High blood pressure (BP) or hypertension (BP of >140/90 mmHg) is a major public health burden. Worldwide, in 2001, 13·5 % of all premature deaths were attributed to high BP(Reference Lawes, Hoorn and Rodgers1). Moreover, hypertension is a major risk factor for the progression of CVD(Reference Lewington, Clarke and Qizilbash2–Reference Vasan, Larson and Leip4), the leading cause of death in England. Individuals with uncontrolled hypertension are at an almost 3-fold higher risk of developing CVD compared with normotensive individuals(Reference Wang, Lee and Fabsitz5). Evidence suggests that a reduction in mean diastolic BP (DBP) by 5–6 mmHg over 5 years would decrease the incidence of stroke and CHD(Reference Collins, Peto and MacMahon6). Recent reports have suggested that the treatment of hypertension should occur before the disease evolves, in patients with prehypertension or in normotensives(Reference Julius, Nesbitt and Egan7). Therefore, novel cost-effective strategies are needed for the treatment of this condition.
Increased fruit and vegetable consumption protects against CVD and hypertension(Reference Appel, Moore and Obarzanek8, Reference Zhang, Shu and Xiang9). These cardioprotective effects have previously been attributed to several different nutrients found in vegetables such as antioxidant vitamins and flavonoids. However, clinical trials fail to ascertain the beneficial effects(Reference Vivekananthan, Penn and Sapp10, Reference Habauzit and Morand11). Although the health benefits from a diet rich in fruits and vegetables are likely to be multi-factorial, epidemiological studies have shown specific food groups such as green leafy vegetables to have the greatest protection against CHD and ischaemic stroke risk(Reference Joshipura, Ascherio and Manson12, Reference Joshipura, Hu and Manson13). Along with beetroot (Beta vulgaris), these are among the highest nitrate-accumulating vegetables.
The continuous production of NO by the endothelium plays an important role in the maintenance of vascular homeostasis. Hypertension and other risk factors for CVD may be partly associated with a diminished release of NO into the arterial wall(Reference Cannon14), due to inefficient synthesis or increased oxidative destruction. Increasing evidence suggests that NO can be biosynthesised in vivo following ingestion of inorganic nitrate(Reference Larsen, Ekblom and Sahlin15) or dietary nitrate from beetroot juice (BJ)(Reference Webb, Patel and Loukogeorgakis16), by a process that is independent of the conventional l-arginine NO synthase pathway. The activity of ingested nitrate relies on its conversion to nitrite by commensal Gram-negative bacteria residing in the oral cavity and enters the circulation after absorption in the stomach. In the blood vessel wall(Reference Li, Cui and Kundu17) and erythrocytes(Reference Webb, Milsom and Rathod18), the nitrite is reduced to NO and other nitrogen oxides, ultimately leading to vasodilation, BP maintenance and protection against ischaemia–reperfusion injury(Reference Lundberg, Weitzberg and Gladwin19). Ingestion of 500 ml BJ by normotensives has been shown to reduce BP, inhibit platelet aggregation and prevent endothelial dysfunction induced by an acute ischaemic insult to the forearm(Reference Webb, Patel and Loukogeorgakis16).
In addition, beetroot is particularly rich in betalains, a group of nitrogen-containing colour compounds that are not commonly represented among edible plants. Betalains can be divided into two subclasses: the red/purple betacyanins which are responsible for the colour of red beetroot or the yellow/orange betaxanthins that contribute to the colour of yellow beetroot. White beetroot is deficient in betacyanins, but no other differences between beetroot varieties have been reported in the literature. Betalains have been shown to act as antioxidants, by the donation of electrons(Reference Halvorsen, Holte and Myhrstad21, Reference Wettasinghe, Bolling and Plhak22), and inhibit lipid peroxidation and haem decomposition in vitro (Reference Kanner, Harel and Granit23), suggesting a role of these compounds in protection against certain oxidative stress-related diseases, such as hypertension and CVD.
In the present study, two novel studies were performed. The aim of study 1 was to determine the dose-dependent effect of nitrate-rich BJ on 24 h ambulatory BP (ABP) to establish the lowest dose of nitrate contained within BJ to have a functional effect on BP. Bread is a versatile and widely consumed food and may be an effective vehicle to increase vegetable consumption without significant dietary changes. Study 2 was performed to determine whether beetroot-enriched bread had similar beneficial effects on BP as BJ, and as a secondary objective to assess the impact of betalains on BP reduction.
Methods and procedure
Subjects
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the University of Reading Research Ethics Committee (approval no.: study 1, 09/25; study 2, 09/48). All subjects gave their written informed consent. Separate recruitment was carried out for each of the studies. The subjects were recruited from the University of Reading and the surrounding Reading areas by email, posters and Internet advertisements. Potential subjects attended a screening visit following a 12 h fast. BP and anthropometric measurements were taken followed by two blood samples, one of which was sent to the local hospital (Royal Berkshire Hospital) for full blood count analysis and the second for in-house analysis of fasting blood lipids, glucose, liver and kidney function using the ILAB 600 (Instrumentation Laboratories Limited). Subjects were selected if they met the study criteria of the following: no known liver disease, diabetes mellitus or a diagnosed mycocardial infarction; no gall bladder problems or abnormalities of fat metabolism; no subjects on weight-reducing diets or taking medication likely to affect BP; no vigorous exercise (>3 times/week, < 20 min each session) or excess consumption of alcohol (>150 ml/week); BMI < 30 kg/m2; BP < 150/90 mmHg and Hb in the range of 138–182 g/l. None of the subjects smoked or had a history of recent serious acute or chronic illness.
Experimental design
Subjects were recruited to take part in two separate randomised, controlled, single-blind, cross-over, test meal studies conducted at the Hugh Sinclair Unit of Human Nutrition at the University of Reading, during two separate periods from June to September 2008 and June to November 2009. There were at least 7 d washout period between each study day. All subjects were asked to refrain from consuming alcohol and caffeine or undertake any strenuous physical activity for 24 h before each intervention day and were provided with a standard evening meal supplied before each visit. On each study day, subjects arrived at 08.00 hours at the Hugh Sinclair Unit of Human Nutrition at the University of Reading after a 12 h fast. After a baseline urine sample, subjects were fitted with an ABP monitor and four baseline measurements were taken.
Study 1
The first study investigated the dose-dependent effects of BJ in eighteen healthy normotensive males. They were randomised to receive one of four different intervention drinks: 0 g BJ (500 g water) as a control; 100 g BJ with 400 g water; 250 g BJ with 250 g water and 500 g BJ (100 % BJ; James White Drinks Limited). The control drink and BJ drinks were all diluted with low-nitrate mineral water (The Buxton Mineral Water Company Limited).
Study 2
A further study was performed in fourteen healthy males to investigate the effects of red or white beetroot-enriched bread products on BP. Subjects were randomly assigned to one of three different intervention bread products (Eccentricities Limited): 200 g white bread (control, 0 g beetroot enrichment), or 200 g bread enriched with either white or red beetroot (comprising 50 % of the total weight of dough before baking). In both studies, ABP was measured automatically every 15 min from 08.00 hours until 13.00 hours, every 30 min from 13.00 hours until 22.00 hours, and every 60 min from 22.00 hours until 08.00 hours. Additionally, urine samples were collected at baseline, 2 and 4 h after intervention food consumption, and as an overnight collection (24 h), which was returned the following morning. The sample volumes were recorded, the urine was divided into aliquots and frozen at − 20°C for later analysis. During the study days, subjects remained in a semi-recumbent position.
Dietary protocol and intervention meals
In both studies, subjects consumed a low-nitrate and -nitrite diet 1 d before each study day. All study subjects were instructed not to take any vitamins or minerals, or other dietary supplements for 1 week before and during the intervention period. Standard, low-nitrate meals were provided for the night before the study day, and lunch and dinner on study days (Sainsbury's ‘Be Good to Yourself’ range; Sainsbury's Limited).
The intervention meals given in study 1 consisted of dose 1: 0 g BJ (500 g water) as a control; dose 2: 100 g BJ with 400 g water; dose 3: 250 g BJ with 250 g water and dose 4: 500 g BJ (100 % BJ; James White Drinks Limited), corresponding to < 0·05 mmol nitrate, 2·3 mmol nitrate, 5·7 mmol nitrate and 11·4 mmol nitrate for dose 1: 0 g BJ; dose 2: 100 g BJ; dose 3: 250 g BJ and dose 4: 500 g BJ, respectively. The control drink and BJ drinks were all diluted with low-nitrate mineral water (The Buxton Mineral Water Company Limited). On each study day, subjects received a standardised breakfast (50 g Nestlé Shreddies and 125 ml semi-skimmed milk).
The intervention meals given in study 2 consisted of the following: 200 g white bread (control, 0 g beetroot enrichment), or 200 g bread enriched with either white or red beetroot (comprising 50 % of the total weight of dough before baking). The bread (200 g) was served as sandwiches with 25 g Philadelphia cheese spread. The beetroot used in the bread was grown on the same farm under identical conditions and soil (Marsh Produce). In addition, the quantity of beetroot in the bread products was equivalent to 100 g BJ, administered as the lowest dose from study 1 to have a functional effect in human subjects. The nitrate concentrations of the beetroot-enriched breads (200 g) were as follows: < 0·05 mmol nitrate, 1·8 and 1·6 mmol nitrate for no-beetroot, red beetroot-enriched bread and white beetroot-enriched bread, respectively. The composition of the intervention meals is shown in Table 1.
Table 1 Nutritional compositions in 500 g (study 1) and 200 g (study 2) servings of the intervention products

BJ, beetroot juice.
* 0 g BJ (500 g water), as a control (The Buxton Mineral Water Company Limited), 100 g BJ with 400 g water; 250 g BJ with 250 g water and 500 g BJ. All beetroot juice was supplied by James White Drinks Limited.
† 200 g white bread (control, 0 g beetroot enrichment), or 200 g bread enriched with either 100 g white or 100 g red beetroot. The bread (200 g) was served as sandwiches with 25 g Philadelphia cheese spread. All bread intervention products were supplied by Eccentricities Limited.
‡ Chemical analysis of intervention drinks and bread products carried out by Leatherhead Food Research.
§ Photometric quantification of total betacyanins within intervention drinks and bread products carried out using UV/vis spectrophotometry.
Low-nitrate water was provided, which subjects were asked to consume for the same period as the low-nitrate diet (The Buxton Mineral Water Company Limited) and to drink throughout the study day. The intervention foods were given immediately after four baseline BP readings and a baseline urine sample, at approximately 09.00 hours on study days. Participants were asked to finish their intervention foods within 15 min. To ensure compliance to the low-nitrate diet, subjects were asked to fill in a 24 h dietary recall for 1 d before the study days and after each study day.
Ambulatory 24 h blood pressure measurements
BP was measured using a non-invasive ABP monitor (ABPM TM-243; A&D Instruments Limited), which met the British Hypertension Society standards for accuracy and performance. ABP monitors were fitted by one researcher at the start of each study day and subjects were asked to leave the monitors on for a period of 24 h. Subjects rested for at least 15 min before the BP monitors were fitted. All readings were performed on the left arm on each study day. The cuff was programmed to inflate automatically for a total period of 24 h post-juice or bread consumption. Once the volunteers left the Nutrition Unit, they were instructed to avoid any strenuous cardiovascular exercise, until the end of the 24 h period. At each subsequent BP measurement, volunteers were asked to hang their arm loosely down the side of their body, while keeping still until the end of the measurement. If walking, the volunteers were instructed to stop and remain standing while the measurement was being recorded. Volunteers were provided with a 24 h BP diary card, which they were required to fill in with information on their daily activities throughout the 24 h period.
Total urinary nitrate/nitrite measurements
Nitrate/nitrite (NOx) measurements were carried out on urine samples using a NO quantification kit (Active Motif). Creatinine was determined for all urine samples, using the ILAB 600 (Instrumentation Laboratories Limited). Total urinary NOx determination was used as a surrogate marker of systemic NO production. It should be noted that this measurement is not a reliable index of NO bioactivity in vivo, but may be used as an indicator of NO production.
Statistical analysis
All subjects who completed the study were included in the data analysis. Data were analysed using Excel (Microsoft Office; Microsoft Limited) and SPSS 18 for Microsoft Windows (SPSS, Inc.). All data are expressed as means with their standard errors of the mean and checked for normality using the Shapiro–Wilk test, as the number of subjects was less than fifty. Unpaired t tests were used to compare the two study groups at baseline. The AUC was calculated as a summation measure for BP. The period between 0 and 4 h represents the time the participants spent in the Nutrition Unit under standard resting conditions, 0 and 13 h represents the total awake period, and 0 and 24 h includes the total 24 h period after intervention food ingestion. One-way ANOVA was used for AUC comparisons, and subsequently Bonferroni multiple pairwise comparison post hoc tests to identify the differences between the control (no beetroot) and 100, 250 and 500 g BJ and beetroot-enriched bread, 200 g white beetroot (100 g)-enriched bread and 200 g red beetroot (100 g)-enriched bread. Urine sample data were compared using repeated-measures ANOVA. In all cases, P ≤ 0·05 was considered as statistically significant unless otherwise stated.
Results
Subject characteristics
A total of eighteen men completed study 1 and fourteen men completed study 2. There were no adverse events reported in response to intervention products, apart from beeturia (red urine), which is a common side effect of beetroot consumption. Table 2 shows the mean and range of baseline characteristics of the subjects. There were no significant differences in the general characteristics of the individuals recruited for the two separate studies.
Table 2 Baseline characteristics of the subjects before ingestion of intervention juice (study 1) or bread products (study 2) (Mean values with their standard errors, n 18 (study 1), n 14 (study 2))
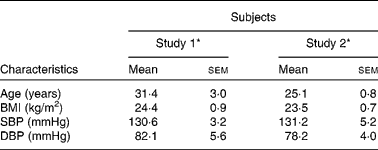
SBP, systolic blood pressure; DBP, diastolic blood pressure.
* There were no significant differences in baseline characteristics between the participants in study 1 and study 2 (unpaired t test): P = 0·05.
24 h ambulatory blood pressure
There were no significant differences in systolic BP (SBP) and DBP between the two groups during the hour before consumption of BJ, beetroot-enriched bread or controls (Table 2).
Study 1 (beetroot juice dose–response study)
Postprandial SBP and DBP after the ingestion of 0 g BJ (control), 100, 250 and 500 g BJ are shown in Fig. 1. SBP and DBP began to decrease 90 min after the ingestion of all BJ intervention drinks (100, 250 and 500 g BJ), in accordance with the increasing dose of BJ. The peak reduction in SBP and DBP occurred at 2–3 h in the order of 13·1, 20·5 and 22·2 mmHg and 16·6, 14·6 and 18·3 for 100, 250 and 500 g BJ, respectively, compared with the water control (0 g BJ). There was a significant effect of treatment on the AUC for both SBP from 0 to 4 h (Fig. 1(a), P = 0·008), 0 to 13 h (Fig. 1(c), P = 0·001) and 0 to 24 h (Fig. 1(e), P = 0·006) and DBP from 0 to 4 h (Fig. 1(b), P = 0·001), 0 to 13 h (Fig. 1(d), P = 0·001) and 0 to 24 h (Fig. 1(f), P = 0·001) after the consumption of 100, 250 and 500 g BJ, respectively. The Bonferroni post hoc test revealed that after ingestion of 100, 250, 500 g BJ, SBP was significantly lower from 0 to 4 h (Fig. 1(a), 250 g BJ, P = 0·04; 500 g BJ, P = 0·008), 0 to 13 h (Fig. 1(c), 250 g BJ, P = 0·02; 500 g BJ, P = 0·001) and for the remaining 24 h period (Fig. 1(e), 500 g BJ, P = 0·004) compared with the control drink (water). DBP was significantly lower from 0 to 4 h after ingestion of 100 g BJ (Fig. 1(b), P = 0·002) and 500 g BJ (Fig. 1(b), P = 0·001), 0 to 13 h (Fig. 1(d), 100 g BJ, P = 0·003; 250 g BJ, P = 0·03; 500 g BJ, P = 0·001) and for the remaining 24 h period (Fig. 1(f), 100 g BJ, P = 0·001; 250 g BJ, P = 0·02; 500 g BJ, P = 0·001) compared with the control drink (water). In addition, mean heart rate did not change significantly over the 24 h period post-ingestion of water control, 100, 250 or 500 g BJ (69·9 (sem 1·2), 69·2 (sem 1·2), 70·9 (sem 1·8) and 71·3 (sem 1·3), respectively). There appeared to be a near dose-dependent effect from 0 to 24 h, as shown by the pattern of decreasing AUC for increasing BJ dose (Fig. 1(e) and (f)).

Fig. 1 Study 1: mean change in area under the postprandial systolic blood pressure (SBP) curve from (a) 0 to 4 h, (c) 0 to 13 h and (e) 0 to 24 h and diastolic blood pressure (DBP) from (b) 0 to 4 h, (d) 0 to 13 h and (f) 0 to 24 h following consumption of dose 1 (control, water, 0 g beetroot juice (BJ)), dose 2 (100 g BJ with 400 g water), dose 3 (250 g BJ with 250 g water) and dose 4 (500 g BJ). All intervention drinks were made up to 500 g with low-nitrate water (The Buxton Mineral Water Company Limited). Values are means, with standard errors of the mean represented by vertical bars. Mean values were significantly different from the control drink (water): *P < 0·05, **P < 0·01, ***P < 0·001; Bonferroni post hoc test.
Study 2 (beetroot-enriched bread study)
Postprandial SBP and DBP after the consumption of three different intervention breads enriched with no beetroot (control), 100 g white beetroot or 100 g red beetroot are shown in Fig. 2. BP started decreasing approximately 60 min after the ingestion of beetroot-enriched breads compared with the bread control (no beetroot). Peak differences in SBP and DBP occurred between 2 and 3 h in the order of 19·3 and 23·6 mmHg and 16·5 and 23·2 mmHg for white and red beetroot-enriched breads, respectively, compared with the control bread (0 g beetroot). The AUC for SBP from 0 to 4 h following beetroot-enriched bread consumption was lower for both beetroot-enriched breads compared with the control bread (Fig. 2(a)), which continued from 0 to 13 h (Fig. 2(c)) and for the remaining 24 h period (Fig. 2(e)), but did not reach significance. However, there was a significant effect of treatment on the AUC for DBP from 0 to 4 h (Fig. 2(b), P = 0·03), 0 to 13 h (Fig. 2(d), P = 0·04) and from 0 to 24 h (Fig. 2(f), P = 0·03). Following ingestion of bread enriched with 100 g red beetroot, DBP was significantly lower from 0 to 4 h (P = 0·05), 0 to 13 h (P = 0·04) and 0 to 24 h (P = 0·03), compared with the control bread (no beetroot). Following ingestion of 200 g bread enriched with 100 g white beetroot, DBP was lower compared with the control (0 g beetroot) but did not reach significance. SBP was lower for both beetroot-enriched bread interventions at all time points compared with the control bread (0 g beetroot), but failed to reach statistical significance. There were no statistical differences between the red and white beetroot-enriched breads for both DBP and SBP over the 24 h period after ingestion of the intervention breads. In addition, mean heart rate did not change significantly over the 24 h period after consumption of the control bread (0 g beetroot), red or white beetroot-enriched breads (73·6 (sem 1·2), 74·9 (sem 1·2) and 74·2 (sem 1·1)).

Fig. 2 Study 2: mean change in area under the postprandial systolic blood pressure (SBP) curve from (a) 0 to 4 h, (c) 0 to 13 h and (e) 0 to 24 h and diastolic blood pressure (DBP) from (b) 0 to 4 h, (d) 0 to 13 h and (f) 0 to 24 h following consumption of 200 g control bread (CB) (0 g beetroot), 200 g white beetroot (WBB) and red beetroot (RBB) enriched bread (100 g red or white beetroot). Values are means, with standard errors of the mean represented by vertical bars. * Mean values were significantly different from the control bread (0 g beetroot) (P < 0·05; Bonferroni post hoc test).
Total urinary nitrate/nitrite
Study 1 (beetroot juice dose–response study)
Total urinary NOx were significantly higher for the 100 and 250 g BJ doses from 0 to 4 h post-consumption compared with the 0 g BJ control (100 g BJ, P < 0·01; 250 g BJ, P < 0·001; Fig. 3(a)). Total urinary NOx increased significantly from 0 to 4 h post-consumption of 500 g BJ and remained significantly higher at 24 h compared with the 0 g BJ control (P < 0·001; Fig. 3(a)).

Fig. 3 Mean total urinary nitrate/nitrite (NOx) concentrations for (a) dose 1 (control, water, 0 g beetroot juice (BJ; ![]() )), dose 2 (100 g BJ with 400 g water;
)), dose 2 (100 g BJ with 400 g water; ![]() ), dose 3 (250 g BJ with 250 g water;
), dose 3 (250 g BJ with 250 g water; ![]() ) and dose 4 (500 g BJ;
) and dose 4 (500 g BJ; ![]() ) and (b) 200 g control bread (0 g beetroot;
) and (b) 200 g control bread (0 g beetroot; ![]() ), 200 g bread enriched with either 100 g white beetroot (
), 200 g bread enriched with either 100 g white beetroot (![]() ) or 100 g red beetroot (
) or 100 g red beetroot (![]() ) at baseline (0 h) and 2, 4 and 24 h consumption of intervention juices or breads. Values are means, with standard errors of the mean represented by vertical bars. Mean values were significantly different between the groups, i.e. control juice (water), bread with BJ or beetroot-enriched bread: *P < 0·05, **P < 0·01, ***P < 0·001 (Bonferroni post hoc test); †P < 0·05, †††P < 0·001(repeated-measures ANOVA).
) at baseline (0 h) and 2, 4 and 24 h consumption of intervention juices or breads. Values are means, with standard errors of the mean represented by vertical bars. Mean values were significantly different between the groups, i.e. control juice (water), bread with BJ or beetroot-enriched bread: *P < 0·05, **P < 0·01, ***P < 0·001 (Bonferroni post hoc test); †P < 0·05, †††P < 0·001(repeated-measures ANOVA).
Study 2 (beetroot-enriched bread study)
Following consumption of the beetroot-enriched breads, total urinary NOx increased from 0 to 4 h, but only reached significance for the red beetroot-enriched bread (P < 0·05; Fig. 3(b)).
Discussion
One of the largest public health schemes in the Western world in the past decade has been to increase fruit and vegetable consumption. However, despite this, recent data from the National Diet and Nutrition Survey highlight that the average British adult consumes less than four portions of fruit and vegetables a day. Interestingly, 64 g of white bread are consumed by almost 80 % of British adults daily(Reference Bates, Lennox and Swan24). Taken together, these data suggest that enriching bread with fruit or vegetables may be a novel and useful strategy to increase the consumption of fruit and vegetables.
The protective effects of diets rich in fruit and vegetables have previously been attributed to several antioxidant vitamins. However, large-scale trials have failed to ascertain these beneficial effects. Recently, it has been postulated that the cardioprotective effects seen from consumption of green leafy vegetables and beetroot may be due to their high dietary nitrate content(Reference Webb, Patel and Loukogeorgakis16, Reference Lundberg and Weitzberg25). Dietary nitrate generates NO independently of the conventional endogenous NO synthase pathway, by reduction to nitrite and subsequently to NO in vivo (Reference Lundberg and Weitzberg25). Since hypertension is associated with a diminished endogenous NO production, the development of novel cost-effective strategies to incorporate dietary nitrate and therefore additional NO into the diet is of considerable interest.
Herein, we demonstrated that consumption of 100, 250 and 500 g BJ reduced SBP and DBP over a period of 24 h when compared with the control drink (0 g beetroot). There was a clear trend for BP reduction with increasing BJ dose, with a dose as low as 100 g resulting in a significant BP-lowering effect. A novel finding from these studies was that both SBP and DBP were substantially lower following consumption of bread enriched with either 100 g white or red beetroot, compared with the control bread (0 g beetroot). Although this only reached significance for red beetroot-enriched bread, it suggests that processing had minimal impact on the physiological effects of beetroot on BP. Therefore, enriching bread with fruit and vegetables may prove to be a good vehicle for increasing the intake of fruit and vegetables with minimal impact on habitual diet. Previous studies have found a peak decrease in SBP of 5·4 (sem 1·5) and 10·4 (sem 3·0) mmHg 2 to 3 h following consumption of 250 ml (5·5 mmol nitrate)(Reference Kapil, Milsom and Okorie20) and 500 ml (22·5 mmol nitrate)(Reference Webb, Patel and Loukogeorgakis16) BJ. In the present studies, the peak SBP reductions were of greater magnitude. This may be due to differences in baseline BP of the subjects in the studies. Kapil et al. (Reference Kapil, Milsom and Okorie20) found that the magnitude of BP response was directly related to baseline BP. Thus, a higher baseline BP would result in a greater magnitude of BP reduction.
Total urinary NOx concentrations were measured as an indicator of systemic NO production and increased in accordance with reductions in BP after consumption of BJ and beetroot-enriched breads. These results are in accordance with previous findings which suggest that urinary nitrate reaches peak concentrations 4–6 h and returns to baseline 24 h after supplementation with nitrate(Reference Bartholomew and Hill26, Reference Cortas and Wakid27) and dietary nitrate(Reference Pannala, Mani and Spencer28). The metabolic fate of NO includes oxidation to nitrate by oxyhaemoglobin in erythrocytes and autoxidation to nitrite. Nitrate and nitrite circulate in the blood and are ultimately excreted in the urine. Total urinary NOx determination may be used as an index of systemic NO production, but is not necessarily an indicator of endothelial NO synthase activity in vivo.
Interestingly, white beetroot, a betacyanin-deficient variety, lowered BP to a similar extent as red beetroot, a betalain-abundant variety, compared with the control (0 g beetroot). This observation suggests that components other than betalain compounds are likely to be responsible for the BP-lowering effects observed. Although beetroot contains other potentially bioactive compounds such as vitamin C, folate, K, dietary fibre and polyphenols, we suggest that it is the dietary nitrate that is partly responsible for the beneficial effects observed from beetroot consumption by bioconversion to nitrite and then to NO in vivo. In humans, 20–25 % of ingested nitrate is secreted in saliva and approximately 20 % of this is converted to nitrite by commensal Gram-negative bacteria on the dorsal surface of the tongue, resulting in nitrite-rich saliva(Reference Lundberg, Weitzberg and Lundberg29). The salivary nitrite is absorbed in the stomach and enters the circulation where NO synthesis occurs by the reduction of nitrite within the blood vessel wall(Reference Li, Cui and Kundu17) and erythrocytes(Reference Webb, Milsom and Rathod18). It is possible that the nitrite derived from ingested nitrate, in the present studies, provided an intravascular source of NO that resulted in vasodilation within the microcirculation to produce a decrease in peripheral resistance and therefore a reduction in BP.
The hypothesis that it was the dietary nitrate present in the BJ and beetroot-enriched breads that caused the beneficial effects on BP was supported by a study showing that supplementation of healthy volunteers with sodium nitrate (0·1 mmol/kg per d) over 3 d reduced DBP by 3·7 mmHg, compared with NaCl control(Reference Larsen, Ekblom and Sahlin15). Moreover, the magnitude of BP reduction and plasma nitrite concentration was diminished when the enterosalivary circulation was interrupted by spitting out all saliva following consumption of 500 ml BJ(Reference Webb, Patel and Loukogeorgakis16).
In the present studies, subjects were in a controlled environment, sitting or in a semi-recumbent position, for the first 4 h after intervention drink and bread consumption. However, the AUC for SBP remained lower following consumption of both beetroot-enriched breads for up to 13 h post-consumption, compared with the no-beetroot condition. This is noteworthy, as it indicates that the BP-lowering effect was sustained throughout the afternoon period, although understandably less marked than the initial effect due to the confounding effect of daily activities. This prolonged effect is suggestive of the impact of colonic metabolic products causing further release of NO into the circulation. It has been previously reported that anaerobic gut flora can reduce nitrate to nitrite(Reference Sobko, Reinders and Jansson30). It is therefore possible that nitrite is further converted to bioactive NO in the colon, which after absorption exerts vasodilatory effects, therefore prolonging the effects of BJ and bread on BP.
A limitation of these pilot studies is the lack of postprandial blood samples. As a result, it is currently unknown how the plasma nitrate and nitrite are affected by BJ or beetroot-enriched bread ingestion and whether they occur in association with changes in BP. Although determination of urinary NOx concentrations is suggestive of an association between BP-lowering effects of BJ and breads and nitrate consumption. Another possible limitation is the relatively small number of subjects who took part in these studies. However, even with the limited number of participants, both studies were suitably powered to detect significant BP changes. A larger postprandial test meal study is being performed to verify these findings and to investigate other parameters of vascular function such as micro-vessel endothelial function.
In conclusion, results from these studies are the first to demonstrate that acute ingestion of BJ lowered BP in a near dose-dependent manner in healthy normotensive individuals. In addition, novel bread enriched with either white or red beetroot, equivalent to 100 g BJ, resulted in similar BP reductions. This may suggest that nitrate in the beetroot is a major contributor to the BP reduction observed, whereas betalains had minimal impact, and that processing beetroot during bread production did not have a significant impact on the BP-lowering effects. Therefore, enriching bread with beetroot may provide a useful vehicle for the delivery of dietary nitrate and increasing vegetable consumption, with minimal impact on dietary habits.
Acknowledgements
This study was supported by grants from the University of Reading Research Endowment Trust Fund (RETF) and Eccentricities Limited. We thank Jan Luff and Cecilia Shen for technical assistance and James White Drinks Limited and Eccentricities Limited for supplying the juice and breads. The authors' responsibilities were as follows: D. A. H., J. A. L., T. W. G. and L. M. designed the studies; D. A. H. and N. K. conducted the research; D. A. H. analysed the data; D. A. H., J. A. L. and T. W. G. wrote the paper. J. A. L. had primary responsibility for the final content. All authors read and approved the final manuscript. As regards conflicts of interest, T. W. G. is the managing director of Eccentricities Limited. There are no other conflicts of interest.







