Introduction
As experience with the diagnosis and management of patients with autoimmune encephalitis (AE) continues to improve,Reference Graus, Titulaer and Ramani 1 the need to characterise the long-term outcomes in recovering patients has become important. This need is most apparent in patients with AE associated with autoantibodies against cell-surface receptors who retain the greatest potential for meaningful recovery following diagnosis and treatment. To date, several large studies have reported “good” long-term outcomes in patients recovering from N-methyl-D-aspartate receptor (NMDAR) encephalitis,Reference Titulaer, McCraken, Inigo, Armangue and Glaser 2 - Reference Wang, Li and Hu 4 and AE associated with voltage-gated potassium channel complex (VGKC) autoantibodies.Reference Arino, Armangue, Petit-Pedrol, Sabater, Martinez-Hernandez and Hara 5 - Reference Gadoth, Pittock and Dubey 8 These studies confirm that between 70% and 80% of patients regain the ability to ambulate within 2 years of first presentation, defined as a score of ≤2 on the modified Rankin Scale (mRS).Reference Titulaer, McCraken, Inigo, Armangue and Glaser 2 - Reference Wang, Li and Hu 4 , Reference Irani, Alexander and Waters 7 - Reference Quinn, Dawson, Walters and Lees 9 However, it is increasingly apparent that “good outcomes,” as quantified by measures emphasising motor function, may overlook outcomes of importance to recovering patients—particularly those related to cognitive capacity.Reference Day 10 In support of this statement, studies applying comprehensive cognitive assessments report multi-domain dysfunction in the acute phase of NMDAR encephalitis, with persistence of deficits in memory, executive function, and attention at long-term follow-up.Reference McKeon, Robinson and Ryan 11 - Reference Finke, Kopp, Pruss, Dalmau, Wandinger and Ploner 13 Similar patterns of deficits are observed in patients with encephalitis associated with leucine-rich glioma inactivated 1 (LGI1) autoantibodies.Reference Irani, Stagg, Schott, Rosenthal, Schneider and Pettiingill 14
Although sensitive and informative, full neuropsychological assessment is not practical in most clinical settings owing to the length and high cost associated with administration. There is a need to develop and validate measures that can be reliably and rapidly applied to assess and monitor progression in recovering AE patients. The Montreal Cognitive Assessment (MoCA) is a sensitive and adaptable method for detecting mild cognitive impairment, with normative data available.Reference Nasreddine, Phillips and Bedirian 15 , Reference Roalf, Moberg, Xie, Wolk, Moelter and Arnold 16 In this study, we used the MoCA to estimate cognitive impairment in patients recovering from AE and determined the clinically measurable factors that affect performance on the MoCA.
Methods
Study Population and Data Collection
Data were ascertained through chart review from patients assessed and diagnosed with AE at our tertiary care AE clinic (Toronto Western Hospital, Toronto, Ontario, Canada) from September 1, 2012, to September 1, 2017. Patients included in the study presented with a clinical diagnosis of “probable” or “definite” AE.Reference Graus, Titulaer and Ramani 1 The MoCA is routinely administered in the clinic. In addition, a 1-minute animal category word generation task was performed in the majority of patients. Information was collected through chart review concerning patient demographics and disease characteristics, including antibody type, presence of tumour, type of symptoms at onset, delay to first treatment, nature of first treatment, and number of relapses. Study protocols were approved by the University Health Network Research Ethics Board.
Data Analysis
MoCA scores were stratified into “early” (the earliest MoCA collected within 1 year of symptom onset) and “long-term” intervals (the latest MoCA collected after 1 year from symptom onset). Clinical data were analysed using SPSS Statistics (IBM Corp., Version 24.0. Armonk, NY, USA). Correlations between disease characteristics and MoCA scores were evaluated using a Pearson’s correlation coefficient. Correlated factors were subsequently analysed using regression analysis and a Mann-Whitney U-test. Statistical significance was defined as p<0.05, unless otherwise stated.
Results
In total, 22 patients were diagnosed with AE during the study period (Table 1). One patient was excluded from further analyses as accurate data outlining the treatment course were not available. In total, 21 patients were therefore included in the study.
Table 1 Patients’ characteristics

Early MoCA scores were available from 13 subjects. Early scores were lower than long-term MoCA scores (p=0.045). Visuospatial/executive abilities, language, attention, and delayed recall were predominantly affected in the early phase of the disease. A similar pattern was observed at last follow-up, with the exception of delayed recall, which demonstrated a trend towards improvement (p=0.0949) (Table 2). Letter-fluency was consistently more impaired than category fluency. The most common source of errors for visuospatial/executive skills involved reproducing a geometrical shape, either a cube, rectangular prism, or cylinder, depending on the MoCA version used. In total, 11 (52%) patients had persistent cognitive impairment at their last follow-up, defined as a MoCA score of <26/30.
Table 2 Montreal Cognitive Assessment (MoCA) results by cognitive domain
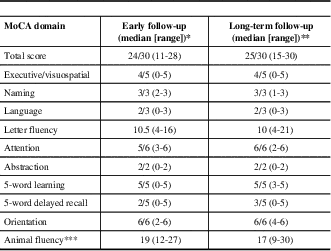
LGI1=leucine-rich glioma inactivated 1; NMDAr=N-methyl-D-aspartate receptor
* For the first MoCA obtained before 1 year, median delay to testing=6 months, range 2-12 months
** For the last MoCA obtained after 1 year, median delay to testing=20 months, range 13-182 months
*** Not part of original MoCA testing
Patients with status epilepticus at presentation had lower total MoCA scores at last follow-up (median 21, range 15-29) compared with patients without status epilepticus at presentation (median 27.5, range 21-30; r 2=0.366, p=0.004). There was a trend towards lower long-term MoCA scores in patients with heavier antiepileptic burden (r=−0.408, p=0.104). No correlation was found between long-term MoCA scores and admission to the intensive care unit (r=−0.117, p=0.613), or length of hospital stay (r=−0.237, p=0.3). Delays >60 days from symptom onset to initiation of treatment were associated with a higher probability of MoCA score <26 at last follow-up (r 2=0.253, p=0.0239; z-score=−2.01, p=0.044). In the long term, there was no statistically significant difference between the MoCA scores of patients with cell-surface antibodies (median 27, range 21-29) and patients with intracellular antibodies (median 23.5, range 22-25, p=0.502). Furthermore, no difference was observed when seronegative cases (median=25, range 21-30, p=0.55) were considered. The long-term MoCA score was not correlated with the first total MoCA score (r 2=0.12, p=0.22), female gender (r 2=0.03, p=0.44), presence of a tumour (r 2=0.069, p=0.249), or occurrence of relapses after initial presentation (r 2=0.032, p=0.44). There was a trend towards lower long-term MoCA scores in older patients (r 2=0.16, p=0.07). Time from symptom onset to testing predicted higher total MoCA scores (r 2=0.16, p=0.002).
Discussion
In all, 52% of recovering adult AE patients had cognitive impairment at their last follow-up, defined as a total MoCA score <26. This compares to rates of ~50% reported in other studies that have considered long-term outcomes in a predominantly paediatric population with cell-surface antibody-mediated AE.Reference Hacochen, Wright and Waters 17 , Reference Pillai, Hacochen and Tantsis 18 In the acute phase of AE, delayed recall was more severely affected in our cohort. Impaired recall presumably reflected impairment in retrieval of stored information, recognising that most patients successfully encoded the five-word list following presentation (median 5/5 words in both trials; range 0-5 early, and 3-5 late at long-term follow-up). Performance on measures of visuospatial/executive, language, and attention were mildly impaired, whereas naming, abstraction, and orientation were largely preserved. This cognitive pattern was relatively preserved at the last follow-up, except for delayed recall impairments, which tended to improve. The persistent discrepancy between letter fluency and animal fluency suggests preferential frontal impairment.Reference Martin, Wiggs, Lalonde and Mack 19 These findings are similar to what was previously reported in the literature in patients with various forms of antibody-mediated AE,Reference Saiz, Blanco and Sabater 20 including NMDARReference Vahter, Kannel, Sorro, Jaakmees, Talvik and Gross-Paju 12 , Reference Finke, Kopp, Pruss, Dalmau, Wandinger and Ploner 13 and VGKC encephalitis.Reference Irani, Stagg, Schott, Rosenthal, Schneider and Pettiingill 14
In the subgroup of 10 patients with NMDAR encephalitis, 30% of patients had cognitive impairment at last follow-up, higher than the 10% reported in the literature using the MMSE (defined as total score <26).Reference Dalmau, Gleichman and Hughes 3 , Reference Wang, Li and Hu 4 Three of four (75%) patients recovering from LGI1 encephalitis exhibited cognitive impairment at a median follow-up of 35 months (range 18-100 months). By comparison, a rate of 29% of moderate to severe cognitive impairment has been reported at 24 months in 76 patients with LGI1 encephalitis, using a non-validated measure of cognitive outcomes,Reference Arino, Armangue, Petit-Pedrol, Sabater, Martinez-Hernandez and Hara 5 and 28% (19/67) of patients were shown to have some form of cognitive impairment using the Clinical Dementia Rating (CDR≥0.5).Reference Gadoth, Pittock and Dubey 8 , Reference Hughes, Berg, Danzgier, Coben and Martin 21
Delay to initial treatment >60 days was a measured potentially modifiable risk factor associated with long-term cognitive impairment (MoCA<26). This finding adds to a growing body of literature emphasising the importance of early consideration of AE, and initiation of empiric treatment.Reference Graus, Titulaer and Ramani 1 - Reference Dalmau, Gleichman and Hughes 3 , Reference Finke, Kopp, Pruss, Dalmau, Wandinger and Ploner 13 A statistically significant difference was observed in early and long-term MoCA scores, suggesting that overall performance on cognitive testing tended to improve over time.
The presence of status epilepticus at onset was identified as a potential risk factor associated with poorer long-term cognitive outcomes, with a trend towards poorer performance on the MoCA in the long term with greater burden of anti-epileptic drug required. There was a non-statistically significant trend towards poor long-term cognitive performance in older patients. Although no statistically significant difference was observed in this study between long-term MoCA scores of patients with cell-surface antibodies compared with other types of encephalitides, we suspect we would find such a difference if our sample size was larger.Reference Saiz, Blanco and Sabater 20 , Reference Bataller, Kleopa, Wu, Rossi, Rosenfeld and Dalmau 22 Unlike previous studies,Reference Titulaer, McCraken, Inigo, Armangue and Glaser 2 - Reference Wang, Li and Hu 4 we did not find that extended hospital stay was related to poorer long-term cognitive impairment.
Rates of cognitive impairment reported in this study (52%) are considerably lower than the 80-90% prevalence reported in smaller case series (n≤10 patients) using formal neuropsychological testing.Reference Vahter, Kannel, Sorro, Jaakmees, Talvik and Gross-Paju 12 - Reference Irani, Stagg, Schott, Rosenthal, Schneider and Pettiingill 14 , Reference Saiz, Blanco and Sabater 20 These differences may be attributed to the lower sensitivity of the MoCA for the detection of cognitive impairment. This limitation may be counterbalanced by relative advantages of the MoCA as applied to routine follow-up in the outpatient population, including the low cost of use, the wide availability of published normative data across different ethnicities and languages,Reference Rahman and El Gaafary 23 - Reference Tsai, Lee, Wang, Shia, Nasreddine and Fuh 26 and the ease of administration. In addition, the MoCA offers comparative advantages to other widely used bedside screening measures, particularly the MMSE.Reference Nasreddine, Phillips and Bedirian 15 Compared with the MMSE, the MoCA exhibits superior sensitivity for the detection of incipient cognitive decline,Reference Roalf, Moberg, Xie, Wolk, Moelter and Arnold 16 while also permitting more detailed assessment of visuospatial, attention, and memory functions—of particular relevance when assessing patients recovering from AE, who may manifest with disproportionate dysfunction in these areas.Reference Nasreddine, Phillips and Bedirian 15 , Reference Roalf, Moberg, Xie, Wolk, Moelter and Arnold 16 Nevertheless, there is a clear need to develop and validate cognitive assessment tools for outpatient follow-up of patients with AE, including direct comparisons between performance on bedside measures and formal neuropsychological tests. In lieu of these data, we advocate for the routine use of the MoCA in outpatient follow-up of patients with AE, with addition of a word-category generation task.
Limitations of this study include its retrospective nature, and reporting within a single outpatient specialty clinic. As a result, our results may overestimate the rate of cognitive impairment seen in other clinical environments, recognising that patients who fully recover following their hospitalisation may be less likely to be referred for further assessment. Furthermore, rates and types of AE may vary across centres, challenging the generalisability of our results. Prospective studies in other clinical environments are ultimately required to confirm our findings. Another limitation of our study is its modest sample size. As a result, this study was probably underpowered for the detection of the relationship between potentially important modifiers of outcomes (e.g., effect of antibody subtype on cognitive outcomes, duration of treatment, medication use, and so on). A final limitation is that data for early MoCA testing were not available for all patients. This is unsurprising given that, during the acute phase of the disease, inpatient health care teams often focus on stabilising more pressing issues such as seizure activity and dysautonomia, and it may be impossible to administer formal cognitive testing in patients who are sedated and intubated.
Conclusions
The MoCA may be used to evaluate cognition in recovering patients with AE. Shorter delays to treatment and absence of status epilepticus at onset predict better performance on the MoCA ≥1 year following acute presentation, and may predict better long-term cognitive outcomes.
Acknowledgements
The authors would like to acknowledge the contributions of the patients to this study.
Funding
None.
Disclosures
JH, CS, RAW, and DTW do not have any conflicts of interest. GSD is the Clinical Director of the Anti-NMDA Receptor Encephalitis Foundation, Inc. (Canada). The Foundation is supported by private donations; GSD is not directly compensated for his work with the Foundation. None of the authors are US government employees.
Statement of Authorship
JH: data collection, design of study, drafting of manuscript, and revision of the manuscript; GSD and CS: data collection, study design, and revision of the manuscript; RAW and DTW: Study design and revision of the manuscript.




