmaceuticals (employed as President and owns stock from Pherin Pharmaceuticals and VistaGen Therapeutics). In addition, Dr. Monti has patents issued for Treatment of Social Phobia (US Patent 8,309,539) and for Treatment of Depressive Disorders (US Patent 10,322,138).
Dr. Liebowitz reports financial support from Pherin Pharmaceuticals (stock options) and from VistaGen Therapeutics (stock options and consulting fees). VistaGen Therapeutics has licensed PH94B and PH10 from Pherin Pharmaceuticals.





