Introduction
Oilseed rape production has increased all around the world in the past 10 years (USDA, 2011), especially in Europe (30% of the worldwide production) where this crop protects against water pollution by nitrates by covering soils during the winter season. The increasing demand for biofuel and industrial oils has resulted in a further strong increase in production of this species, by up to almost 70% in Europe since 2003. More than half of the European production is distributed between Germany and France (Eurostats, 2010). In seed production, France is the top European producer with a positive seed turnover and exports which place it third in the world (ISF, 2009). Export of oilseeds makes up 12% of total seed exports, behind maize and vegetable seeds (GNIS, 2009).
Oilseed rape, particularly winter rape, needs fast seed quality testing because of the short time period between harvest and the next sowing. Due to its importance, Groupe d'Etude et de Contrôle des Variétés et Semences (GEVES) has allocated 8% of its research programmes since 1999 to oilseed rape for both varieties and seeds. Developing better, more reliable and efficient, and less expensive tests is one of the tasks of GEVES Research and Development on seeds. The first studies on oilseed rape were dedicated to understanding how and when seed quality was established on the mother plant. This work monitored seed quality during the maturation stage and, as previously reported for other crops (Bailly et al., Reference Bailly, Audigier, Ladonne, Wagner, Coste, Corbineau and Côme2001), the onset of the ability to germinate and to tolerate desiccation were investigated in relation to abscisic acid (ABA) accumulation (Juricic et al., Reference Juricic, Orlando and Le Page-Degivry1995). For seed producers it was important to identify stages of heterogeneity in seed quality and to anticipate optimum harvest time to achieve high seed quality. The genetic and physiological potential of seeds, interacting with environmental factors prior to or during storage, both determine seed longevity (Bewley and Black, Reference Bewley and Black1994). The main source of quality loss is due to seed ageing, to which oilseeds are particularly susceptible, since their major constituent is lipids, which increase the seeds' predisposition to ageing (Priestley, Reference Priestley1986). Consequently, GEVES programmes on oilseed rape were aimed at maintaining high seed quality and to develop new seed testing methods to provide information in addition to germination analyses performed under ISTA (International Seed Testing Association) international rules.
Oilseed rape seed development and maturation
Changes in oilseed rape (Brassica napus L.) quality were monitored during seed development and maturation in 2-year field and glasshouse experiments. The onset of the ability to germinate and to tolerate desiccation was investigated in relation to ABA accumulation. Two morphologically different cultivars, an apically dominant one and a bushy type, were studied in this work. Samples of seeds were harvested by hand at different levels from the plants, once a week in the field and every 4 d in the greenhouse, between days 30 and 90 after flowering. Comparison of development between varieties and years of production was monitored with a temperature scale based on the first flowering of the primary raceme for 50% of the plant population (Fig. 1). ABA accumulation (Le Page-Degivry et al., Reference Le Page-Degivry, Duval, Bulard and Delaage1984) was observed at 700–800 degree days after first flowering (DAF) for both cultivars, the delay between them being related to their flowering precocity. Mass maturity (end of the seed-filling phase) was obtained by fitting seed dry weight during development (Fig. 1b) and occurred between 900 DAF and 1000 DAF. Seed moisture contents (wet basis) at this stage were about 45%, while the onset of germinability occurred 200 DAF prior to mass maturity. The ability to germinate was acquired before the end of the seed filling and required no loss in water. However maximum germination after desiccation was obtained in the latest stages of development: 1100–1200 DAF.

Figure 1 Seed moisture content (a), mean seed dry weight (b), ABA content (c), ability to germinate after seed drying (d) and total germination (e) for two cultivars: apical dominant (diamonds) and bushy type (circles). Seeds were collected weekly during their development in the field in 1998. Means of germination and ABA content are represented ± SD. (A colour version of this figure can be found online at http://journals.cambridge.org/ssr).
Subsequent programmes on oilseed rape produced in the field began with harvest around 900 DAF, when mass maturity was completed for seeds, whatever the cultivar. Collaborations with seed physiologists allowed adding some biochemical tests to the global characterization of seed quality. Among them, a number of late embryogenesis abundant proteins (Duval et al., Reference Duval, Dehaye, Alban, Derose, Douce, Job, Job, Ellis, Black, Murdoch and Hong1997) and enzymes involved in oxidative stress (Bailly, Reference Bailly2004) were studied.
Seed ageing markers for biochemical seed testing
Biotinylated late embryogenesis abundant (LEA) proteins have been described in many species as seed-specific proteins (Job et al., Reference Job, Laugel, Duval, Gallardo and Job2001). They appear during seed development together with the acquisition of desiccation tolerance and are accumulated only in seeds. They disappear quickly when seeds germinate (Alban et al., Reference Alban, Job and Douce2000). They can be detected quite easily owing to the fact that they covalently bind biotin, thereby enabling their detection by avidin–biotin technology. Hence, biotinylated LEA proteins were considered potential candidates for seed quality markers. They were studied over 2 years in oilseed rape during seed development and maturation. The accumulation of biotinylated LEA proteins was concomitant with desiccation tolerance and highly correlated with the maturity of seeds sorted by chlorophyll fluorescence and with the storage potential of seed lots, as determined by controlled deterioration. Thus, the overall results of this study have provided new seed quality markers for oilseed rape with such biotinylated LEA proteins. These proteins can be monitored routinely through an enzyme-linked immunosorbent assay (ELISA), using streptavidin conjugated to peroxidase, and its robustness, sensitivity and simplicity make it an interesting assay for further development in seed testing laboratories. Work is still in progress due to the necessary introduction of a control sample to calibrate serial assays when several seed lots have to be compared. Determination of a threshold for the relative abundance of seed biotinylated proteins is in progress in order to rank seed lots in two or three quality classes.
Over the same period as the above work, seed ageing was widely studied in sunflower seeds (Bailly et al., Reference Bailly, Leymarie, Rousseau, Côme, Feutry, Corbineau, Nicolas, Bradford, Côme and Pritchard2003) through scavenging of reactive oxygen species (ROS) by antioxidant enzymes, or lipid deterioration (Vertucci and Roos, Reference Vertucci and Roos1990). Since lipids are important storage compounds in rapeseed, the role of antioxidant enzymes in seed quality (Bailly et al., Reference Bailly, Bogatek-Leszczynska, Côme and Corbineau2002) was investigated a potential marker of seed quality. This was completed using two kinds of material: seed samples with quality adjusted in the laboratory, using a controlled deterioration test; and seed lots of various ages provided by seed companies and stored under two conditions (control: 10°C, relative humidity (RH) 50%; unfavourable storage: 30°C, 75% RH). Among the antioxidant enzymes studied in two cultivars, catalase gave the best predictive results for oilseed rape germination after ageing in both artificial and storage deterioration. These results are relevant to recent work showing that molecular events occurring in artificial ageing or natural ageing are similar in Arabidopsis thaliana (Rajjou et al., Reference Rajjou, Lovigny, Groot, Belghazi, Job and Job2008).
Vigour testing and germination monitoring
In the 1990s our lab was involved in several ISTA working groups on vigour testing in order to standardize some seed vigour methods and to incorporate them in the ISTA rules (Matthews and Powell, Reference Matthews and Powell2005). The conductivity test is the quickest, based on solute leakage from both living and dead cells (Matthews and Powell, Reference Matthews and Powell2006), and we have attempted to extend its use to several crops with varying chemical seed composition. Oilseed rape was the first among the main French crops to give encouraging results with leakage measurements. A good correlation was obtained with the standard germination test (Fig. 2) and, hence, seed lot quality can be checked within a shorter time frame. An international comparative test has validated the conductivity method for rape (Wagner and Ducournau, Reference Wagner and Ducournau2007), but further experiments have to be performed in order to obtain correlation with field emergence or seed storage ability and to determine partition values for interpretation of conductivity results.
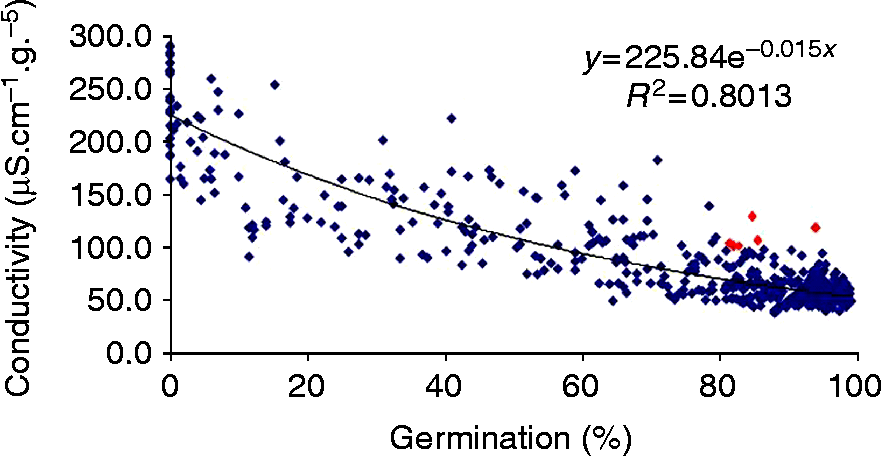
Figure 2 Correlation between standard germination test of oilseed rape (ISTA, 2011) and conductivity test. Data of 520 samples analysed from 2002 to 2010 at the French seed testing station. (A colour version of this figure can be found online at http://journals.cambridge.org/ssr).
Another method to measure seed vigour is to determine rate and uniformity of seed germination and seedling growth. This has been achieved using a prototype computer imaging system described by Ducournau et al. (Reference Ducournau, Feutry, Plainchault, Revollon, Vigouroux and Wagner2004, Reference Ducournau, Feutry, Plainchault, Revollon, Vigouroux and Wagner2005), with a single camera above a Jacobsen table. The complete monitoring system has been improved to study imbibition, germination and the first step of seedling elongation of 20 species, including oilseed rape. Two Jacobsen incubators were exposed to four colour cameras each in order to automate germination time courses for a large number of seeds (up to 400 per camera).
In order to compare controlled deterioration methods in oilseed rape, a large screening with this computer video equipment was carried out to select 15 samples from the same range of vigour. The germination time courses of 42 seed lots were compared and the related parameters, such as percentiles, mean germination time (MGT), final germination, etc., were obtained with a high precision due to the high frequency of images. The older samples displayed the longer germination times and the highest heterogeneity (Table 1). Nevertheless, despite being 7 years old, two seed lots from the same cultivar harvested in 2002 (A5 and A6, in bold in Table 1) germinated as rapidly and homogeneously as more recent seed lots. The germination monitors are used to answer seed companies' requests and to rank seed lots according to their rate and uniformity of germination, but they are also useful for seed research to phenotype mutants or genotypes during this first stage of plant development (Joosen et al., Reference Joosen, Kodde, Willems, Ligterink, van der Plas and Hilhorst2010). The tools have already been used for phenotyping a model crop for its establishment, and some quantitative trait loci related to germination and heterotrophic growth have been determined (Menna-Barreto-Dias et al., 2011).
Table 1 Main characteristics obtained at 20°C for 42 samples of oilseed rape. Seed lots with the same letter are from the same cultivar. Values are means of 50 seeds ± standard error of the means (SEM); the general mean is of the 42 lots ± standard deviation (SD)
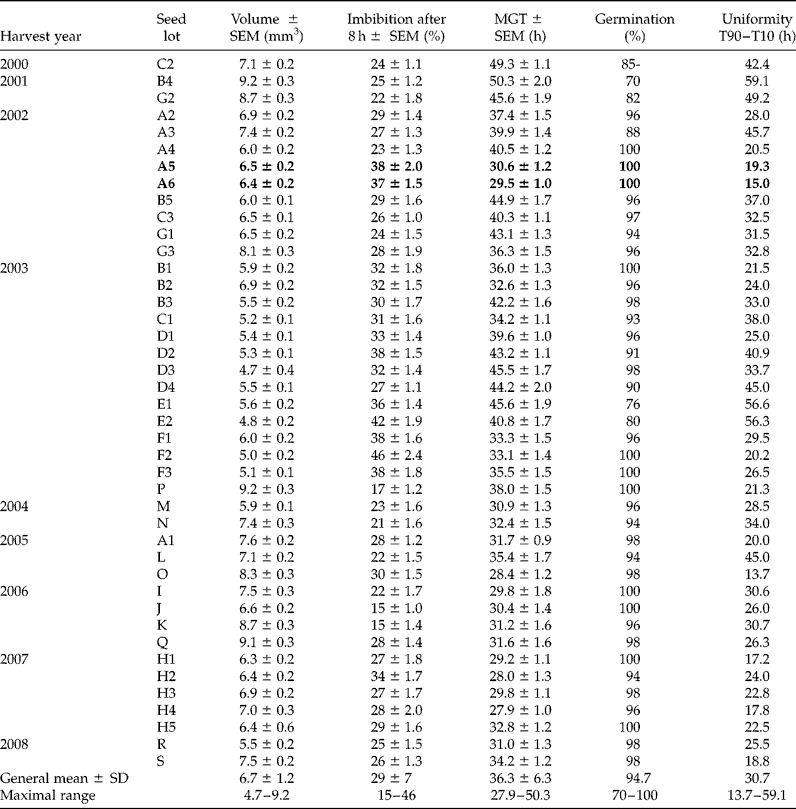
MGT, mean germination time.
In the experiments presented here tools have been developed for analysing seed quality, which are faster, more reliable and less empirical than the existing tools. They have also contributed to an increased understanding of the seed physiology of oilseed rape. Evidently, the testing methods based on seed markers need further development before becoming routine. In addition, seed science in progress will provide new approaches to seed testing.
Acknowledgements
We would like to acknowledge Dr Alison Powell, University of Aberdeen for her helpful review of this paper. As methodological research in our laboratory is carried out through much collaboration, we are grateful to many people. Chronologically, we thank Elisabeth Le Page-Degivry and Ginette Garello (Université de Nice), the first to join us and work on rapeseed. Then we collaborated with Dominique and Claudette Job (CNRS), Olivier Leprince and Pascale Satour (Agrocampus, Université d'Angers), Christophe Bailly and Françoise Corbineau (Université Pierre et Marie Curie) and, more recently, Nathalie Nesi and Michel Renard (INRA), we thank all of them. We have always worked with seed companies from which some people are also to be thanked: Bernard Baduffle, Armand Feutry, Elisabeth Lasserre, Anne-Sophie Lory, Isabelle Pauchet and François Poutoire. Our colleagues from GEVES or INRA whom we enjoy working with: Didier Demilly, Carolyne Dürr, Marie-Claire Gâtineau and Lydie Ledroit. Finally, we proudly remember Professor D. Côme who transmitted to us his passion for seed science. The various research programmes were carried out with financial support from the Ministère de l'Agriculture, de l'Alimentation, de la Pêche, de la Ruralité et de l'Aménagement du territoire, and the forthcoming international project ANR-10-KBBE-002 with public funds allocated to research and development from Agence Nationale de la Recherche (ANR).





