Introduction
Schistosomatids (Digenea: Schistosomatidae) are digenetic flukes inhabiting the venous and arterial systems of birds and mammals, with the medically noteworthy genus Schistosoma Weinland, 1858 as the most well studied taxon. This family is currently composed of 17 genera, 13 of which are found parasitising different orders of freshwater and marine birds (Gibson et al., Reference Gibson, Jones, Bray, Gibson, Jones and Bray2002; Horák et al., Reference Horák, Mikeš, Lichtenbergová, Skála, Soldánová and Brant2015; Flores et al., Reference Flores, Viozzi, Casalins, Loker and Brant2021; Lorenti et al., Reference Lorenti, Brant, Gilardoni, Diaz and Cremonte2022). As part of the phylogenetic clade “Derived avian schistosomes”, hereafter clade DAS (sensu Brant & Loker, Reference Brant and Loker2013), the genus Trichobilharzia Skrjabin & Zakharow, 1920 is considered the most speciose with about 40 species, mostly parasitising birds of order Anseriformes, and is globally distributed (Brant & Loker, Reference Brant and Loker2009, Reference Brant and Loker2013; Horák et al., Reference Horák, Mikeš, Lichtenbergová, Skála, Soldánová and Brant2015; Ebbs et al., Reference Ebbs, Loker, Bu, Locke, Tkach, Devkota, Flores, Pinto and Brant2022).
The life cycle of avian schistosomatids is not as complex compared to other digeneans because it requires only one intermediate host, an aquatic snail, rather than two or three. The snail hosts become infected with schistosomatids when miracidia in feces or nasal secretions of the infected bird hosts are released into the water. In the snail, sporocysts and then furcocercariae will develop, the latter is the infective stage to the birds (Horák & Kolářová, Reference Horák and Kolářová2011; Horák et al., Reference Horák, Schets, Kolářová, Brant and Liu2012). Once the furcocercaria penetrates the skin of the new bird host, it transforms into a schistosomula and migrates through the bloodstream to reach sexual maturation in the nasal, arterial, or venous system depending on the schistosomatid species. However, when these furcocercariae accidentally penetrate humans, it causes a zoonotic allergic condition of a cutaneous manifestation called cercarial dermatitis or swimmer’s itch (Horák et al., Reference Horák, Mikeš, Lichtenbergová, Skála, Soldánová and Brant2015). The zoonotic cercarial dermatitis has been reported in both Argentina and Chile (Flores et al., Reference Flores, Brant and Loker2015; Oyarzún-Ruiz et al., Reference Oyarzún-Ruiz, Thomas, Santodomingo, Collado, Muñoz and Moreno2022).
Host specificity of avian schistosomatids to their mollusk intermediate host has been shown to be somewhat narrow but often not restricted to a single species (Horák & Kolářová, Reference Horák and Kolářová2011; Horák et al., Reference Horák, Mikeš, Lichtenbergová, Skála, Soldánová and Brant2015). Unlike the mollusk hosts, reports suggest that the specificity of schistosomatids to their avian hosts is not as restricted to a single species of host or host group (Brant & Loker, Reference Brant and Loker2009; Ebbs et al., Reference Ebbs, Loker, Davis, Flores, Veleizán and Brant2016). The appearance of schistosomatid host specificity in the absence of experimental work can be due to consistent ecological and behavioral traits of their avian hosts (Ebbs et al., Reference Ebbs, Loker, Davis, Flores, Veleizán and Brant2016). Although more studies are needed, there are examples of species that only have been found in a particular host group such as Allobilharzia visceralis Kolářová, Rudolfová, Hampl & Skírnisson, Reference Kolářová, Rudolfová, Hampl and Skírnisson2006 parasitising only swans in the Northern hemisphere (Kolářová et al., Reference Kolářová, Rudolfová, Hampl and Skírnisson2006; Brant, Reference Brant2007; Hayashi et al., Reference Hayashi, Ichikawa-Seki, Ohari, Mohanta, Aita, Satoh, Ehara, Tokashiki, Shiroma, Azuta, Oka, Watanabe, Harasawa, Inohana, Ichijo and Furuhama2017), Anserobilharzia brantae (Farr & Blankemeyer, 1956) Brant, Jouet, Ferte & Loker, Reference Brant, Jouet, Ferte and Loker2013 in geese, Trichobilharzia physellae (Talbot, 1936) McMullen & Beaver, Reference McMullen and Beaver1945 in diving ducks and Trichobilharzia querquedulae McLeod, Reference McLeod1937 in blue-winged ducks (Brant & Loker Reference Brant and Loker2009, Reference Brant and Loker2013; Ebbs et al., Reference Ebbs, Loker, Davis, Flores, Veleizán and Brant2016). Although these species represent a few examples, the continued use of molecular tools and phylogenetic analyses can help establish if this phenomenon is more or less widespread in the avian schistosomes (Brant & Loker, Reference Brant and Loker2009, Reference Brant and Loker2013).
Flores et al. (Reference Flores, Brant and Loker2015) summarised the schistosomatid taxa reported in South America but in the past decade since that summary, there have been several more investigations into the systematics of schistosomatids parasitising aquatic birds (see Ebbs et al., Reference Ebbs, Loker, Davis, Flores, Veleizán and Brant2016; Pinto et al., Reference Pinto, Pulido-Murillo, de Melo and Brant2017, Reference Pinto, Tenório Mati, Melo and Brant2022; Flores et al., Reference Flores, Viozzi, Casalins, Loker and Brant2021; Lorenti et al., Reference Lorenti, Brant, Gilardoni, Diaz and Cremonte2022) revealing considerable new diversity. The focus of this work will be on waterfowl hosts (Anseriformes) in the Neotropics because much of the discovered diversity has come from this group of avian hosts. These hosts are also the host most likely to overlap with people in recreating or working aquatic areas; thus, the zoonotic potential to transmit cercarial dermatitis.
In Neotropical waterfowl there have been six species of avian host reported to have schistosomatids; white-cheeked pintail (Anas bahamensis Linnaeus), yellow-billed pintail (Anas georgica Gmelin), blue-winged teal [Spatula discors (Linnaeus)], silver teal [Spatula versicolor (Vieillot)], muscovy duck [Cairina moschata (Linnaeus)], and the black-necked swan [Cygnus melancoryphus (Molina)] from Argentina (Szidat, Reference Szidat1951; Flores et al., Reference Flores, Brant and Loker2015, Reference Flores, Viozzi, Casalins, Loker and Brant2021; Ebbs et al., Reference Ebbs, Loker, Davis, Flores, Veleizán and Brant2016), Brazil (Travassos et al., Reference Travassos, Teixeira de Freitas and Kohn1969; Freitas & Costa, Reference Freitas and Costa1972; Leite et al., Reference Leite, Costa and Costa1978, Reference Leite, Costa and Costa1979; Pinto et al., Reference Pinto, Pulido-Murillo, de Melo and Brant2017), Chile (Oyarzún-Ruiz et al., Reference Oyarzún-Ruiz, Muñoz, Paredes, Valenzuela and Ruiz2019), and Cuba (Sánchez et al., Reference Sánchez, Alba, García, Cantillo, Castro and Vázquez2018).
If the species richness of Neotropical waterfowl is considered, only a small fraction of these have been reported as definitive hosts of avian schistosomatids. Therefore, it is not a case of low species richness of these schistosomatids in birds, but rather a minimal effort in the study of their helminth fauna, particularly for schistosomatids (Agüero et al., Reference Agüero, Gilardoni, Cremonte and Diaz2016; Oyarzún-Ruiz & González-Acuña, Reference Oyarzún-Ruiz and González-Acuña2021). To date, despite only a small proportion of anseriform host diversity having been examined, there are already five species belonging to three genera of avian schistosomatids that have been described: Trichobilharzia, Nasusbilharzia Flores, Viozzi, Casalins, Loker & Brant, Reference Flores, Viozzi, Casalins, Loker and Brant2021 and Dendritobilharzia Skrjabin & Zakharov, 1920 (Leite et al., Reference Leite, Costa and Costa1978; Ebbs et al., Reference Ebbs, Loker, Davis, Flores, Veleizán and Brant2016; Flores et al., Reference Flores, Brant and Loker2015), with Nasusbilharzia restricted to the Neotropics (Flores et al., Reference Flores, Viozzi, Casalins, Loker and Brant2021). Only two of the five species, both from Argentina, have been molecularly characterised: T. querquedulae and Nasusbilharzia melancorhypha Flores, Viozzi, Casalins, Loker & Brant, Reference Flores, Viozzi, Casalins, Loker and Brant2021 (Ebbs et al., Reference Ebbs, Loker, Davis, Flores, Veleizán and Brant2016; Flores et al., Reference Flores, Viozzi, Casalins, Loker and Brant2021). In Chile there are two previous records of avian schistosomatids parasitising aquatic birds (i.e., Chilean flamingo [Phoenicopterus chilensis Molina] and C. melancoryphus), but none of these studies included a morphological or molecular characterisation, only the histopathological discoveries from the infected birds (Paré & Black, Reference Paré and Black1999; Oyarzún-Ruiz et al., Reference Oyarzún-Ruiz, Muñoz, Paredes, Valenzuela and Ruiz2019). In Argentina and Brazil, studies have found avian schistosomatids in gulls and penguins (Brant et al., Reference Brant, Loker, Casalins and Flores2017; Vanstreels et al., Reference Vanstreels, Gardiner, Yabsley, Swanepoel, Kolesnikovas, Silva-Filho, Ewbank and Catão-Dias2018; Lorenti et al., Reference Lorenti, Brant, Gilardoni, Diaz and Cremonte2022).
To continue to advance our impoverished understanding of the evolutionary ecology and life history of avian schistosomatids in the Neotropics, this study aimed to use both morphological and molecular features to characterise the avian schistosomatids parasitising waterfowl and freshwater snails in Chile and Argentina.
Material and methods
Sampling and necropsy of waterfowl
The sampling of waterfowl from Chile (Ñuble region, Biobío region, and Los Ríos region) and Argentina (La Pelada, Corrientes province) was performed between January 2019 and January 2021. Five carcasses of C. melancoryphus (died by natural causes) from the Ramsar site Carlos Anwandter Nature Sanctuary, Los Ríos region, Chile, were retrieved following an official permission for research by the state organisation CONAF (Permission number 1201020). Seven wild black-necked swans from different localities from Ñuble and Biobío regions, which were euthanised for humanitarian reasons at Centro de Rehabilitación de Fauna Silvestre, Universidad de Concepción, Chillán, Chile, were also included in this study. In addition, the carcasses of six wild cinnamon teal [Spatula cyanoptera (Vieillot)] from Ñuble region hunted by certified hunters, following the hunting state law Ley de Caza no. 19.473 (SAG, 2018), were also included. For Argentina, one rosy-billed pochard [Netta peposaca (Vieillot)] from La Pelada, Corrientes province, collected during 2013, and two S. versicolor from Estancia Santa Rita, Buenos Aires province, and Gualeguaychú, Entre Ríos province, both collected during 2014, were included as part of this study (Figure 1).
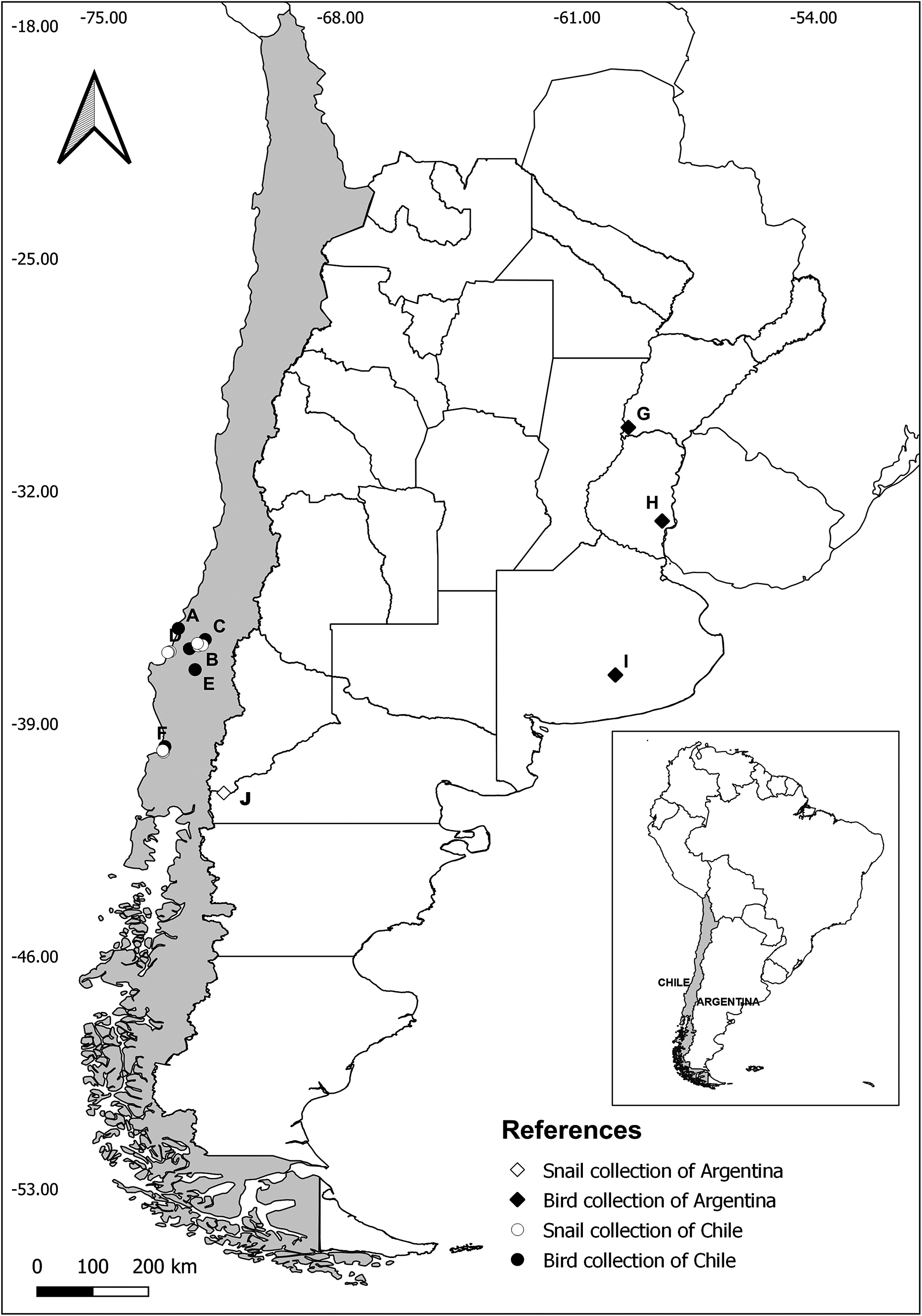
Figure 1. Map of sampled localities in Chile and Argentina. Circles indicate collections from Chile (A-F). Ñuble region: A = Cobquecura; B = Nebuco; C = Ninquihue; D = Quillón; Biobío region: E = Santa Clara; Los Ríos region: F = Carlos Anwandter Nature Sanctuary. Diamonds indicate collections from Argentina (G-J). G = La Pelada Lodge; H = Gualeguaychú; I = Estancia Santa Rita; J = Laguna Fantasma.
For the necropsy, the nasal mucosa and turbinate, heart and associated blood vessels, mesenteric blood vessels and its ramifications, liver, lungs, and kidneys were examined for the presence of avian schistosomatids following Lutz et al. (Reference Lutz, Tkach, Weckstein and Webster2017). The isolated trematodes were relaxed in citrated saline and fixed and preserved in 80% ethanol. Worms for molecular analyses were preserved in absolute ethanol and kept at -20°C (Christiansen et al., Reference Christiansen, Olsen, Buchmann, Kania, Nejsum and Vennervald2016; Kolářová et al., Reference Kolářová, Horák and Skírnisson2010; Horák et al., Reference Horák, Schets, Kolářová, Brant and Liu2012). An additional pool of worms was preserved in 80% ethanol for scanning electron microscopy (SEM) (Oyarzún-Ruiz et al., Reference Oyarzún-Ruiz, Thomas, Santodomingo, Collado, Muñoz and Moreno2022). Small fragments of worms aimed for SEM were previously sliced and preserved for molecular analyses. Parasitological descriptors such as prevalence (P), mean intensity (MI) and mean abundance (MA) were estimated and interpreted following Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997).
Cercarial emergence and dissection of snails
In an attempt to elucidate the life cycle of the avian schistosomatids belonging to clade Q, sensu Brant & Loker (Reference Brant and Loker2009), a total of 1,390 Physa cf. acuta Draparnaud (Physidae) snails were collected between 2019 and 2020 in different freshwater bodies from Ñuble region (n = 351), Biobío region (n = 819), and Los Ríos region (n = 220) in Chile. In addition, during 2015, 10 Physa sp. snails were collected from Laguna Fantasma, Río Negro province, Argentina (Figure 1).
To examine snails for infections, they were pooled in cell culture plates (maximum five snails per well) under artificial light for a 12-hour light:12-hour night period during three consecutive days. Once a well was found with furcocercariae, the snails were arranged in individual wells to identify the ones that were parasitised. When the cercarial emergence period completed, all snails were dissected under stereomicroscope to look for prepatent infections (i.e., presence of sporocysts in the tissues) (Oyarzún-Ruiz et al., Reference Oyarzún-Ruiz, Thomas, Santodomingo, Collado, Muñoz and Moreno2022). The cercariae were identified morphologically following Schell (Reference Schell1985) and Ostrowski de Núñez (Reference de Núñez M and de Castellanos1992).
Because just one snail from Argentina released too few cercariae, all these cercariae had to be preserved in absolute ethanol at -20°C for molecular analyses. Snail shells from Chile were deposited at Museo de Zoología, Universidad de Concepción, Concepción, Chile (accession number MZUC-UCCC 47890). The whole Physa sp. snail from Argentina was deposited at the Museum of Southwestern Biology Division of Parasites, New Mexico, USA (catalog number MSB:Host:21642).
Morphological identification of avian schistosomatids
Adult worms were stained with Alum carmine, dehydrated in increasing concentrations of ethanol (70%-100%), cleared in oil clove, and mounted in Canada balsam (Lutz et al., Reference Lutz, Tkach, Weckstein and Webster2017). Avian schistosomatids were photographed and measured using the software Motic Images Plus 2.0 associated with the light microscope MOTIC BA310. Morphological traits and measurements of these worms were compared with the taxonomic keys and descriptions by McLeod (Reference McLeod1937), McLeod & Little (Reference McLeod and Little1942), Schell (Reference Schell1985), Gibson et al. (Reference Gibson, Jones, Bray, Gibson, Jones and Bray2002), Kolářová et al. (Reference Kolářová, Rudolfová, Hampl and Skírnisson2006), Brant & Loker (Reference Brant and Loker2009), and Flores et al. (Reference Flores, Viozzi, Casalins, Loker and Brant2021). SEM was performed with the scanning electron microscope HITACHI SU 3500 following Oyarzún-Ruiz et al. (Reference Oyarzún-Ruiz, Thomas, Santodomingo, Collado, Muñoz and Moreno2022). Worms were disposed in an ionic solution for a maximum of 4 hours to achieve an appropriate conduction of electrons in the SEM equipment. Because of the equipment specifications, there was no need for bathing the worms in gold. The stub with worms was frozen at -30°C with vacuum sealing inside the cool stage. Values for spot values, variable pressure (VP-SEM), kilovolts (Kv), and pascals (Pa) were stated.
Slides and vouchers of avian schistosomatids were deposited at Museo de Zoología, Universidad de Concepción, Concepción, Chile (accession numbers MZUC-UCCC 47891-47897). Fragments of the avian schistosomatid collected from N. peposaca and Physa sp. from Argentina were deposited at the Museum of Southwestern Biology Division of Parasites (catalog number MSB:Para:23182 and MSB:Para:25363, respectively) (Supplementary Table S1).
DNA extraction, polymerase chain reaction, and phylogenetic analyses
Genomic DNA was extracted using DNeasy blood and animal tissue kit (QIAGEN, Germany) following the manufacturer’s instructions. The quantity and quality of DNA for each extracted sample was measured with a spectrophotometer EpochTM Microplate. Samples with values between 1.6 and 2.0 and an absorbency proportion A260/A280 were considered pure and suitable for amplification through polymerase chain reaction (PCR) (Khare et al., Reference Khare, Raj, Chandra and Agarwal2014). Extracted DNA was kept at -20°C until molecular analyses were performed.
A Touchdown PCR was performed to amplify partial sequences of the cytochrome c oxidase subunit I gene (hereafter COI; expected length of band 600-1,000 bp) and 28S rDNA gene (hereafter 28S; expected length of band 1,500 bp) (Brant & Loker, Reference Brant and Loker2009; Oyarzún-Ruiz et al., Reference Oyarzún-Ruiz, Thomas, Santodomingo, Collado, Muñoz and Moreno2022). Primers were the following: for COI Cox1_schis’_5’ and Cox1_schis’_3’ (Lockyer et al., Reference Lockyer, Olson, Østergaard, Rollinson, Johnston, Attwood, Southgate, Horák, Snyder, Le, Agatsuma, McManus, Carmichael, Naem and Littlewood2003), and CO1F15, CO1R15, and CO1RH3R internal (Brant & Loker, Reference Brant and Loker2009); for 28S U178, L1642, DIG12 internal, and ECD2 internal (Tkach et al., Reference Tkach, Pawlowski and Mariaux2000; Lockyer et al., Reference Lockyer, Olson, Østergaard, Rollinson, Johnston, Attwood, Southgate, Horák, Snyder, Le, Agatsuma, McManus, Carmichael, Naem and Littlewood2003; Olson et al., Reference Olson, Cribb, Tkach, Bray and Littlewood2003). PCR was performed following Dvořák et al. (Reference Dvořák, Vaňáčová, Hampl, Flegr and Horák2002) and Horák et al. (Reference Horák, Schets, Kolářová, Brant and Liu2012): 3 μL of template DNA was added into a mix of 0.3 μL DreamTaq Polymerase (Thermo Fisher Scientific, Waltham, MA, USA), 0.5 μL dNTPs (0.2 mM), 2.5 μL DreamTaq Buffer, 1 μL of each primer (10 pmol), and 16.7 μL of ultra-pure water to achieve a final volume of 25 μL.
PCR thermal conditions were different according to each locus, for COI there were 15 hybridisation cycles at 50°C, 49°C, 48°C, 47°C, and 46°C with three cycles each for 30 seconds. Then 20 cycles of hybridisation at 45°C for 30 seconds. For 28S the protocol considered 15 hybridisation cycles at 55°C, 54°C, 53°C, 52°C, and 51°C with three cycles each for 30 seconds. Then 20 hybridisation cycles at 50°C for 30 seconds. For both loci, extension temperature was at 72°C and denaturation was at 95°C. Amplicons were submitted to electrophoresis in 2% agarose gel, stained with GelRed® (Biotum, Tehran, Iran), and visualised in an ENDUROTM GDS UV transilluminator. Amplicons of expected size were purified and sequenced in both directions at Macrogen (South Korea).
The obtained sequences were verified and edited with Geneious Prime® v. 2021.2.2 to get the consensus sequences. A basic local alignment search was performed with BLASTn tool (https://blast.ncbi.nlm.nih.gov) and similar sequences were downloaded from GenBank (https://www.ncbi.nlm.nih.gov/genbank/) to build multiple alignments with the former software using the MAFFT algorithm (Katoh & Standley, Reference Katoh and Standley2013). Phylogenetic analyses were performed using Bayesian Inference (BI) with the software BEAST v2.5 and substitution model rates were estimated with BEAUTi v2.6.7 (Bouckaert et al., Reference Bouckaert, Vaughan, Barido-Sottani, Duchêne, Fourment, Gavryushkina, Heled, Jones, Kühnert, De Maio, Matschiner, Mendes, Müller, Ogilvie, du Plessis, Popinga, Rambaut, Rasmussen, Siveroni, Suchard, Wu, Xie, Zhang, Stadler and Drummond2019).
The genetic distances between the sequences generated in the present study and sequences from GenBank were estimated using MEGA7 (Kumar et al., Reference Kumar, Stecher and Tamura2016). The sequences obtained in the present study were deposited in the NCBI GenBank database (accession numbers: PP333189-PP333197 for the 28S gene; PP333615-PP333623, PP334484-PP334485 for the COI gene).
Results
Four distinct adult schistosomatid taxa, one nasal and three visceral, were isolated from the anatid hosts examined. From Argentina, N. peposaca (P = 1/1) and S. versicolor (P = 2/2), only a few fragments of visceral schistosomatids were retrieved from their liver, precluding a formal morphological assessment. From Chile, in S. cyanoptera, two taxa were recovered, both visceral; one was a pool of unidentifiable juvenile avian schistosomatids (P = 1/6; MI = 5; MA = 0.83) isolated from liver, and the second was T. querquedulae (P = 6/6; MI, MA = 68.83) that was isolated mostly from the mesenteric vessels of small intestine and viscera, but also the liver and kidneys, heart, caeca and colon mucosa. Morphology and morphometric data of the recovered T. querquedulae (Supplementary Table S2) agreed with the description of McLeod (Reference McLeod1937), McLeod & Little (Reference McLeod and Little1942), and Brant & Loker (Reference Brant and Loker2009). The SEM images of T. querquedulae showed additional putative diagnostic characters that are not included in the generic or specific diagnosis of this T. querquedulae: tegument covered by small spines that alternate with papillae-like structures of greater diameter distributed from the oral sucker to posterior end, irregularly altern on the tegument. On the lateral border of the gynaecophoric canal, papillae-like structures were observed, with several distributed around the genital papilla. The tegument of the ventral surface of the gynaecophoric canal area is corrugated with deep folds over its complete extension, then resumes its spinous surface on body surface. Oral sucker showed triangular spines on the anterior border of oral aperture, which are directed internally, and several circumoral papillae were seen on the anterior border of oral sucker (Figure 2).

Figure 2. Scanning electron microscopy images of Trichobilharzia querquedulae isolated from Spatula cyanoptera from Chile. (A) Oral sucker with small triangular spines on its ventral surface, which are also directed to its aperture (*). Note the small papillae immediately in the posterior border of oral sucker (arrowheads). (B) Dorsal surface of oral sucker with evident papillae at its base (*) as well as on the tegument, immediately posterior to it (arrowhead). (C) Presence of circumoral papillae slightly anterior-dorsal to the border of oral sucker (black arrowheads), also note a pair of dorsal papillae (white arrowhead). (D) Detail of gynaecophoric canal, which is densely covered by small fine spines. Also note the presence of small papillae covering the border of gynaecophoric canal (*). (E) Dorsal surface of gynaecophoric canal densely covered by small, rounded spines and characterised by a notorious folded tegument (arrowheads). (F) Spatulated posterior end with its tegument densely covered by small, rounded spines and papillae randomly distributed on its surface (*).
For the swan host, C. melancoryphus, from Chile, two taxa were recovered. The first was N. melancorhypha (P = 50%, 6/12; MI = 15; MA = 7.5) in the nasal blood vessels of swans from the Ramsar site Carlos Anwandter wetland (Los Ríos) and San Fabián commune (Ñuble); small fragments of this same species (based on genetic data) were retrieved from the kidney of one bird (A92R; Figure 3), which could represent a migrating worm to the nasal blood vessels. The morphology and morphometric data of recovered worms (Supplementary Table S3) agreed with the description by Flores et al. (Reference Flores, Viozzi, Casalins, Loker and Brant2021). From the same locality as described previously, the second schistosomatid taxon was a visceral schistosomatid recovered from the liver of two swans. However, it could not be formally described because only fragments were retrieved from the previously frozen birds. No co-infections between these two avian schistosomatid taxa were recorded.

Figure 3. Phylogenetic 28S gene tree placing the new taxa among the available sequences of avian schistosomatid taxa in GenBank. Specimens from this study are in bold and the clades containing the new taxa are highlighted in gray boxes. The “*” represent significant posterior probability support for the Bayesian analysis, values lower than 0.95 are not indicated. GenBank accession numbers follow the taxon names. To generate the file for BEAST, BEAUTi v2.6.7 was used with Substitution Model GTR rates estimated, substitution rate estimated, for Priors Yule Model default, MCMC chain length 10,000,000, starting tree random, to generate the xml file for BEAST v2.6.4 (Bouckaert et al., Reference Bouckaert, Vaughan, Barido-Sottani, Duchêne, Fourment, Gavryushkina, Heled, Jones, Kühnert, De Maio, Matschiner, Mendes, Müller, Ogilvie, du Plessis, Popinga, Rambaut, Rasmussen, Siveroni, Suchard, Wu, Xie, Zhang, Stadler and Drummond2019). The tree was visualised in FigTree v1.4.4.
All taxa recovered from the hosts belonged to the DAS clade of avian schistosomatids (sensu Brant & Loker, Reference Brant and Loker2013; Ebbs et al., Reference Ebbs, Loker, Bu, Locke, Tkach, Devkota, Flores, Pinto and Brant2022) with robust nodal support (Figure 3). Phylogenetic analysis of BI for both loci supported the morphological identification of T. querquedulae and the placement of Trichobilharzia sp. within clade Q (Figure 4). The worms of T. querquedulae isolated from S. cyanoptera grouped with other conspecific sequences with morphological confirmation and museum vouchers, as a monophyletic clade with robust nodal support for both loci (Figures 3-4). For COI analysis of the larvae and adult fragments, the furcocercariae from Physa sp. W915 from Laguna Fantasma, Argentina, fragments from S. cyanoptera sequence (A61H), and the sequences isolated in S. versicolor from Argentina (W787, W791) were also grouped within the T. querquedulae clade (Figure 4).
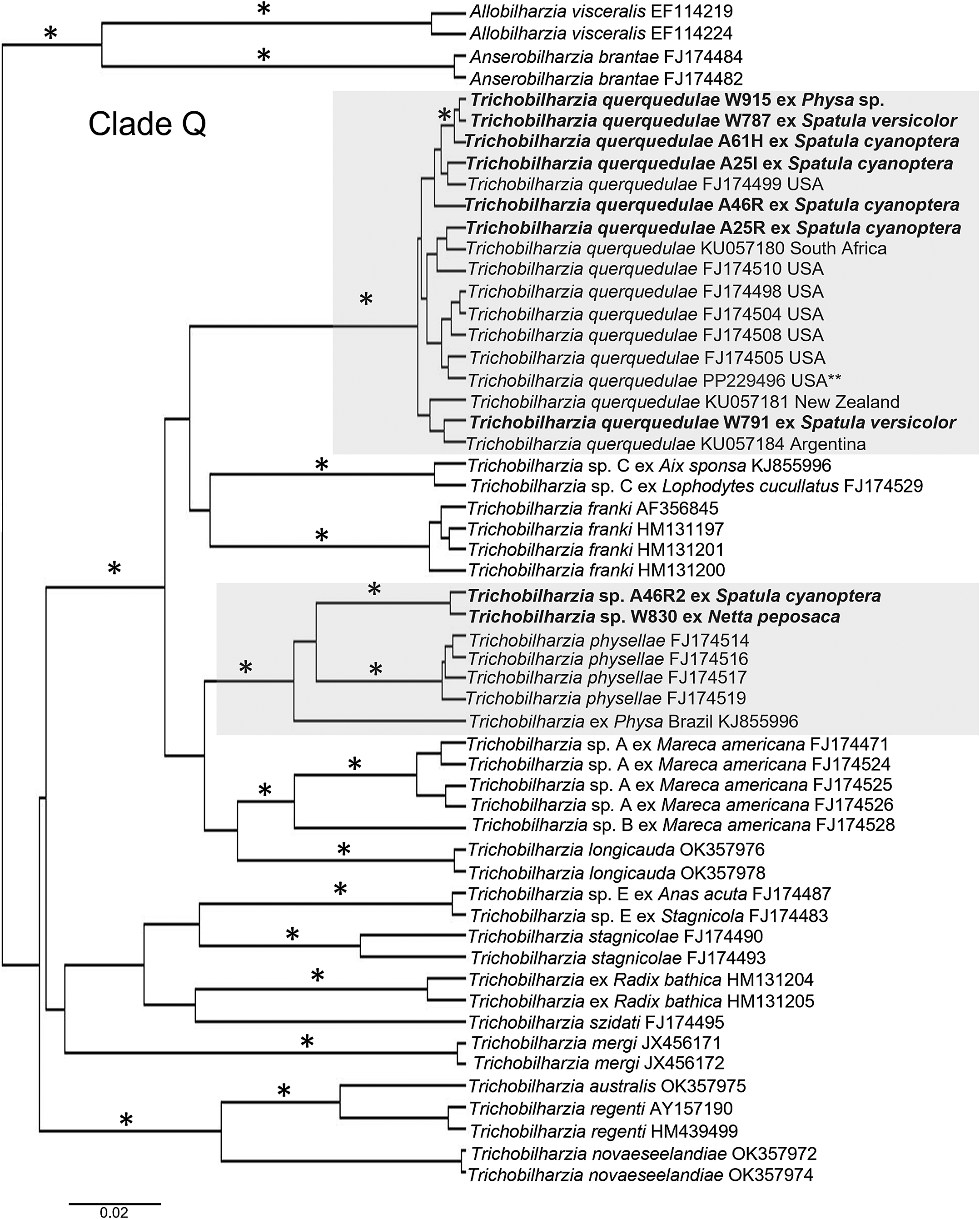
Figure 4. Phylogenetic COI gene tree placing the new taxa among the available sequences of avian schistosomatid taxa in GenBank. Specimens from this study are in bold and the clades containing the new taxa are highlighted in gray boxes. The “*” represent significant posterior probability support for the Bayesian analysis, values lower than 0.95 are not indicated. GenBank accession numbers follow the taxon names. The “**” denotes a sequence from a specimen that was also identified morphologically and is vouchered at Museum of Southwestern Biology (MSB:Para:181). Bayesian inference analysis performed in BEAST v2.6.4 (Bouckaert et al., Reference Bouckaert, Vaughan, Barido-Sottani, Duchêne, Fourment, Gavryushkina, Heled, Jones, Kühnert, De Maio, Matschiner, Mendes, Müller, Ogilvie, du Plessis, Popinga, Rambaut, Rasmussen, Siveroni, Suchard, Wu, Xie, Zhang, Stadler and Drummond2019). To generate the file for BEAST, BEAUTi v2.6.7 was used with Substitution Model GTR rates estimated, substitution rate estimated, for Priors Yule Model default, MCMC chain length 10,000,000, starting tree random. Tree was visualised in FigTree v1.4.4.
According to both 28S and COI analyses, the unidentified avian schistosomatid fragments from S. cyanoptera and N. peposaca did not group with any currently available sequences of morphologically described Trichobilharzia sp. in GenBank. The phylogeny of 28S placed those fragments of Trichobilharzia sp. from N. peposaca as part of a monophyletic clade with a furcocercaria isolated from Stenophysa (= Physa) marmorata (Guilding) snail from Brazil (KJ855994). Furthermore, for COI, the Trichobilharzia sp. from the N. peposaca formed a monophyletic clade with the pool of juvenile worms of Trichobilharzia sp. isolated in a S. cyanoptera from Chile, suggesting they might be conspecifics but await morphological identification (Figure 4). This was also supported by the genetic distances (0.5%; Supplementary Table S5). Unfortunately, we could not get a 28S sequence for the Trichobilharzia sp. from S. cyanoptera.
The sequences of N. melancorhypha formed a monophyletic clade with previously morphologically (also vouchered in a museum) and genetically described individuals for 28S, with a robust nodal support. This clade also includes the sequences of adult worms from the same avian host and furcocercariae of Chilina gibbosa Sowerby snails from Argentina (Flores et al., Reference Flores, Brant and Loker2015, Reference Flores, Viozzi, Casalins, Loker and Brant2021), and furcocercariae isolated from Chilina dombeiana (Bruguière) from Chile (Oyarzún-Ruiz et al., Reference Oyarzún-Ruiz, Thomas, Santodomingo, Collado, Muñoz and Moreno2022). This clade based on genetic data falls basal to the rest of clade DAS, as was shown by Flores et al. (Reference Flores, Brant and Loker2015, Reference Flores, Viozzi, Casalins, Loker and Brant2021) and Oyarzún-Ruiz et al. (Reference Oyarzún-Ruiz, Thomas, Santodomingo, Collado, Muñoz and Moreno2022).
In the 28S phylogenetic analysis, the unidentified taxon, Schistosomatidae gen. sp. A91H (PP333192), isolated from a C. melancoryphus, formed an independent clade with sequences of an unidentified furcocercariae isolated from freshwater snails Chilina spp. from Argentina (Lineage 2) (Flores et al., Reference Flores, Brant and Loker2015) and Chile (Schistosomatidae gen. sp. Lineage II) (Oyarzún-Ruiz et al., Reference Oyarzún-Ruiz, Thomas, Santodomingo, Collado, Muñoz and Moreno2022). This finding represents the first molecular characterisation of the adult worm from its bird host and thus the life cycle is illuminated. Future collections should uncover more adults suitable for a formal morphological description.
A total of 20 furcocercariae morphologically identified to Schistosomatidae were isolated from one Physa sp. (P= 10%, 1/10) collected at Laguna Fantasma, Argentina. No P. cf. acuta from Chile was found parasitised by furcocercariae. Because of the small number of furcocercariae, no morphological characterisation was done because they were used for molecular analyses. The molecular phylogeny showed that these larvae were part of the clade Q (sensu Brant & Loker, Reference Brant and Loker2009), particularly in the well-supported clade of T. querquedulae (Figure 4). The genetic distances for comparative purposes as proxies for how taxa are related, for each gene, are reported and detailed in Supplementary Tables S4-S5.
Discussion
This study represents the first morphological and molecular characterisation of adult avian schistosomatids from Chilean anseriform hosts. A high proportion of the bird species examined were found infected with the three visceral and one nasal schistosomatids. Based on our results in Chile, the geographical distribution of T. querquedulae is extended (from Canada to Patagonia in western hemisphere), N. melancorhypha was isolated in the swan, and both S. cyanoptera and N. peposaca are recorded as additional definitive hosts for species of Trichobilharzia in the Neotropics (see Flores et al., Reference Flores, Brant and Loker2015; Ebbs et al., Reference Ebbs, Loker, Davis, Flores, Veleizán and Brant2016).
Genetically the specimens of T. querquedulae isolated in S. cyanoptera grouped with those from Brant & Loker (Reference Brant and Loker2009) and Davis et al. (Reference Davis, Blair and Brant2022). Morphologically, the specimens were mostly similar to the descriptions of Brant & Loker (Reference Brant and Loker2009) and McLeod (Reference McLeod1937) but there were subtle differences. The specimens from this paper had a longer gynaecophoric canal and seminal vesicle, a wider distance between acetabulum and gynaecophoric canal, and fewer testes (180 vs 210 testes) in comparison to the previously mentioned authors (see Supplementary Table S2). Notwithstanding, this morphological identification was supported by the phylogenetic analyses of both loci, placing our specimens in the T. querquedulae clade (that includes a sequence from a museum voucher that was morphologically identified, GenBank PP229496). Therefore, based on the known data, with published sequences and vouchers from the blue-winged ducks (Spatula spp.) clade such as S. versicolor from Argentina (Ebbs et al., Reference Ebbs, Loker, Davis, Flores, Veleizán and Brant2016), S. discors and S. cyanoptera from the USA (Brant & Loker, Reference Brant and Loker2009; Garvon et al., Reference Garvon, Fedynich, Peterson and Pence2011), and the Australian shoveler [Spatula rhynchotis (Latham)] from New Zealand (Davis et al., Reference Davis, Blair and Brant2022), they are most likely T. querquedulae. Interestingly, previous records from other species of Spatula from the USA (Brant & Loker, Reference Brant and Loker2009), and the recent proposal by Ebbs et al. (Reference Ebbs, Loker, Davis, Flores, Veleizán and Brant2016), stated that T. querquedulae has a cosmopolitan distribution in Spatula spp. The specimens found here represent the first records from Chile, widening its known geographic distribution in the Southern Cone, plus representing the second record and an additional host species in the Neotropics. The high prevalence of T. querquedulae in S. cyanoptera (6/6) and intensity of infection (MA = 68.83) are remarkable similar to what has been reported previously (Brant & Loker, Reference Brant and Loker2009; Davis et al., Reference Davis, Blair and Brant2022).
Szidat (Reference Szidat1951) recorded a visceral Trichobilharzia sp. from S. versicolor collected in Tapalqué, Argentina, which might represent the T. querquedulae mentioned here. However, no morphological description, images, and most importantly, no vouchers exist; thus, new collections are warranted to stablish its specific identity.
The SEM characterisation of T. querquedulae highlighted additional morphological traits that were not mentioned in the original description by McLeod (Reference McLeod1937) and McLeod & Little (Reference McLeod and Little1942) or Gibson et al. (Reference Gibson, Jones, Bray, Gibson, Jones and Bray2002), but that will be useful in future diagnoses. There are only two publications that used SEM features for Trichobilharzia species characterisation. These SEM features include the presence of circumoral papillae in Trichobilharzia australis Blair & Islam, Reference Blair and Islam1983, a nasal avian schistosomatid from Australia (Blair & Islam, Reference Blair and Islam1983). These same papillae distributed over the tegument were also described by SEM images in Trichobilharzia arcuata Islam, Reference Islam1986. These papillae in T. arcuata were restricted between the oral sucker and gynaecophoric canal and thus used as a distinguishing character from T. australis SEM (Islam, Reference Islam1986). SEM is underused for the differentiation of avian schistosomatids; it provides additional diagnostic features to the existing keys (e.g. Blair & Islam, Reference Blair and Islam1983; Islam, Reference Islam1986; Flores et al., Reference Flores, Viozzi, Casalins, Loker and Brant2021; Lorenti et al., Reference Lorenti, Brant, Gilardoni, Diaz and Cremonte2022; Oyarzún-Ruiz et al., Reference Oyarzún-Ruiz, Thomas, Santodomingo, Collado, Muñoz and Moreno2022) for species with few features visible in light microscopy. The present study emphasises the importance to include features visible in SEM for the morphological description of both adult and larval schistosomatids, with an eventual amendment of T. querquedulae at least, but also the genus Trichobilharzia, based on SEM features.
In the COI phylogenetic tree (Figure 4), there are two unidentified clades, Trichobilharzia sp. Brazil KJ855996 (ex physid snail) and two immature specimens from this study (A46R ex S. cyanoptera, W830 ex N. peposaca) that group with but not within T. physellae. The North American T. physellae is most often found in an ecological group of diving ducks (usually Aythya spp.), and cycles through Physa spp. snails (Brant & Loker, Reference Brant and Loker2009; Pinto et al., Reference Pinto, Brant and Melo2014), though a recent record from the snail P. acuta was found in Europe (Helmer et al., Reference Helmer, Blatterer, Hörweg, Reier, Sattmann, Schindelar, Szucsich and Haring2021). Thus far, no genetically or morphologically identified specimens have been found in South America that correspond to T. physellae. One of the specimens collected herein was from a diving duck, N. peposaca, but those worms were immature and did not form a clade with T. physellae or the physid specimen of Trichobilharzia from Brazil. Thus, more specimens from diving ducks are needed to understand the species diversity of Trichobilharzia in these ducks from South America. It would be interesting to show if the clade of Trichobilharzia found here, and that of T. physellae, are exclusive to the ecological clade of diving duck hosts (see Johnsgard, Reference Johnsgard2010), similar to what has been shown for T. querquedulae in Spatula spp.
Clade Q is composed of species of Trichobilharzia that are transmitted by aquatic snails in the families Physidae (T. querquedulae and T. physellae) and Lymnaeidae (Trichobilharzia franki Müller & Kimmig, 1994, Trichobilharzia longicauda [Macfarlane, 1944] Davis, Reference Davis2006, Trichobilharzia regenti Horák, Kolářová & Dvořák, 1998, Trichobilharzia novaeseelandiae Davis & Brant, Reference Davis, Blair and Brant2022, Trichobilharzia sp. A), with most taxa transmitted by lymnaeid snails (Brant & Loker, Reference Brant and Loker2009; Jouet et al., Reference Jouet, Skírnisson, Kolářová and Ferté2010a, Reference Jouet, Skírnisson, Kolářová and Fertéb; Brant et al., Reference Brant, Bochte and Loker2011; Horák et al., Reference Horák, Mikeš, Lichtenbergová, Skála, Soldánová and Brant2015; Ebbs et al., Reference Ebbs, Loker, Davis, Flores, Veleizán and Brant2016; Ashrafi et al., Reference Ashrafi, Sharifdini, Darjani and Brant2021; Davis et al., Reference Davis, Blair and Brant2022). In South America there are three previous reports of physid snails as intermediate hosts of avian schistosomatids, all of which have been recorded from S. marmorata. Those furcocercariae were identified as Cercaria I from Argentina (Ostrowski de Núñez, Reference Ostrowski de Núñez1978), and Trichobilharzia jequitibaensis Leite, Costa & Costa, Reference Leite, Costa and Costa1978 (Leite et al., Reference Leite, Costa and Costa1979) and Trichobilharzia sp. from Brazil (Pinto et al., Reference Pinto, Brant and Melo2014). However, some caution with T. jequitibaensis should be taken as the authors report both a physid and lymnaeid serving as intermediate hosts. In Argentina and Chile there are four native species of Physidae, with P. acuta as the invasive species for both (Valdovinos, Reference Valdovinos2006; Rumi et al., Reference Rumi, Gutiérrez Gregoric, Núñez and Darrigran2008). Physa acuta has been recorded as an intermediate host of T. physellae in its natural distribution in North America (Brant & Loker, Reference Brant and Loker2009; Brant et al., Reference Brant, Bochte and Loker2011), but also recently in Europe, where it is considered invasive (Ebbs et al., Reference Ebbs, Loker and Brant2018; Helmer et al., Reference Helmer, Blatterer, Hörweg, Reier, Sattmann, Schindelar, Szucsich and Haring2021).
The present study represents the first record of T. querquedulae recovered from a physid snail in the Neotropics. Unfortunately, no specific identification was achieved for the snail host, but it has been vouchered in a museum collection (Museum of Southwestern Biology, Division of Parasites, MSB:Host:21642) and is available for specific identification. Availability of physical and curated specimens is particularly important considering the high intraspecific variations for these snails (Cuezzo, Reference Cuezzo, Domínguez and Fernández2009; Collado et al., Reference Collado, Vidal, Aguayo, Méndez, Valladares, Cabrera, Pastenes, Gutiérrez Gregoric and Puillandre2019). Transmission dynamics of Trichobilharzia spp in physids is important, for example, to determine if P. acuta from Chile is refractory to the infection with the native avian schistosomatids from Clade Q (e.g. Stanicka et al., Reference Stanicka, Cichy, Bulantová, Labecka, Ćmiel, Templin, Horák and Żbikowska2022), or if Physa sp. from Argentina is a native species, which might explain the infection despite the small sample size examined. In summary for Clade Q in South America T. querquedulae from Spatula spp. and Physidae, Trichobilharzia sp. (W830/A46R) from Argentina and Chile, together with Trichobilharzia sp. (KJ855994) from Brazil (Pinto et al., Reference Pinto, Brant and Melo2014; Ebbs et al., Reference Ebbs, Loker, Davis, Flores, Veleizán and Brant2016; this study) have been reported.
In addition to the species lineages of Trichobilharzia recovered in Chile, two other taxa of avian schistosomatids were recovered, one nasal and one visceral schistosomatid from C. melancoryphus. The morphology and genetics of the nasal schistosomatid, N. melancorhypha, was in accordance with Flores et al. (Reference Flores, Viozzi, Casalins, Loker and Brant2021), although with some minor differences such as a smaller acetabulum, smaller distance between oral sucker and acetabulum, longer seminal vesicle, and a slightly shorter gynaecophoric canal. The small size of certain structures could be due to how these worms were preserved before observation, for example, the specimens in Flores et al. (Reference Flores, Viozzi, Casalins, Loker and Brant2021) were measured from hot 5% formaldehyde fixed material. Notwithstanding the previous information, both phylogenetic analyses of 28S and COI supported the morphological diagnosis as N. melancorhypha. The confirmation of N. melancorhypha parasitising C. melancoryphus, together with the recent description of its furcocercariae from C. dombeiana (Oyarzún-Ruiz et al., Reference Oyarzún-Ruiz, Thomas, Santodomingo, Collado, Muñoz and Moreno2022), make this taxon the first avian schistosomatid with its life cycle elucidated in Chile.
The finding of N. melancorhypha extends its geographic distribution, originally from Argentina (Flores et al., Reference Flores, Viozzi, Casalins, Loker and Brant2021), to Central and Southern Chile, maintaining its monotypic status. This is noteworthy because its intermediate host, snails of genus Chilina Gray, and its definitive host, C. melancoryphus, are both endemic to South America (Cuezzo, Reference Cuezzo, Domínguez and Fernández2009; Fuentealba et al., Reference Fuentealba, Figueroa and Morrone2010; Johnsgard, Reference Johnsgard2010), suggesting the life cycle is restricted to the distribution of Chilina spp. Cygnus melancoryphus is the only native swan in the Neotropics (Johnsgard, Reference Johnsgard2010), which might limit the possibility of finding N. melancorhypha in other anatids. This is in contrast with Allobilharzia Kolářová, Rudolfová, Hampl & Skírnisson, Reference Kolářová, Rudolfová, Hampl and Skírnisson2006, which exclusively parasitises swans (Cygnus spp.) in the Northern hemisphere, from North America, Europe, and Japan (Kolářová et al., Reference Kolářová, Rudolfová, Hampl and Skírnisson2006; Brant, Reference Brant2007; Hayashi et al., Reference Hayashi, Ichikawa-Seki, Ohari, Mohanta, Aita, Satoh, Ehara, Tokashiki, Shiroma, Azuta, Oka, Watanabe, Harasawa, Inohana, Ichijo and Furuhama2017).
There was an unexpected finding of N. melancorhypha fragments in the kidneys of one of the swans (A92R), with the genetic identification confirmed through phylogenetic analysis. Although the migration route of juvenile worms for this nasal species remains unknown, this observation could represent a systemic migration of the worms. In contrast, T. regenti, another nasal schistosomatid, migrates exclusively through the nervous system before reaching the preferred nasal tissue (Horák et al., Reference Horák, Schets, Kolářová, Brant and Liu2012; Prüter et al., Reference Prüter, Sitko and Krone2017). The explanation of this finding herein requires additional samplings and experimental studies to trace the migration of the schistosomules.
In addition to the finding of the nasal schistosome in C. melancoryphus, a visceral avian schistosomatid was isolated; Schistosomatidae gen. sp. Unfortunately, because of the poor state of the worm fragments from the host, they could not be described properly. Until a morphological assessment is performed genetically, this undescribed visceral schistosomatid phylogenetically groups within the DAS clade. Interestingly, Schistosomatidae gen. sp. grouped in a clade with the undescribed Lineage 2/II of furcocercariae isolated from Chilina fulgurata Pilsbry, Chilina perrieri Mabille and C. gibbosa from Argentina (Flores et al., Reference Flores, Brant and Loker2015), and C. dombeiana from Chile (Oyarzún-Ruiz et al., Reference Oyarzún-Ruiz, Thomas, Santodomingo, Collado, Muñoz and Moreno2022). These results suggest they are likely conspecific and represent a new Neotropical visceral schistosomatid with C. melancoryphus as the definitive host for the furcocercariae identified as Lineage 2/II from Flores et al. (Reference Flores, Brant and Loker2015) and Oyarzún-Ruiz et al. (Reference Oyarzún-Ruiz, Thomas, Santodomingo, Collado, Muñoz and Moreno2022), representing another endemic taxon to the Neotropics.
Considering that most visceral schistosomatids are typically found in the venous system of birds, spreading through parenchymatous organs such as the liver and mesenteric vessels (Horák et al., Reference Horák, Mikeš, Lichtenbergová, Skála, Soldánová and Brant2015; Ashrafi et al., Reference Ashrafi, Sharifdini, Darjani and Brant2021), the schistosomatids reported by Oyarzún-Ruiz et al. (Reference Oyarzún-Ruiz, Muñoz, Paredes, Valenzuela and Ruiz2019) in C. melancoryphus from Southern Chile might also correspond to Schistosomatidae gen. sp. within Lineage 2/II (Figure 3). In the study herein, the specimens of Schistosomatidae gen. sp. were not ideal because of the poor state of preservation of the hosts, which disrupts the integrity of these digeneans for morphological assessment (Lutz et al., Reference Lutz, Tkach, Weckstein and Webster2017). The necropsy of swans recently salvaged or euthanised would offer better material for the morphological description of this undescribed schistosomatid.
Future efforts to classify avian schistosomatids from the Neotropics should consider not only collecting non-studied waterfowl but also other aquatic avian groups that have not been properly considered such as Suliformes, Charadriiformes, Phoenicopteriformes, and Passeriformes (especially those inhabiting wetlands) (Brant, Reference Brant2007; Horák & Kolářová, Reference Horák and Kolářová2011; Flores et al., Reference Flores, Brant and Loker2015; Horák et al., Reference Horák, Mikeš, Lichtenbergová, Skála, Soldánová and Brant2015; Lorenti et al., Reference Lorenti, Brant, Gilardoni, Diaz and Cremonte2022). Even though these groups of birds have been reported as definitive hosts of avian schistosomatids, several of these records have no phylogenetic work, vouchers to reexamine, or genetic data (Horák & Kolářová, Reference Horák and Kolářová2011). Using an integrative approach, Lorenti et al. (Reference Lorenti, Brant, Gilardoni, Diaz and Cremonte2022) described two new genera from two common South American gulls and discussed the entangled systematics of the globally, but poorly understood, schistosomatid genus Gigantobilharzia Odhner. Taking into consideration that Argentina and Brazil concentrate most of the knowledge regarding South American avian schistosomatid diversity (see Pinto et al., Reference Pinto, Brant and Melo2014, 2017; Flores et al., Reference Flores, Brant and Loker2015, Reference Flores, Viozzi, Casalins, Loker and Brant2021; Brant et al., Reference Brant, Loker, Casalins and Flores2017; Lorenti et al., Reference Lorenti, Brant, Gilardoni, Diaz and Cremonte2022), there is a clear need to replicate these efforts in neighboring countries to enhance the understanding of these neglected parasites not only from the biodiversity point of view, but also for the whole understanding of their evolutionary relationships. In addition, we need researchers to deposit museum vouchers for re-examination that is critical to replicating past studies and clarifying authors hypotheses. Expanding collections to other southern hemisphere continents to look at how biogeography and host use has shaped the evolutionary history of these avian schistosomatids, relative to what we know about their distribution in the northern hemisphere (Pinto et al., Reference Pinto, Pulido-Murillo, de Melo and Brant2017, Reference Pinto, Tenório Mati, Melo and Brant2022) is critical.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0022149X2400035X.
Acknowledgements
The authors are grateful to CONAF for permission to collect the swans from Carlos Anwandter Nature Sanctuary (permission number 1201020). To J. Navedo and Centro de Rehabilitación de Fauna Silvestre (Universidad de Concepción) who provided us with some carcasses of black-necked swans for this study, and to the personnel of San Pedro de la Paz municipality, Biobío region, and Cendyr Náutico who allowed us to collect the snails at Laguna Chica. The authors are also grateful to C. Silva-de la Fuente who assisted us with the use of SEM equipment and F. Castro and Ll. Rodríguez for providing us the Epoch™ Microplate Spectrophotometer. In addition to N. Martin, V. Aravena, N. Lizama, N. Inostroza, and P. Muñoz who supported us with field collections and laboratory analyses.
Financial support
This study was funded by ANID Doctorado Nacional fellowship (grant number 21181059) and FONDECYT Postdoctorado fellowship (grant number 3230461) (P. O.-R).
Competing interest
The authors declare no competing interests.
Ethical standard
This study was approved by the Bioethical committee of Facultad de Ciencias Veterinarias, Universidad de Concepción (number CBE-16-2020).






