Highlights
-
The University of Alberta Hospital-based mobile stroke unit (MSU) enrolled patients in the Intravenous Tenecteplase Compared with Alteplase for Acute Ischemic Stroke (AcT) trial.
-
All eligible patients were randomized to the study without MSU workflow issues.
-
An MSU is a feasible site for clinical trials in the hyperacute phase.
Introduction
Intravenous thrombolysis (IVT) is the first-line treatment for eligible patients with acute ischemic stroke within the first 4.5 hours of symptom onset. Reference Heran, Lindsay and Gubitz1 IVT is typically delivered in the emergency department (ED) of a primary or comprehensive stroke center supervised by a neurologist. Rapid administration of IVT closer to symptom onset is associated with better short- and long-term functional outcomes than delayed treatment. Reference Ebinger, Siegerink and Kunz2 The time-limiting step for IVT delivery is the need for a CT scan before IVT and the availability of a trained multidisciplinary team. Mobile stroke units (MSUs) are specially designed ambulances equipped with a CT scanner and trained personnel that may include a physician, nurse, CT technologist and paramedics. The MSU can assess a patient with acute stroke syndrome at the scene of the stroke, at a rendezvous point with another ambulance or at a community hospital without access to a CT scanner. The MSU hastens time to neurological assessment after symptom onset, provides dedicated access to a CT scanner and allows for rapid administration of IVT. Reference Mackey, Yamal and Parker3 Challenges exist in the recruitment of patients in clinical trials in the prehospital setting with respect to the consent process and clinical trial workflow. Reference Broderick, Aziz and Adeoye4,Reference Sandset, Walter and Song5 Few clinical trials have enrolled patients in MSUs. Reference Bivard, Zhao and Churilov6
The pivotal Intravenous Tenecteplase Compared with Alteplase for Acute Ischemic Stroke (AcT) trial was a multicenter, randomized controlled trial that demonstrated non-inferiority of tenecteplase (TNK) compared to alteplase within the first 4.5 hours from stroke onset. Reference Menon, Buck and Singh7 The Edmonton-based MSU transports patients to the University of Alberta Hospital (UAH) and was involved in enrolling patients in the AcT trial. Reference Kate, Jeerakathil and Buck8 We examined the feasibility, impact on workflow and functional outcomes with MSU enrollment in the AcT trial.
Methods
The AcT trial
This is a post hoc analysis of the AcT trial; the protocol and main results have been previously published. Reference Menon, Buck and Singh7,Reference Sajobi, Singh and Almekhlafi9 Briefly, the AcT trial was a Canadian, pragmatic, multicenter, parallel-group, open-label, registry-linked, randomized, controlled, non-inferiority study comparing the efficacy of TNK versus alteplase in eligible patients with acute ischemic stroke. In the AcT trial, patients were recruited in 1 MSU, 5 primary stroke centers and 17 comprehensive stroke centers between November 2020 and January 2022. Eligibility for IVT and post-thrombolysis care was based on the Canadian Stroke Best Practices Guidelines. Reference Heran, Lindsay and Gubitz1,Reference Gladstone, Patrice Lindsay and Douketis10 Patients were randomized 1:1 to receive either TNK at a dose of 0.25 mg/kg according to weight brackets up to a maximum of 25 mg as bolus dose or alteplase 0.9 mg/kg up to a maximum of 90 mg (10% as a bolus dose and the remaining 90% over 1 hour as an infusion). The primary outcome for the AcT trial was the proportion of patients with a modified Rankin score (mRS) of 0–1 at 90–120 days after randomization.
Workflow of Edmonton mobile stroke unit
The Edmonton MSU has been operational since February 2017, servicing both urban and rural areas within a 250 km radius of the UAH in Alberta, Canada. Reference Shuaib and Jeerakathil11 The MSU team consists of a stroke fellow, ED nurse, CT technologist, primary care paramedic and advanced care paramedic. The MSU operates on weekdays between 8 a.m. to 4 p.m. and is alerted by the Emergency Medical Services (EMS) dispatch or a critical care phone consultation service regarding patients with an acute stroke syndrome who are within 4.5 hours of symptom onset or last known well time. For calls within the city of Edmonton, both a regular ambulance and MSU are co-dispatched. For rural locations, the MSU and EMS ambulance meet at a rendezvous point approximately midway between the site of the initial assessment and the UAH. On arrival, if the patient is suspected to be an acute stroke eligible for thrombolysis, the patient is then transferred to the MSU for a CT scan. Once completed, the team reviews the case and CT scan with a telestroke physician. If eligible for IVT, alteplase or TNK is delivered in the field, and if required, the infusion is continued as the patient is transported to the comprehensive stroke center for further evaluation and admission.
Workflow of trial enrollment
The UAH MSU was included as a site in the AcT trial and screened consecutive patients for trial enrollment between November 2020 and January 2022. Patients evaluated on the MSU, similar to other sites in the AcT trial, were enrolled using a deferred consent process in accordance with the Tri-Council Policy Statement – Ethical Conduct for Research involving human guidelines and the Helsinki Declaration. Reference Faris, Dewar and Fedyk12 The deferred consent process was designed to allow for rapid treatment of time-critical conditions for patients being enrolled in randomized clinical trials. The deferred consent process was approved at all sites in the AcT trial except for two centers in Quebec. Patients were screened for trial eligibility by stroke fellows who were on board the MSU. If the patient was deemed eligible for IVT, the stroke fellow randomized the patient with the help of a secure, real-time, web-based server that could be accessed via a web browser on a mobile phone. Randomization was also available through secure text messaging and automated telephone calls. The randomizing physician received instant information about the treatment allocation and dose. The study drug was stored on the MSU. The study coordinator would meet the patient and/or the family member(s) on arrival at the UAH site at the earliest feasible time to obtain written informed consent.
Study outcome measures
The primary outcome was the proportion of MSU-administered IVT patients during the study period who were enrolled in the trial. Secondary outcomes were door-to-needle time (min), CT-to-needle time (min), the proportion of patients with symptomatic intracerebral hemorrhage (ICH) at 24 hours and mRS 0–1 at 90–120 days.
Statistical analyses
The study participants were divided into MSU-treated, UAH site and non-UAH sites. Age was described as mean SD. Baseline NIHSS and workflow metrics were non-normally distributed and described as median with interquartile range (IQR). Sex, presence of intracranial occlusion, patients undergoing endovascular thrombectomy (EVT), symptomatic ICH and patients with mRS of 0–1 and 0–2 at 90–120 days were expressed as proportions. Univariable analysis to compare proportions was performed with the chi-square test. To assess between-group differences in workflow measures (min), a linear regression was used. Logistic regression assessed between-group differences on 90-day mRS 0–1. Similarly, a binary logistic regression was used to assess between-group differences in patients achieving excellent outcomes (mRS 0–1), good outcomes (mRS 0–2) and deaths. The reference category was a non-UAH site. All regression analyses were adjusted for prespecified variables, including age (years), sex, baseline NIHSS, visible symptomatic arterial occlusion site and IVT drug type (TNK or alteplase). Statistical significance was defined as a p-value < 0.05. All analyses were conducted using STATA 18.0 BE (StataCorp LLC, Texas, USA).
Results
Of the 1577 patients (after excluding the 23 patients who did not consent) in the AcT trial, 43 (2.7%) were MSU-treated, 273 (17.3%) were treated at the UAH site and 1261 (80%) were treated at non-UAH sites. 100% of eligible MSU patients during the data collection period of the AcT trial were enrolled. No issues were noted with randomization or internet connectivity during enrollment. There were no issues related to drug availability or drug administration at the MSU. The baseline characteristics of participants in the MSU-treated, UAH and non-UAH groups are described in Table 1 and outcomes in Table 2.
Table 1. Baseline characteristics in MSU-treated, UAH site and non-UAH site groups
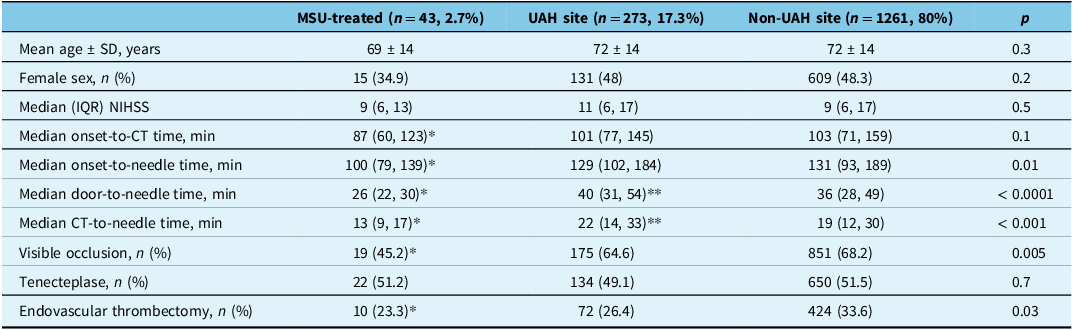
MSU = mobile stroke unit; UAH = University of Alberta Hospital; NIHSS = National Institute Health Stroke Scale. *p < 0.05 in MSU compared to other groups, **p < 0.05 in UAH compared to non-UAH.
Table 2. Outcomes in MSU-treated, UAH site and non-UAH site groups
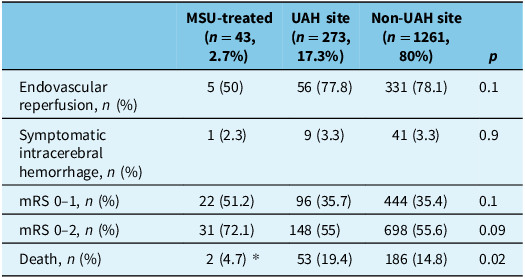
MSU = mobile stroke unit; UAH = University of Alberta Hospital; mRS = modified Rankin scale. *p < 0.05 in MSU compared to other groups.
On linear regression, door-to-needle time (min) was lower in the MSU-treated group by 17.2 (95% confidence interval [CI] 9.7–24.6) min compared to the non-UAH sites (Supplementary Table 1). CT-to-needle time (min) was lower in the MSU-treated group compared to the non-UAH site by 10.7 (95% CI 4.2–17.1) min (Supplementary Table 2; Figure 1). CT-to-needle time was higher at the UAH site than the non-UAH sites by 3.1 (95% CI 0.4–5.9) min. There was no statistically significant interaction noted between study groups and IVT drug type.

Figure 1. Box plot (A) CT-to-needle time (min) in different groups (MSU-treated, UAH site, non-UAH site) and different intravenous thrombolysis groups (alteplase, tenecteplase); (B) door-to-needle time (min) in different groups (MSU-treated, UAH site, non-UAH site) and in different intravenous thrombolysis groups (alteplase, tenecteplase). MSU = mobile stroke unit; UAH = University of Alberta Hospital.
Symptomatic ICH at 24 hours, odds of excellent outcome (mRS 0–1) and odds of good outcome (mRS 0–2) showed no difference between the MSU site and non-UAH site (Supplementary Table 3–4; Figure 2). A total of 241 (15.3%) died during the study period. The odds of death were increased in the UAH-treated group (aOR 1.57 [1.08–2.26]) (Supplementary Table 5) compared to non-UAH site.

Figure 2. Distribution of the modified Rankin scale scores at 90–120 days in MSU-treated, UAH site and non-UAH site. MSU = mobile stroke unit; UAH = University of Alberta Hospital.
Discussion
This post hoc analysis of MSU-enrolled patients included in the AcT trial indicates that it is feasible to rapidly screen and enroll patients with acute ischemic stroke into clinical trials on an MSU without significantly disrupting thrombolysis workflow. During the study period, all stroke patients eligible for IVT evaluated by the MSU were enrolled in the trial. The MSU-enrolled participants had faster workflow metrics, such as CT-to-needle time (min) and door-to-needle time (min), allowing rapid delivery of reperfusion therapy after symptom onset. No difference was observed in functional outcome measures assessed at 90–120 days. No safety concerns were observed.
The prehospital stroke trial enrollment with an emergency medical systems ambulance is challenging due to the issues of consent, CT scanner availability and randomization. Reference Saver, Starkman and Eckstein13,Reference Bath, Scutt and Anderson14 The Prehospital Acute Neurological Treatment and Optimization of Medical Care in Stroke study (PHANTOM-S), conducted in Germany, and the Benefits of Stroke Treatment Delivered by an MSU Compared with standard management by Emergency Medical Services (BEST-MSU) trial, conducted in the USA, consistently demonstrated that it is possible to enroll patients safely in the CT-equipped MSU. Reference Ebinger, Kunz and Wendt15,Reference Grotta, Yamal and Parker16 Both the BEST-MSU trial and PHANTOM-S prospectively designated weeks of enrollment by MSU versus standard of care in a quasi-experimental fashion. These primary studies randomized patients to receive IVT either in MSU or in the closest stroke center and observed the efficacy of MSU in delivering acute care. In the comparison of TNK with alteplase for the early treatment of ischemic stroke in the Melbourne MSU (TASTE-A) trial, the investigators were able to conduct an MSU-only trial. Reference Bivard, Zhao and Churilov6 The investigators randomized 104 patients 1:1 with the help of sealed, opaque envelopes that contained the treatment allocation, organized in sequential order as internet connectivity concerns were expected. The TASTE-A study was able to randomize all eligible patients. In the AcT study, patients were randomized in the MSU as a site along with the other stroke centers, thus allowing MSU participation in the acute stroke multicenter trial. In this analysis, we demonstrated the feasibility of randomizing patients in the MSU with the help of a web browser. rFVIIa for Acute Hemorrhagic Stroke Administered at Earliest Time (FASTEST) is another ongoing global study randomizing patients with acute spontaneous ICH in the first 2 hours of symptom onset to either receive rFVIIa or placebo. Reference Naidech, Grotta and Elm17 The study is enrolling patients at 15 MSUs worldwide. Like the AcT trial, a deferral of consent is used at applicable sites. The randomization procedure involves the delivery of a fixed number of predetermined drug kits to each site, which are sequentially numbered, blinded and dispensed by the site pharmacy. Thus, the MSU will carry only one sequential kit at any given time. This is an example of innovative ideas that can be successfully implemented in recruiting patients in the MSU environment. The deferral of the consent and presence of a stroke fellow may have facilitated the enrollment process. In the absence of a physician at the MSU, enrollment is still possible if a study stroke physician is available via telehealth and the patient is being transferred to a study-site ED. It is also possible to enroll patients in implementation studies by time-based cluster randomization, where the intervention and control can be provided on certain days of the week.
MSU allows for faster and earlier delivery of thrombolysis in eligible acute ischemic stroke patients. MSU has been associated with better utility-weighted disability outcomes derived from the mRS at 90 days. Reference Turc, Hadziahmetovic and Walter18 Furthermore, it has also been demonstrated that IVT with TNK is associated with better early reperfusion than alteplase on the MSU. Reference Bivard, Zhao and Churilov6 In our analysis, CT-to-needle time and door-to-needle times were quicker than other sites. Faster CT-to-needle time could be MSU workflow related as the patient is not moved from the stretcher bed, and the MSU team can deliver dedicated care to one patient without simultaneity, which may be presented in ED care. Reference Sarmiento, Wagner and Sheriff19 The AcT was conducted during the COVID-19 pandemic, and it may have affected workflow metrics. In a study from Alberta, the first year of COVID-19 pandemic was associated with delays in CT-groin puncture, door-to-groin puncture and groin puncture-to-reperfusion times compared to the pre-pandemic period. However, no delays were noted in the door-to-CT and door-to needle times during the first year of the pandemic. Reference Ganesh, Stang and McAlister20 Our results demonstrated that there was no significant difference between the site of enrollment and the proportion of patients that had an mRS of 0–1 at 90–120 days. Due to the small sample size, the results can only be considered exploratory.
With respect to measures of safety, the proportion of symptomatic ICH was not different between groups, and the proportion of favorable outcomes (mRS 0–1 or mRS 0–2) was not different among groups (Table 2). Interestingly, the rates of visible occlusions were significantly lower in the MSU (45.2%) compared to UAH (64.6%) and non-UAH sites (68.2%) as well as the proportion of patients that received EVT: MSU 23.3%, UAH 26.4% and non-UAH 33.6%. Recent evidence that TNK reduces perfusion lesion volume as compared to alteplase does not explain this group difference as the proportion of TNK use was similar among groups: MSU 51.2%, UAH 49.1% and non-UAH 51.5%. In a recent meta-analysis, IVT resulted in successful reperfusion in 1 in 10 patients averting the need for EVT. Reference Tsivgoulis, Katsanos and Schellinger21 However, sub-group analyses of time interval from stroke onset to IVT did not significantly change rates of successful reperfusion, and thus, faster onset-to-needle times cannot adequately explain the proportional differences in visible occlusions and EVTs in our MSU group from existing data. As the MSU is treating earlier than patients included in the above analyses, it is possible that ultra-early thrombolysis could have a higher recanalization rate in the setting of a threshold effect but that remains unproven. In this exploratory analysis, there was increased death in the UAH ED-treated group compared to the non-UAH site and MSU site. Possible considerations include that patients treated in MSU site may have stable hemodynamic parameters, be younger in the current observation, have a lower intracranial occlusion and exhibit mild to moderate severity of baseline neurological deficits as assessed by NIHSS (Table 1).
Our study has limitations. First, this is a secondary analysis of the AcT trial and as such is underpowered to detect differences in patients’ functional outcomes treated via the MSU in relation to other sites, though point estimates favor the MSU. Furthermore, the small sample size also limits the evaluation of workflow metrics beyond door-to-needle and CT-to-needle times. Unexplained lower proportions of visible occlusions and EVT may be confounding the lower rates of death observed in the MSU group. As MSU care expands globally, the feasibility of enrollment will enhance the inclusion of stroke patients in the prehospital space and ultra-early time period in trials of important stroke therapies.
Conclusion
Enrollment into the AcT trial from the MSU was feasible. MSU-enrolled patients demonstrated no difference in a favorable outcome but had faster door-to-needle and CT-to-needle times, resulting in earlier IVT and similar rates of symptomatic ICH. Clinical trial enrollment in MSU will allow for the inclusion of patients in the hyperacute phase of stroke.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/cjn.2024.354.
Author contributions
GC and AP contributed equally as the first authors. MK led the conceptualization and creation of the study design with assistance from GC and AP. AP and MK led the statistical analysis, with critical input from TTS and TJ. GC, AP and MK prepared the first draft. BHB, TJ, AS, MA, RS, AB, LC, NS, AT, TTS and BKM critically revised the final manuscript.
Funding statement
This research study received no funding.
Competing interests
MA has received grants from Canadian Institute of Health Research (CIHR) and has participation on a data safety monitoring board or advisory board with Palmera Medical and Fluid Biomed. RS discloses salary support from Sunnybrook Research Institute, grants from CIHR, participation on an advisory board for Hoffman-La Roche (< $5000) and stock ownership in FollowMD Inc. BKM discloses grants from CIHR, consulting fees from Roche, Boehringer Ingelheim, Circle CVI and Biogen, honoraria from Roche and Boehringer Ingelheim, participation on an advisory board with EMMA-CAN and INSTRUCT and stock ownership in Circle CVI. LC discloses payments received from Servier and consulting fees from Ischaemavie RAPID, Circle NV and the Canadian Medical Protective Association. AS discloses honoraria from Bayer Daiichi; advisory board membership for Bayer; consulting fees from Bayer, Servier Canada, Daiichi Sanyko Compan, AstraZeneca, VarmX and Takeda; and stock options in Ensho. TTS discloses consulting feeds from Circle NVI. All other authors have nothing to disclose.






