Under the global coronavirus disease 2019 (COVID-19) pandemic, school closure was conducted in many countries in 2020(Reference Viner, Russell and Croker1). For example, almost all primary and secondary schools in Japan were closed from early March to the end of May. The school schedule is one of the major factors associated with the sleep and dietary habits of school-age children. For example, studies have consistently shown that school starting time is associated with sleep patterns(Reference Knutson and Lauderdale2,Reference Carissimi, Dresch and Martins3) . In addition, previous studies have reported differences in sleep patterns(Reference Mathew, Hale and Chang4), physical activity(Reference Jose, Blizzard and Dwyer5), time of eating breakfast(Reference Fleig and Randler6) and dietary intake(Reference Rothausen, Matthiessen and Hoppe7,Reference Asakura and Sasaki8) between weekdays with school and weekends without school among children. Thus, the long-term school closure due to the pandemic might have altered sleep and dietary habits, including the time of sleep and eating among school-age children.
Recent studies concerning the influence of the COVID-19 pandemic have examined changes in lifestyle behaviours and dietary habits before and during school closure or lockdown among children. These studies mainly focused on screen time(Reference ten Velde, Lubrecht and Arayess9,Reference Moore, Faulkner and Rhodes10) , physical activity(Reference ten Velde, Lubrecht and Arayess9–Reference Censi, Ruggeri and Galfo11), sleep habits(Reference Moore, Faulkner and Rhodes10,Reference Ruiz-Roso, Knott-Torcal and Matilla-Escalante12) and dietary habits(Reference Censi, Ruggeri and Galfo11–Reference Rodríguez-Pérez, Molina-Montes and Verardo13). However, to our knowledge, no study has investigated circadian rhythms of sleep and eating as well as their associations with lifestyle behaviours and dietary intake among children during long no-school days. In the context of an emerging focus on the whole dietary pattern(Reference O’Hara and Gibney14,Reference Leech, Worsley and Timperio15) and chrono-nutrition(Reference Asher and Sassone-Corsi16), dietary intake should be examined at the level of specific eating occasions with the timing and distribution of daily eating(Reference O’Hara and Gibney14,Reference Leech, Worsley and Timperio17) . Considering the circadian system of the human body(Reference Asher and Sassone-Corsi16), the daily timing of eating should be captured in combination with sleep habits, which are associated with the time of eating meals(Reference Baron, Reid and Van18), breakfast skipping(Reference Yu, Yeung and Ho19,Reference Sato-Mito, Sasaki and Murakami20) , dietary intake(Reference Mathew, Hale and Chang4,Reference Sato-Mito, Sasaki and Murakami20–Reference Gariépy, Doré and Whitehead23) and longer screen time(Reference Gariépy, Doré and Whitehead23). Moreover, previous studies primarily assessed dietary variables, such as dietary intake(Reference Ruiz-Roso, Knott-Torcal and Matilla-Escalante12), eating frequency(Reference Censi, Ruggeri and Galfo11) or diet quality scores(Reference Rodríguez-Pérez, Molina-Montes and Verardo13), with simple and/or non-validated questions. Quantitative assessments of dietary intake have rarely been conducted.
In a usual school year, Japanese school-aged children wake up early(Reference Oka, Suzuki and Inoue24,Reference Ojio, Kishi and Sasaki25) and eat school lunch(Reference Asakura and Sasaki8) regularly on weekdays. The absence of a school schedule and school lunch during school closure might alter the patterns of sleep and eating among children on weekdays. Further, irregular sleep and eating habits possibly resulted in unfavourable lifestyle behaviours among school-age children during school closure. The aim of this cross-sectional study was (i) to identify daily temporal patterns of sleep and eating among Japanese school-age children during school closure and (ii) to examine the associations between temporal patterns of sleep and eating and lifestyle behaviours and dietary intake.
Methods
Study design and participants
The target population of this cross-sectional survey was school-aged children (i.e. from third to sixth graders of elementary schools and from first to third graders of secondary schools; aged 8–15 years). First, we announced the conduct of the survey via a website, social media and direct e-mail to previous collaborators. As a result, forty-three elementary and secondary schools, one sports club and four research collaborators in fourteen out of forty-seven prefectures showed interest in the survey. Questionnaires were then distributed to school-age children by the schools, the sports club or research collaborators. The aim and procedure of the study were explained to children and their parents using a document attached to the questionnaires. The participants were considered to agree with participation in the survey when they answered and submitted the questionnaires. However, provided data from participants were excluded from the analysis when the participants indicated disagreement against using their data in the research even if they answered questionnaires. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki. All procedures involving human subjects were approved by the Ethics Committee of the University of Tokyo, Faculty of Medicine (approval no. 2020056NI, 25 May 2020).
The information on the timing of school closure in the schools attended by the study participants was collected through the schools, sports club and research collaborators participating in this study. Although the date of closure started and reopened slightly differ by school or region, all schools attended by the participants started school closure in the first week of March 2020 and ended in the last week of May or the first week of June 2020. According to the Ministry of Education, Culture, Sports, Science and Technology in Japan, 99·0 % of all primary and secondary schools in Japan closed as of 16 March 2020(26).
The survey was conducted immediately after schools reopened in June 2020. Participants were instructed to answer two types of questionnaires: a questionnaire for lifestyle behaviours and a brief-type self-administered diet history questionnaire for children and adolescents (BDHQ15y)(Reference Okuda, Asakura and Sasaki27,Reference Okuda, Sasaki and Bando28) . Participants were asked to recall and answer their lifestyle behaviour and dietary intake in the previous month during school closure. Among 11 958 participants invited to the survey, 8512 participants (71 %) answered both questionnaires distributed in June 2020.
Measurements
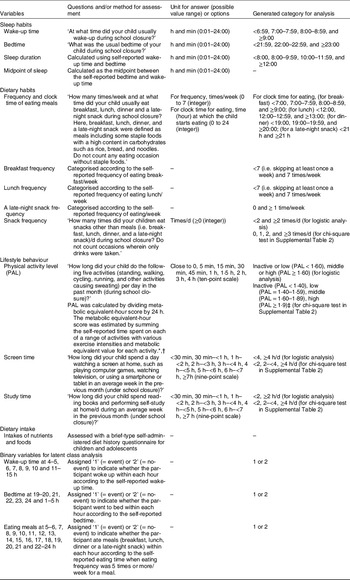
The questionnaire for lifestyle behaviours during school closure included questions on clock time for waking up and going to bed; frequency and clock time for eating breakfast, lunch, dinner and a late-night snack; the frequency of snacks; physical activity; screen time and study time. The participants were asked to recall and answer their lifestyle behaviour listed above in the previous month under school closure. In addition, parents were instructed to ask their children for their lifestyle if needed when answering the questionnaire. The questionnaire was answered by one of the family members of the participating children who mainly prepared meals at home. Table 1 summarises the analysed variables, the questions and/or methods used for measurement and generated categories for analysis. In brief, questions on clock times for waking up and going to bed were answered with units in h and min (hh:mm) and then categorised into <7:00, 7:00–7:59, 8:00–8:59 and ≥9:00 for wake-up time and <22:00, 22:00–22:59 and ≥23:00 for bedtime. Sleep duration was calculated using the wake-up time and bedtime. The midpoint of sleep was calculated as the midpoint between bedtime and wake-up time. Questions on the frequencies of eating breakfast, lunch, dinner and a late-night snack/week were answered in integer values from 0 to 7. Questions on clock times for eating breakfast, lunch, dinner and a late-night snack/week were answered in integer values from 0 to 24 h, as the clock time when the participants started to eat the concerned meal during school closure. Questions on snack frequency/d were answered in integer values, which were categorised into 0, 1, 2 and ≥3 times/d. Physical activity level (PAL) was calculated by dividing the metabolic equivalent-h score by 24 h. Metabolic equivalent-hour score was estimated by summing the self-reported time spent on each of a range of activities with various exercise intensities and metabolic equivalent value for each activity(Reference Ainsworth, Haskell and Herrmann29,Reference Murakami, Sasaki and Okubo30) . Participants with PAL < 1·40, 1·40–1·59, 1·60–1·89 and ≥1·9(31) were categorised as inactive, low, middle and high, respectively. Questions on screen time and study time were answered using a nine-point scale; the answers of questions pertaining to both parameters were categorised into <2, 2–<4 and ≥4 h/d.
Table 1 Summary of analysed variables, questions and/or methods for measurement and generated category for analysis

* Ainsworth BE, Haskell WL, Herrmann SD et al. (2011) Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc 43, 1575–1581.
† Murakami K, Sasaki S, Okubo H et al. (2007) Association between dietary fiber, water and magnesium intake and functional constipation among young Japanese women. Eur J Clin Nutr 61, 616–622.
‡ Ministry of Health Labour and Welfare (2020) Dietary reference intakes for Japanese. (in Japanese).
Dietary intake during school closure was assessed using the BDHQ15y, which was filled by participating children themselves, one of the children’s family members (who was mainly responsible for meal preparation), or children and their family members together. The participants were asked to answer their dietary habits in the previous month under school closure. The BDHQ15y is a four-page fixed-portion questionnaire concerning the consumption frequency of selected foods commonly consumed in Japan, general dietary behaviours and usual cooking methods during the previous month. The BDHQ15y was developed based on the sixteen-page comprehensive(Reference Sasaki, Yanagibori and Amano32) and four-page brief versions(Reference Kobayashi, Murakami and Sasaki33,Reference Kobayashi, Honda and Murakami34) of a validated self-administered diet history questionnaire for Japanese adults. Estimates of daily intakes of foods (ninety food items in total), energy and selected nutrients were calculated using an ad hoc computer algorithm for the BDHQ15y based on the Standard Tables of Food Composition in Japan(35) and sex-specific fixed portion size. The BDHQ15y was validated for selected nutrients, including protein, fatty acids and carotenoids, using biomarkers (erythrocyte fatty acid and serum carotenoid levels) as a gold standard(Reference Okuda, Asakura and Sasaki27,Reference Okuda, Sasaki and Bando28) .
Date of birth, sex, body weight and height were self-reported as part of the BDHQ15y. Living status and sibling status were determined based on the relationship of family members living with the participating children asked with the questionnaire for lifestyle behaviours. Living status and sibling status were determined based on the relationship of family members living with the participating children for whom the questionnaire for lifestyle behaviours was filled. Living status was categorised into two groups: (i) living with both parents or (ii) living with a single parent and/or other relatives (including those living with a mother or father, a mother or father and/or other relatives and other relatives without both parents). Sibling status was categorised into two groups: (i) having at least one sibling at or under primary school age or (ii) not having any siblings at or under primary school age (i.e. not having any siblings or having an only sibling(s) over primary-school-age).
Analysed participants
Participants who indicated disagreement in using their data (n 402) were excluded from the analysis. Furthermore, we excluded participants who were not in the targeted grades (n 88) and had missing information on the variables used (n 1763; n 1 for sex; n 3 for identification number; n 186 for frequency and eating time of breakfast, lunch or dinner; n 528 for snack frequency; n 773 for wake-up time or bedtime; n 378 for anthropometric data; n 10 for family structure, n 863 for physical activity; n 162 for screen time; n 78 for study time and n 3 for dietary data; some participants had more than one missing value).
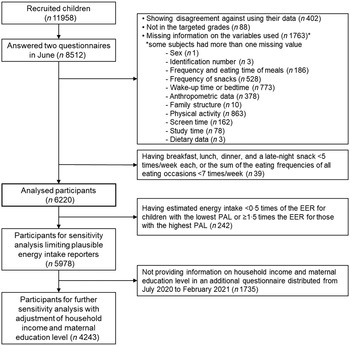
To identify temporal patterns of sleep and eating with regard to the usual time of eating meals among participants, we excluded participants whose eating frequencies/week were less than five times for all eating occasions, or in whom the sum of the eating frequencies of all eating occasions was less than 7 times/week (n 39). For example, participants were excluded when they ate breakfast, lunch, dinner and a late-night snack 3, 4, 3 and 1 time(s)/week, respectively, or 0, 1, 5 and 0 time(s)/week, respectively. Finally, the analysed participants were 6220 children of 8512 potentially eligible participants who replied to the surveys in June 2020 (Fig. 1).

Fig. 1 Flow chart of the participant for analysis. EER, estimated energy requirement, PAL, physical activity level. The thick line box shows the participants for the main analysis
Statistical analyses
Latent classes of temporal patterns of sleep and eating
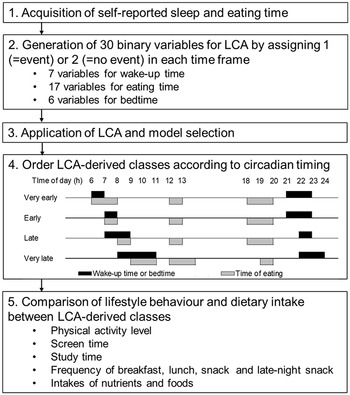
Summary of the analytical method was shown in Fig. 2. Based on the clock time for wake-up; going to bed and eating breakfast, lunch, dinner and a late-night snack; binary variables (1 = event, 2 = no-event) were generated as input variables for latent class analysis (LCA). For clock time for wake-up and going to bed, binary variables indicated whether or not wake-up and going to bed had occurred within each hour of the day. For example, for the participants having clock time for wake-up at 7:45 am, a value of ‘1’ was assigned to the generated binary variable ‘wake-up time at 7 h’. A value of ‘2’ was assigned for the other binary variables for wake-up time. For the clock time of eating meals, binary variables indicated whether or not meal consumption had occurred within each hour of the day. To identify temporal eating patterns according to the usual clock time of eating meals, a value of ‘1’ was assigned when the eating frequency was 5 or more times/week for a particular meal; otherwise, a value of ‘2’ was assigned. Examples of variables generating eating time are shown in Supplemental Fig. 1. During the variables generating process, the self-classification of meals was not distinguished. For example, a value of ‘1’ was assigned to the binary variable ‘eating time at 11 h’ for participants who reported a clock time for eating breakfast at 11 am and those who reported a clock time for eating lunch at 11 am. Subsequently, generated binary variables with a few events were integrated to establish the number of input variables that gave the feasible solution in LCA. For example, going to bed from 1:00 am to 5:00 am was expressed in one binary variable, ‘bedtime at 1–5 h’. Binary variables with no events were excluded from the input variables for the LCA. Finally, thirty binary variables were generated: seven, six, and seventeen variables for wake-up time, bedtime and eating meals, respectively.

Fig. 2 Summary of the analytical method. LCA, latent class analysis
LCA was performed using SAS statistical software (version 9.4; SAS Institute Inc.) with the PLOC LCA procedure (version 1.3.2)(Reference Lanza, Lemmon and Dziak36,Reference Lanza, Collins and Lemmon37) to identify temporal patterns of sleep and eating. Models with two to six latent classes were tested. The Bayesian information criterion, Akaike information criterion (AIC), adjusted AIC and entropy were computed for each LCA. Models with lower Bayesian information criterion and AIC values suggested better goodness of fit. The four-class solution was chosen considering a combination of model fit and the interpretability of the classes(Reference Lanza, Collins and Lemmon37). The values of Bayesian information criterion, AIC and adjusted AIC decreased as the number of classes increased, but the decrease of the values levelled off among four, five and six classes (Table 2). Some patterns of five or six classes were less interpretable and resulted in classes representing <10 % of the sample (see online Supplemental Fig. 2). Thus, the four-class solution was better than the other solutions for further analysis.
Table 2 Model-fit indices for the latent class analysis model

AIC, the Akaike information criterion; BIC, Bayesian information criterion.
To interpret the identified patterns of sleep and eating, three or four peaks in the conditional probability of eating events were labelled as breakfast, lunch, dinner and a late-night snack, according to the chronological order in each latent class.
Associations between latent classes and socio-demographic and eating pattern indicators
All statistical analyses were performed using SAS 9.4 statistical software. All reported P-values were two-tailed, and a P-value < 0·05 was considered statistically significant. Data are presented as means and sd for continuous variables and as numbers and percentages for categorical variables. The mean (sd) clock times of eating meals were calculated for participants who ate each type of meal ≥5 times/week. Mean differences in continuous variables (i.e. clock times for wake-up, going to bed and eating, as well as basic characteristics) among LCA-derived classes were tested with linear regression models using the PROC GLM procedure. The ordinal scale (i.e. 1 to 4) according to the circadian timing was assigned for each class and used as a continuous variable in the linear regression models. Differences in categorical variables were tested using the χ 2 test.
The risks of classifying participants as having unfavourable lifestyles were tested using logistic regression. Examined unfavourable lifestyles were as follows: physical inactivity or low PAL; longer screen time (4 h or more/d); shorter study time (less than 2 h/d); skipping breakfast at least once/week; skipping lunch at least once/week; having a late-night snack at least once/week and having a snack at least twice/d. First, crude odds ratios (OR) and 95 % CI for the risk of having unfavourable lifestyles were calculated for each LCA-derived class; the LCA-derived class having the earliest circadian timing was used as the reference. Thereafter, multivariate-adjusted OR and 95 % CI were calculated by entering the following confounding factors into the regression model: age, sex, living status and sibling status.
Linear regression models were constructed to examine the association between LCA-derived classes and intakes of nutrient and food using the ordinal scale of LCA-derived classes as a continuous variable. A multivariate-adjusted model analysis was also performed with age, sex, living status and sibling status as confounding variables.
Sensitivity analysis
Sensitivity analysis was performed only on participants with more plausible reported energy intake (n 5978) (Fig. 1). Participants were excluded if their energy intake estimated using the BDHQ15y was <0·5 times the estimated energy requirement for children with the lowest PAL or ≥1·5 times the estimated energy requirement for those with the highest PAL. Further sensitivity analysis was performed with adjustments for household income and maternal education level (n 4243) (Fig. 1). Information regarding household income and maternal education level was collected with an additional questionnaire distributed to the participants several months after the first survey, from July 2020 to February 2021. Participants who did not answer the additional questionnaire were excluded from the analysis.
Results
The mean (sd) age, height and weight of participants were 11·0 (1·9) years, 144·8 (12·5) cm and 38·4 (10·7) kg, respectively. Fifty-one percent of the participants were boys and 67 % were primary school-aged.
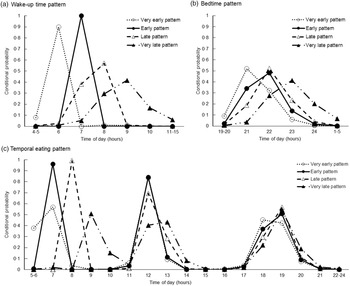
Using LCA, four temporal patterns of sleep and eating were derived. Each class was labelled based on distinguishing features, as shown by high or low conditional probability for wake-up, going to bed and consuming a meal at each time frame of a usual day during school closure. Figure 3 shows the confidence probabilities for each pattern. The first pattern was labelled ‘Very early’, as participants in this class (20 % of participants) woke up and ate breakfast earlier than those in the other classes. The second pattern was labelled ‘Early’, as participants in this class (24 % of participants) woke up and ate breakfast 1 h later than those in the first pattern but earlier than those in the other two patterns. The third pattern was labelled ‘Late’, as participants in this pattern (30 % of participants) woke up and ate breakfast 1–2 h later than those in the first pattern. Peaks in the conditional probability of eating lunch at 12 pm were similar among the ‘Very early,’ ‘Early’ and ‘Late’ patterns. The fourth pattern labelled ‘Very late’ (26 % of participants) was characterised by participants with the latest timings of wake-up and eating breakfast. Latter patterns were also characterised by participants with later timings of eating dinner or a late-night snack and bedtime. However, differences in the timings of eating dinner or a late-night snack and bedtime were less distinctive among classes compared with those of wake-up and eating breakfast.

Fig. 3 Conditional probabilities of: (a) wake-up time; (b) bedtime and (c) eating time across the day according to latent class analysis-derived temporal patterns of sleeping and eating among 6220 school-aged children. Dashed lines with a white circle represent the ‘Very early’ pattern, solid lines with a black circle represent the ‘Early’ pattern, dashed lines with a white triangle represent the ‘Late’ pattern, and dashed lines with a black triangle represent the ‘Very late’ pattern
Among all participants, the mean (sd) clock times were 7:39 (1:10) for wake-up and 22:18 (1:01) for going to bed (Table 3). The mean (sd) sleep duration was 9:21 (0:58), and the midpoint of sleep was 2:58 (0:58). The mean (sd) clock times for each eating occasion were 8 (1) h (n 5650) for breakfast, 12 (1) (n 6108) h for lunch, 19 (1) h (n 6180) for dinner and 20 (1) h (n 69) for a late-night snack, among participants eating the concerned meals ≥5 times/week. The clock times for wake-up, going to bed and eating meals, as well as the midpoint of sleep were later for children with latter patterns than for those in earlier patterns (P < 0·0001). Sleep duration was longer in participants with the latter patterns than those with earlier patterns (P < 0·0001).
Table 3 Sleep habits and clock time for eating in 6220 school-age children (third to sixth grade of primary school and first to third grade of secondary school) according to latent class analysis-derived temporal patterns of sleep and eating

* For continuous variables, P values represent P for trend in temporal patterns of sleep and eating. For categorical variables, P values were tested using the χ 2 test. The trend of association was examined using a linear regression model with the ordinal scale of temporal patterns of sleep and eating (1 = ‘Very early’ pattern, 2 = ‘Early’ pattern, 3 = ‘Late’ pattern and 4 = ‘Very late’ pattern) as a continuous variable.
† In the questionnaire, the clock time for eating was answered with an integer value of hour by participants (e.g. breakfast consumed between 7:00 and 7:59 was answered as ‘7’).
‡ Participants who ate breakfast, lunch, dinner or late-night snacks less than 5 times/week were excluded from the analysis.
Participants with latter patterns tended to be older and were predominantly girls (Table 4). The proportion of participants living with a single parent and/or other relatives and having siblings aged at or under primary school age was higher in the latter patterns (P < 0·0001). Latter patterns had higher proportions of participants who were inactive or had a low PAL (P < 0·0001), had a screen time of 4 h or more (P < 0·001), had a study time of less than 2 h (P < 0·0001), skipped breakfast (P < 0·001) and lunch (P < 0·0001), had a late-night snack at least once/week (P < 0·001) and had snacks at least twice/week (P = 0·001) (Table 4 and see online Supplemental Table 2).
Table 4 Characteristics of 6220 school-aged children (third to sixth grade of primary school and first to third grade of secondary school) according to latent class analysis-derived temporal patterns of sleeping and eating

* For continuous variables, P values represent P for trend in temporal patterns of sleep and eating. For categorical variables, P values were tested using the χ 2 test. The trend of association was examined using a linear regression model with the ordinal scale of temporal patterns of sleeping and eating (1 = ‘Very early’ pattern, 2 = ‘Early’ pattern, 3 = ‘Late’ pattern and 4 = ‘Very late’ pattern) as a continuous variable.
† Including participants living with mother (or father), living with mother (or father) and/or other relatives (e.g. living with mother and grandparents) and living with other relatives without both parents (e.g. living with grandparents).
‡ Participants with physical activity level (PAL) <1·60(31) were categorised as ‘inactive or low PAL.’ PAL was calculated by dividing the metabolic equivalent-hour score by 24 h. The metabolic equivalent-hour score was estimated by summing the product of the time spent on each of a range of activities (sleeping, standing, walking, cycling, running and other activities causing sweating) with various exercise intensities and metabolic equivalent values for each activity(Reference Ainsworth, Haskell and Herrmann29,Reference Murakami, Sasaki and Okubo30) .
§ Screen time included the time spent in watching television; using a computer, smartphone or tablet and playing video games.
║ Study time included time spent reading books and self-studying.
¶ A late-night snack was defined as a meal including staple foods (i.e. rice, bread or noodles).
The risk of having unfavourable lifestyle behaviours was high in participants with latter patterns compared with those with the ‘Very early’ pattern (all P for trend <0·0001) (Table 5). The results were similar after adjustment for confounding factors (all P for trend <0·0001, except for having snacks ≥2 times/d (P for trend was 0·003)). Compared with the ‘Early’ and ‘Late’ patterns, the ‘Very late’ pattern had much higher OR for longer screen time (adjusted OR = 3·13, 95 % CI (2·66, 3·68)), skipping breakfast (adjusted OR = 12·28, 95 % CI (9·28, 16·26)), skipping lunch (adjusted OR = 3·71, 95 % CI (2·63, 5·12)) and having a late-night snack (adjusted OR = 3·86, 95 % CI (2·62, 5·69)).
Table 5 Odds ratios for unfavourable lifestyle and dietary habits according to latent class analysis-derived temporal patterns of sleeping and eating among 6220 school-age children (third to sixth grade of primary school and first to third grade of secondary school)

* Logistic regression models were used with the ordinal scale of temporal patterns of sleeping and eating (1 = ‘Very early’ pattern, 2 = ‘Early’ pattern, 3 = ‘Late’ pattern and 4 = ‘Very late’ pattern) as a continuous variable.
† Participants with physical activity level (PAL) <1·60(31) were categorised as ‘Inactive or low PAL.’ PAL was calculated by dividing the metabolic equivalent-hour score by 24 h. The metabolic equivalent-hour score was estimated by summing the product of the time spent on each of a range of activities (sleeping, standing, walking, cycling, running and other activities causing sweating) with various exercise intensities and metabolic equivalent values for each activity(Reference Ainsworth, Haskell and Herrmann29,Reference Murakami, Sasaki and Okubo30) .
‡ In the adjusted model, sex, age, living status and sibling status were adjusted.
Participants with latter patterns had lower intakes for protein; dietary fibre; vitamins A, C, B6 and B12; thiamine; riboflavin; niacin; folate; potassium; Ca; Mg; Fe; pulses; vegetables; fruits; fish and shellfish and dairy products and higher intakes of carbohydrate, sugars and confectionaries and sweetened beverages compared with those with earlier patterns (all P for trend < 0·0001 except for carbohydrates) (Table 6). Dietary intakes did not significantly differ among participants with different patterns for total fat, saturated fat, Na and meat. After adjusting for confounding factors, the direction of association between LCA-derived classes and dietary intake did not change (see online Supplemental Table 2). The statistical significance of P for trend for cereal intake disappeared after the adjustment, but the results of other nutrients and foods did not change before and after the adjustment.
Table 6 Dietary intakes according to latent class analysis-derived temporal patterns of sleep and eating among 6220 school-age children (3rd to 6th grade of primary school and 1st to 3rd grade of secondary school)

* Trend of association was examined using a linear regression model with the ordinal scale of temporal patterns of sleeping and eating (1 = ‘Very early’ pattern, 2 = ‘Early’ pattern, 3 = ‘Late’ pattern and 4 = ‘Very late’ pattern) as a continuous variable.
Sensitivity analyses limiting analysed participants with reported energy intake and a further adjustment of household income and maternal education level did not considerably alter the abovementioned findings.
Discussion
To our knowledge, this is the first study that explored temporal patterns of sleep and eating and examined their associations with lifestyle behaviours and dietary intake. In addition, no study has identified temporal eating patterns among school-age children during school closure in any country including Japan. Inconsistent with the findings of a previous study wherein three eating patterns (including irregular eating) were identified among Australian adults using LCA(Reference Leech, Worsley and Timperio17) participants with all four identified patterns in our study had three main meals/d at a regular time. This inconsistency with the previous study might be because we did not include the time of eating snacks in the LCA in this study. Considering the higher snack frequency among participants with the ‘Very late’ pattern than among participants with the other three patterns, different temporal eating patterns might be identified when the time of eating snacks is considered.
Among participants with the four identified patterns, those classified as having ‘Late’ and ‘Very late’ patterns waked up on average at 7:44 am and 9:00 am, respectively. On weekdays during usual school days, Japanese school-age children wake up between 6 am and 7 am on average(Reference Oka, Suzuki and Inoue24,Reference Ojio, Kishi and Sasaki25) . Thus, children with ‘Late’ and ‘Very late’ patterns woke up and ate breakfast much later than they did during usual school days on average, whereas children with ‘Very early’ and ‘Early’ patterns kept their time schedule as they did during usual school days. However, dinner time and bedtime were not as distinctively different as wake-up and breakfast times between participants with different patterns. A possible reason for the less distinctive difference in dinner time and bedtime between participants with different patterns is the frequency of family eating. A previous study reported a higher frequency of family eating at dinner compared with that at breakfast(Reference Takeda, Melby and Ishikawa38). Having family meals at breakfast or dinner might play a role in preventing a delay in eating time, which in turn prevents delays in wake-up time or bedtime. Although the frequency of family eating at each meal was not evaluated among participants in this study, it is possible that participants with earlier patterns frequently had family eating at both breakfast and dinner, whereas those with latter patterns frequently had family meals at dinner but not at breakfast. Consequently, the time of dinner and bedtime would not be delayed and would less distinctively differ between participants with different patterns. In contrast, a lower frequency of family eating at breakfast could delay wake-up and breakfast times in participants with latter patterns, possibly because the management of wake-up and breakfast times highly depends on children themselves.
Regarding sleep habits among participants, children with ‘Late’ and ‘Very late’ patterns might have later chronotypes because they had a later time of midpoint sleep. Previous studies reported associations between later chronotype or later midpoint sleep and longer screen time(Reference Gariépy, Doré and Whitehead23), a higher frequency of skipping meals(Reference Yu, Yeung and Ho19,Reference Sato-Mito, Sasaki and Murakami20) , consuming food at late hours(Reference Baron, Reid and Van18), a lower intake of fruits and/or vegetables(Reference Mathew, Hale and Chang4,Reference Thellman, Dmitrieva and Miller21,Reference Gariépy, Doré and Whitehead23) and a higher intake of soft drinks(Reference Thellman, Dmitrieva and Miller21,Reference Gariépy, Doré and Whitehead23) or lower diet quality(Reference Full, Berger and Erickson22). Thus, our study findings are consistent with those of previous studies. A higher frequency of skipping meals and consuming snacks might have resulted in poor dietary intake (such as lower intakes of vegetables and fruits, as well as higher intakes of sugar, confectionaries and sweetened beverages) among participants with latter patterns. Considering the larger social jet lag among children with later chronotypes(Reference Flanagan, Bechtold and Pot39) and the associations between school time and time for sleep(Reference Knutson and Lauderdale2,Reference Carissimi, Dresch and Martins3) and breakfast(Reference Fleig and Randler6), children with later chronotypes could be more vulnerable to changes in their circadian timing during no-school days.
A possible reason for the association of latter temporal patterns of sleep and eating with unfavourable lifestyles in the present study was the family environment, including parenting practice. Previous studies have shown an association between parenting practice and screen time(Reference Xu, Wen and Rissel40), PAL(Reference Xu, Wen and Rissel40) and sleep habits(Reference Khor, McClure and Aldridge41) among children. Children with latter patterns were possibly less likely to have family rules regarding screen time and sleep habits and less likely to be encouraged to engage in physical activity. During school closure, managing children’s lifestyles might depend highly on their family environment and caregivers. Mothers reportedly have more childcare and household work during lockdown periods than fathers(Reference Xue and McMunn42,Reference Yerkes, André and Besamusca43) . In Japan, working mothers with primary school-age children are more likely to work from home, unlike fathers with primary school-age children and parents with secondary school-age children(Reference Yamamura and Tsustsui44). These results suggest a higher burden and gender inequality in childcare in different households during school closure. Hence, social support for parents of school-age children is needed. In addition, some strategies to encourage children to manage circadian rhythms would be needed during school closure, such as having regular online meetings in the morning, although there is no established causal relationship between the temporal pattern of sleep and eating, lifestyle behaviours and dietary intake.
There are several limitations to the present study. First, our study sample was a convenient, not a nationally representative, sample. Possibility of selection bias needs to be recognised in this study. The study area was limited to only fourteen of the forty-seven prefectures in Japan. In addition, participants included in the analysis possibly had different characteristics from non-participants, although our sensitivity analysis with adjustment for household income and maternal education level did not change the direction of associations between LCA-derived classes and lifestyle behaviours and dietary intake. Thus, the generalisability of our results may be low. Second, the survey period might have affected the answers of participants, as the questionnaires were filled after schools reopened. Thus, participant answers did not fully reflect their lifestyle during school closure. However, four distinctive temporal patterns of sleep and eating were identified among participants. ‘Late’ and ‘Very late’ patterns might be rarely identified in a usual school year. This suggests that the effect of recall bias on the identification of patterns and the association between patterns and lifestyle variables might be low. Third, the variables used for analysis were all self-reported, and the validity of the questionnaires was insufficient or unknown. Previous studies have investigated the validity of the BDHQ15y against several biomarkers; the correlation coefficients were found to be low for all the examined nutrients(Reference Okuda, Asakura and Sasaki27,Reference Okuda, Sasaki and Bando28) . The nonvalidated questions were used to assess sleep habits, physical activity, screen time and study time estimates. A previous systematic review found a high correlation between self-reported sleep time and those assessed with accelerometer among children(Reference Nascimento-Ferreira, Collese and de Moraes45). Thus, analysing patterns of temporal sleep and eating time based on self-reported sleep habits could be acceptable. However, the questionnaire had the potential of measurement error resulting from recall bias and social desirability bias(Reference Hidding, Chinapaw and van Poppel46,Reference Klesges, Baranowski and Beech47) . The parents of participating children possibly reported later wake-up time and earlier bedtime than the time when their children actually went to bed and woke up(Reference Mazza, Bastuji and Rey48,Reference Short, Gradisar and Lack49) . Thus, some participants were possibly misclassified. Regarding physical activity and sedentary behaviours, parents could overestimate their children’s physical activity and study time, while they underestimated screen time due to social desirability bias(Reference Klesges, Baranowski and Beech47). However, it was also possible that physical activity was possibly underestimated and sedentary behaviour was overestimated under the situation of the pandemic. Thus, it is unknown whether a possible measurement error overestimated or attenuated the associations between later timing of sleep and eating patterns and unfavourable lifestyle behaviour. Finally, we could not determine a causal relationship between temporal patterns of sleep and eating and lifestyle behaviours due to the cross-sectional study design. A sedentary lifestyle or longer screen time could have potentially adversely affected sleep habits.
In conclusion, more than half of the children in the present study had later circadian timings, especially for wake-up and eating breakfast and lunch. Later timings for sleeping and eating meals were associated with unfavourable lifestyle behaviours and dietary intake, including longer screen time and short study time, skipping meals, a lower consumption of vegetables and fruits and a higher consumption of sweetened beverages. Further studies are needed to investigate the environmental and social factors that determine differences in the temporal patterns of sleep and eating among children during school closure.
Acknowledgements
Acknowledgements: The authors and their colleagues thank the all participating schools and organizations, the dietitians, school nurses, teachers, research collaborators and stuff who supported the research in each school or organisation and research centre, namely, Elementary School attached to Miyagi University of Education, Fukaya Municipal Fukaya Elementary School, Fukaya Municipal Sakuragaoka Elementary School, Fukui Prefectural Koshi Junior High School, Hikari Elementary School attached to Faculty of Education, Yamaguchi University, Hikari Junior high School attached to Faculty of Education,Yamaguchi University, Komae Municipal Izumi Elementary School, Kurashiki Municipal Kojima Elementary School, Mito Municipal Kasahara Elementary School, Mito Municipal Midorioka Elementary School, Mito Municipal Migawa Elementary School, Mito Municipal Sakado Elementary School, Mito Municipal Sannomaru Elementary School, Mito Municipal Yoshizawa Elementary School, Nara Prefectural School for the Deaf, Nara Prefectural School for the Blind, Non-profit organization ATTAKA, Office Noriko Higuchi (NGO Equalnet Sendai and Sendai Teacher’s Union), Okayama Municipal Oomoto Elementary School, Okegawa Municipal Asahi Elementary School, Ryugasaki Municipal Ryugasaki Elementary School, Saitama Municipal Sashiogi Junior High School, Saitama Municipal Takasago Elementary School, Sakai Municipal Sanbo Elementary School, Setagaya Ward Kitami Junior High School, Shinjuku Ward Kashiwagi Elementary School, Soja Municipal Soja Elementary School, Soja Municipal Soja Higashi Junior High School, Tokashiki Municipal Aharen Elementary School, Tokashiki Municipal Tokashiki Elementary School, and Tokashiki Junior High School, Tsumagoi MunicipalTsumagoi Junior High School, Yamaguchi Elementary School attached to the Faculty of Education, Yamaguchi University, Mieko Aoki, Sachiyo Otani, Fumi Ono, and Shoma Kunisho and other 14 primary and secondary schools. The authors and their colleagues thank Editage (www.editage.jp) for English language editing. Financial support: The present study was supported by the Foundation for Dietary Scientific Research, Institute for Food and Health Science, Yazuya Co., Ltd., Japan Crop Protection Association, Bourbon Corporation, Kao Corporation, ILSI, Japan and Mr. Tatsuya Yoshida. Authorship: M.S. involved in the recruitment of study participants, directed the dietary survey, designed the research, analysed and interpreted the data for the work and prepared the first draft of the manuscript; K.M. designed the research, interpretated of data for the work, provided critical oversight of the project, including critical input into the final draft of the manuscript and S.S. designed and directed the dietary survey, involved in the recruitment of study participants and assisted in the writing. All authors contributed to the development of the manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the Ethics Committee of the University of Tokyo, Faculty of Medicine (approval number: 2020056NI, approval date: 25 May 2020). Written informed consent was obtained from all participants.
Conflicts of interest:
There are no conflicts of interest.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980022001148