Moderate reductions in food/energy ingestion (20–40 %) have been widely associated with increases in lifespan and reductions in the risks of ageing-related chronic disease in animal models( Reference Bordone and Guarente 1 ). A reduction in energy intake promotes several metabolic responses related to substrate mobilisation mediated by hormonal alterations that are well characterised in energy restriction (ER), such as decreased body fat, inflammation and oxidative stress, and increased insulin sensitivity( Reference Bordone and Guarente 1 ). In human subjects, studies of ER have indicated improvements in surrogate markers of ageing, such as glycaemia, blood pressure and cholesterol concentrations( Reference Walford, Mock and Verdery 2 , Reference Heilbronn and Ravussin 3 ). It has been postulated that physiological changes elicited by ER contribute towards a condition of robust health, and that these same changes may trigger greater longevity in certain species( Reference Bordone and Guarente 1 ).
ER also modulates the cellular redox balance. Rats under ER have been reported to show diminished production of mitochondrial reactive oxygen species (ROS) in several tissues( Reference Gredilla, Sanz and Lopez-Torres 4 – Reference Sanz, Caro and Ibanez 6 ). The reduction in mitochondrial electrochemical membrane potential may be one mechanism responsible for the decreased production of ROS in rats under ER( Reference Speakman and Mitchell 7 ), as this would lead to an acceleration of respiration rates. Another antioxidant mechanism of ER is the increase in mitochondrial biogenesis and consequent higher electron transport efficiency( Reference Guarente 8 ).
A widely used ER protocol is obtained by fasting animals every other day, which is also called intermittent fasting (IF). Several studies have demonstrated that IF decreases the risk factors of CVD and diabetes in human subjects and rodents( Reference Varady and Hellerstein 9 ), reduces the production of liver mitochondrial reactive oxygen in mice( Reference Caro, Gomez and Lopez-Torres 10 ) and increases the lifespan of rodents( Reference Martin, Mattson and Maudsley 11 ).
It has previously been demonstrated by our group that LDL-receptor knockout mice, a model of familial hypercholesterolaemia, show tissue mitochondrial oxidative stress( Reference Oliveira, Cosso and Alberici 12 ) and glucose intolerance( Reference Bonfleur, Vanzela and Ribeiro 13 ). Furthermore, these mice show hypertension( Reference Trieu and Uckun 14 ). Therefore, in the present study, we hypothesised that IF-induced ER would ameliorate metabolic disturbances in LDL-receptor knockout mice, and reduce susceptibility to atherosclerosis in this context.
Experimental methods
Animals
LDL-receptor knockout mice and control wild-type mice (C57BL6/J) founders were purchased from Jackson Laboratory. The animal experiments were approved by the University's Committee for Ethics in Animal Experimentation (protocol no. 1969-1; CEUA/UNICAMP). The animals had free access to a standard laboratory rodent chow diet (Nuvital CR1; Quintia, S.A.), and were housed at 22 ± 1°C on a 12 h light–12 h dark cycle. The IF protocol used was an every-other-day fasting regimen in which male mice had free access to food only on alternate days over 12 weeks from 8 weeks of age.
Plasma biochemical analysis
Blood samples were collected from either the retro-orbital plexus or the tail tip of anaesthetised mice. Unless specified, blood samples were obtained after 12 h of fasting. Total cholesterol, TAG and NEFA concentrations were measured in fresh plasma samples using standard commercial kits (Roche-Hitachi® and Wako®). Glucose levels were measured using a hand-held glucometer (Accu-Chek Advantage; Roche Diagnostic). Plasma insulin, leptin and adiponectin (EMD Millipore Corporation), TNF-α (eBioscience, Inc.) and C-reactive protein (Immuno-Biological Laboratories, Inc.) levels were measured by ELISA. Plasma total antioxidant capacity was determined by an enzymatic assay (Cayman Chemical Company). Plasma lipoprotein levels were determined by fast protein liquid chromatography (Amersham-Pharmacia Biotech)( Reference Jiao, Cole and Kitchens 15 ).
Body composition analysis
Mice as well as their food intake were weighed once per week. The epididymal adipose tissue and liver mass of mice were determined gravimetrically. Carcass composition of mice was determined as previously described in detail by Salerno et al. ( Reference Salerno, Silva and Amaral 16 ). Liver lipids were extracted using the Folch method( Reference Folch, Lees and Sloane Stanley 17 ). Liver cholesterol and TAG contents were determined using colorimetric enzymatic assays (Roche-Hitachi®) after dissolving the lipid extracts in a triton-containing buffer.
Liver VLDL-TAG secretion
After 12 h of fasting, a base line blood sample was harvested from the tail tip of mice. Then, mice received an intraperitoneal injection of Triton WR 1339 (Sigma), 500 mg/kg in saline solution, to inhibit lipoprotein lipase activity and TAG hydrolysis and clearance. At 2 h after the injection of Triton, another blood sample was obtained to determine TAG levels. VLDL-TAG secretion was calculated by the difference in TAG concentrations between values at 2 h and baseline. In order to measure the rate of VLDL-TAG secretion during the fed state, an oral glucose load (1·5 g/kg) was given to 12 h fasted mice 30 min before the administration of Triton.
Adipocyte isolation
Adipocytes of mice were isolated using modifications of the established protocol for rat adipocytes( Reference Rodbell 18 ). Briefly, subcutaneous fat samples were cut into small pieces, and the fragments were digested at 37°C with collagenase II (1 mg/ml; Sigma-Aldrich) in Krebs–Ringer bicarbonate buffer containing fatty acid-free albumin (KRBA, 3 %) and glucose (6 mm) at pH 7·4. After 45 min of incubation under continuous vigorous shaking, the fat cells were filtered through a nylon mesh and washed three times with KRBA to eliminate the stroma-vascular fraction and collagenase. The cells were counted in a Neubauer chamber. Adipocyte area (μm2) was analysed (at least 100 adipocytes per mice) with software ImageJ (1.45h; National Institutes of Health).
Oral glucose tolerance and insulin tolerance tests
For the oral glucose tolerance test, mice were fasted for 12 h, and blood samples were harvested before and after an oral glucose load of 1·5 g/kg body weight. For the insulin tolerance test, blood samples of the fed mice harvested before and after an insulin injection (intraperitoneal) of 3·5 pmol/kg body weight (Biohulin®R; Biobrás)( Reference Bonfleur, Vanzela and Ribeiro 13 ).
In vivo CO2 production rates
Mice were adapted to the respirometer chamber for 5 d. After the adaptation period, CO2 production of each mouse was measured in a temperature-monitored respirometer( Reference Alberici, Oliveira and Patricio 19 ). CO2 expiration was quantified during a period of 5 min once per d, between 09.00 and 11.00 hours, for 3 d. The production rate of CO2 of each mouse was calculated as the average of the 3 d measurements.
VLDL oxidative susceptibility
Plasma VLDL fractions were obtained from 12 h fasting mice by ultracentrifugation (d< 1·006, 50 min, 140 000 rpm, 16 °C, micro-ultracentrifuge Hitachi model CS150GXL; Hitachi Koki Co., Ltd), and used in the assay on the basis of TAG concentrations (100 μg/ml). CuSO4-induced oxidation (40 μm, 37 °C) was measured by detecting the formation of conjugated dienes at 234 nm over time (Spectrophotometer Fusion™; Packard BioScience Co.)( Reference Hau, Smelt and Bindels 20 ).
Liver protein carbonyl content
zLiver protein carbonyl content was estimated according to Reznick & Packer( Reference Reznick and Packer 21 ), as modified by Schild et al. ( Reference Schild, Reinheckel and Wiswedel 22 ). Liver homogenate samples were treated with 10 mm-dinitrophenylhydrazine in 2·5 m-HCl for 1 h at room temperature. The reaction was stopped by the addition of 20 % TCA. The pellets were washed twice with absolute ethanol–ethyl acetate (1:1) and once with 10 % TCA. The protein pellets were dissolved in 6 m-guanidine hydrochloride, and absorption at 370 nm was determined. Carbonyl content was calculated using the molar absorption coefficient of aliphatic hydrazones of 0·022/μm per cm.
Isolation of mouse liver mitochondria
Mitochondria were isolated by conventional differential centrifugation at 4°C( Reference Kaplan and Pedersen 23 ). Isolated mitochondria were kept over ice and used within 90 min of preparation.
Reactive oxygen species production
ROS production by mitochondria was monitored using the membrane-permeable fluorescent dye 2′,7′-dichlorodihydrofluorescein diacetate according to Garcia-Ruiz et al. ( Reference Garcia-Ruiz, Colell and Mari 24 ). The rate of H2O2 produced by isolated mitochondria was determined by measuring the conversion of Amplex red (Molecular Probes, Invitrogen), in the presence of horseradish peroxidase, to highly fluorescent resorufin( Reference Paim, Velho and Castilho 25 ).
Histological analysis of atherosclerosis
The heart of anaesthetised mice was perfused in situ with PBS followed by 10 % PBS-buffered formaldehyde. The heart samples were then embedded in the Tissue-Tek® OCT compound (Sakura) and frozen at − 50 °C. Oil red O stainings were performed according to Paigen et al. ( Reference Paigen, Morrow and Holmes 26 ). The lipid-stained lesions were quantified, as described by Rubin et al. ( Reference Rubin, Krauss and Spangler 27 ), using software ImageJ (1.45h; National Institutes of Health) for image analysis. The slides were read without group identification (blind). The areas of lesions were expressed as the sum of lesions in six 10 μm sections, 80 μm apart, along a total aorta length of 480 μm. The segment chosen for analysis started at the aortic sinus.
Statistical analysis
Results are presented as means with their standard errors. Comparisons between the groups were analysed by unpaired Student's t test and correlation analyses by Spearman's r test. The level of significance was set at P≤ 0·025.
Results
Body weight and food intake were measured weekly during a period of 3 months in hypercholesterolaemic LDL-receptor knockout mice under IF (KO-IF) or fed ad libitum (KO-AL). As expected, the KO-IF mice showed a reduction of 20 % in cumulative food intake (263 (sem 2·3) v. 326 (sem 3·3) g, P< 0·0001). Weight gain was reduced by 20 % (3·6 (sem 0·4) v. 4·5 (sem 0·3) g, P= 0·043), and food efficiency (weight gain/food intake × 100) (1·39 (sem 0·15) v. 1·42 (sem 0·08)) was similar between the groups.
Possible parallel changes in body metabolism were evaluated indirectly by measuring CO2 production in vivo, since the IF regimen changes the feeding behaviour. The KO-IF mice showed marked variations in CO2 production rates (g CO2/kg per h) during the fasting and fed days. Compared with the KO-AL mice (12·4 (sem 0·23)), the KO-IF mice showed a 22 % decrease (9·7 (sem 0·25), P< 0·0001) and 10 % increase (13·7 (sem 0·45), P= 0·02) in CO2 production rates during the fasting and fed days, respectively. The KO-IF mice exhibited changes in their body metabolic rates by 40 % between the fasting- and fed-day measurements.
The IF regimen induced changes in plasma lipids and cholesterol distribution in plasma lipoproteins, which are shown in Table 1. The KO-IF mice showed a marked elevation of 37 % in total plasma cholesterol, corresponding to increases of 195 % in VLDL-cholesterol and 50 % in LDL-cholesterol. The plasma levels of HDL-cholesterol, TAG and NEFA were similar between the KO-IF and KO-AL mice (Table 1). The rates of VLDL-TAG secretion in the fasted state were similar between the KO-AL and KO-IF groups (8·2 (sem 1·5) v. 7·6 (sem 0·6) mg/l per min, respectively). However, in the fed state (after an oral glucose dose), the rate of VLDL-TAG secretion was significantly increased by 32 % (P= 0·04) in KO-IF mice compared with KO-AL mice (24·6 (sem 2·4) v. 18·6 (sem 1·6) mg/l per min, respectively). In agreement with this, the hepatic TAG content in KO-IF mice was 35 % lower than that in KO-AL mice (15·8 (sem 1·7) v. 21·3 (sem 2·3) mg/g). The IF regimen did not change the liver mass (4·28 (sem 0·19) v. 4·29 (sem 0·13) % body weight) and the hepatic cholesterol content (2·07 (sem 0·13) v. 2·18 (sem 0·16) mg/g).
Table 1 Plasma lipids, glucose and insulin in LDL-receptor knockout mice under an intermittent fasting regimen (KO-IF) and fed ad libitum (KO-AL) over 3 months (Mean values with their standard errors)
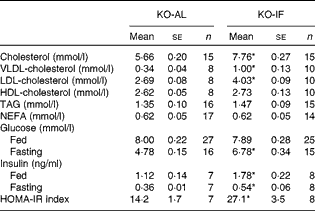
HOMA-IR, homeostatic model assessment for insulin resistance.
* Mean values were significantly different from those of the KO-AL group (P< 0·025; Student's t test).
The IF effects on body composition and adiposity are shown in Fig. 1. Based on the body composition, the KO-IF mice showed an obesity phenotype with significant increases of 15 % in the epididymal adipose tissue (Fig. 1(a)) and 72 % in the carcass fat content (Fig. 1(b)), as well as a decrease of 11 % in the body lean mass relative to the KO-AL mice (Fig. 1(c)). In addition, the mean size of subcutaneous adipocyte was 68 % greater in KO-IF mice than in KO-AL mice (Fig. 1(d)). The adipocyte size distribution shows that IF caused a reduction of 25 % in the number of medium-sized adipocytes and an increase of 260 % in large-sized adipocytes (Fig. 1(e)). Furthermore, the fasting and fed plasma levels of leptin were, respectively, 50 and 35 % higher in KO-IF mice than in KO-AL mice (Fig. 1(f)). The plasma levels of adiponectin were similar in KO-AL and KO-IF mice (7·68 (sem 0·33) v. 8·45 (sem 0·28) μg/ml, respectively).

Fig. 1 (a) Epididymal adipose tissue, (b) carcass fat, (c) lean mass, (d) subcutaneous adipocyte area and (e) adipocyte size distribution and (f) plasma leptin in LDL-receptor knockout mice under an intermittent fasting regimen (KO-IF) and fed ad libitum (KO-AL) over 3 months. L, large adipocyte (>1400 μm2); M, medium adipocyte (465–1400 μm2); S, small adipocyte ( < 465 μm2). Values are means of eleven to twenty-two mice per group, with their standard errors represented by vertical bars. * Mean values were significantly different from those of the KO-AL group (P< 0·025; Student's t test).
The increases in cholesterolaemia and adipose mass in response to IF were quite unexpected. Thus, the control wild-type mice (C57BL6/J) under the same feeding protocol were also studied. As expected, cholesterolaemia was reduced by 28 % (n 9, P< 0·0001) and the epididymal fat pad was decreased by 24 % (n 9, P< 0·01). Therefore, we attribute the IF responses of LDL-receptor knockout mice to their specific genetic defect.
The effects of IF on glucose homeostasis in LDL-receptor knockout mice are shown in Table 1. The baseline fasting glycaemia levels were 42 % higher in KO-IF mice than in KO-AL mice, while in the fed state, the levels of glycaemia were similar in both groups. In addition, there were increases of 50 and 60 % in the fasting and fed plasma levels of insulin, respectively, in KO-IF mice relative to the KO-AL mice. The homeostatic model assessment for insulin resistance index was approximately 2-fold higher in KO-IF mice than in KO-AL mice, indicating that the IF regimen induced insulin resistance (Table 1). Accordingly, after an oral glucose tolerance test, the KO-IF mice showed increased levels of glycaemia at 90 and 120 min post-glucose loading, and a significant increase of 15 % in the area under the glycaemia curve (Fig. 2(a) and (b)). In addition, the KO-IF mice clearly showed an insulin resistance response after the insulin tolerance test, exhibiting an increase of 25 % in the area under the glycaemia curve after the insulin injection (Fig. 2(c) and (d)). Therefore, the IF regimen worsened glucose homeostasis in these hypercholesterolaemic mice, causing peripheral insulin resistance.

Fig. 2 (a, b) Glucose tolerance test (GTT) and (c, d) insulin tolerance test (ITT) in LDL-receptor knockout mice under an intermittent fasting regimen (KO-IF, ![]() ) and fed ad libitum (KO-AL,
) and fed ad libitum (KO-AL, ![]() ) over 3 months. (a) 12 h fasted mice received an oral glucose load of 1·5 g/kg. The GTT was also performed in wild-type C57BL6/J mice (WT,
) over 3 months. (a) 12 h fasted mice received an oral glucose load of 1·5 g/kg. The GTT was also performed in wild-type C57BL6/J mice (WT, ![]() ) as a reference. (b) Fed mice received an intraperitoneal injection of regular insulin (3·5 pmol/kg). (b) AUC GTT curves and (d) AUC ITT curves. Values are means of nine to ten mice per group, with their standard errors represented by vertical bars. * Mean values were significantly different from those of the KO-AL group (P< 0·025; Student's t test).
) as a reference. (b) Fed mice received an intraperitoneal injection of regular insulin (3·5 pmol/kg). (b) AUC GTT curves and (d) AUC ITT curves. Values are means of nine to ten mice per group, with their standard errors represented by vertical bars. * Mean values were significantly different from those of the KO-AL group (P< 0·025; Student's t test).
Correlation analyses showed that the carcass fat content was positively correlated with fasting glycaemia (R 0·65, P= 0·01) and with the degree of glucose intolerance (R 0·88, P< 0·0001). In addition, the lean body mass was inversely correlated with fasting glucose (R − 0·61, P= 0·02) and with the degree of glucose intolerance (R − 0·86, P= 0·0001).
The effects of IF on the markers of oxidative stress are shown in Table 2. The plasma total antioxidant capacity of KO-IF mice was similar to that of KO-AL mice. However, the plasma VLDL of KO-IF mice took a longer time to be oxidised by CuSO4 than that of KO-AL mice, indicating protection against oxidative insult. Compared with KO-AL mice, KO-IF mice showed the same amounts of liver protein oxidation, as indicated by the unaffected protein carbonyl content. However, the overall liver mitochondrial ROS production, indicated by the oxidation of the probe 2′,7′-dichlorodihydrofluorescein, was reduced by 43 % in KO-IF mice relative to KO-AL mice (Table 2). Thus, the IF regimen exerted beneficial effects on the systemic and tissue redox state in hypercholesterolaemic mice.
Table 2 Oxidative stress markers in LDL-receptor knockout mice under an intermittent fasting regimen (KO-IF) and fed ad libitum (KO-AL) over 3 months (Mean values with their standard errors)

ROS, reactive oxygen species; H2DCF, 2′,7′-dichlorodihydrofluorescein.
* Mean values were significantly different from those of the KO-AL group (P< 0·025; Student's t test).
† VLDL was oxidised at 37°C using 40 μm-copper sulphate.
‡ nmol H2DCF/mg protein per min.
§ nmol H2O2/mg protein per min.
The effects of the IF regimen on systemic inflammation markers and on the development of spontaneous atherosclerosis were evaluated. The IF regimen promoted a pro-inflammatory profile in hypercholesterolaemic mice, as shown by a 28 % increase in plasma TNF-α levels (Fig. 3(a)), and a 23 % increase in plasma C-reactive protein levels (Fig. 3(b)) in KO-IF mice compared with the KO-AL mice. Spontaneous atherosclerosis in the aortic root was markedly aggravated in KO-IF mice, which showed a 3-fold increase in the average size of the lesions (Fig. 3(c) and (d)). The atherosclerosis lesion size was positively correlated with plasma cholesterol levels (R 0·60, P= 0·0009), carcass fat content (R 0·60, P= 0·008), plasma leptin levels (R 0·56, P= 0·01) and plasma TNF-α levels (R 0·85, P= 0·004). All of these correlations remained statistically significant after adjusting the analyses for plasma cholesterol concentrations.

Fig. 3 (a) Plasma TNF-α, (b) C-reactive protein (CRP), (c) areas of aortic atherosclerotic lesions and (d) representative histological sections in LDL-receptor knockout mice under an intermittent fasting regimen (KO-IF) and fed ad libitum (KO-AL) over 3 months. Images with 5 × and 20 × magnification. Values are means of seven to eighteen mice per group, with their standard errors represented by vertical bars. * Mean values were significantly different from those of the KO-AL group (P< 0·025; Student's t test).
Discussion
In the present study, we hypothesised that LDL-receptor knockout mice could benefit from IF-induced ER. However, IF actually worsened the metabolic syndrome features of these models, precipitating the appearance of obesity and diabetes and markedly aggravating atherosclerosis. These results are genotype dependent since wild-type mice responded to IF with significant decreases in plasma cholesterol levels and body fat mass.
Varady et al. ( Reference Varady, Hudak and Hellerstein 28 ) showed cardioprotective effects on C57BL6 mice under alternate-day fasting, such as reductions in cholesterol and TAG plasma concentrations and reductions in aortic smooth muscle cell proliferation. In the present study, despite the reduction in cumulative food intake, the KO-IF mice showed a marked elevation in the plasma levels of total cholesterol and VLDL- and LDL-cholesterol, an increased rate of liver VLDL-TAG secretion and a concomitant reduction in the liver content of TAG. These data are consistent with the stimulated rates of liver TAG synthesis and secretion during the fed day. In accordance with the present results, Bruss et al. ( Reference Bruss, Khambatta and Ruby 29 ) demonstrated that 30 % ER in C57BL6 mice stimulates the rates of VLDL secretion for the first 6 h after the commencement of feeding( Reference Bruss, Khambatta and Ruby 29 ). Increased VLDL-TAG secretion shown in KO-IF mice resulted in further elevation of its product LDL that accumulated in the vascular compartment due to the lack of LDL receptors. Besides increasing LDL (and hence hypercholesterolaemia), the increase in VLDL secretion has another consequence, that is, providing more substrates (NEFA) that accumulate and expand the adipose tissues. Thus, in addition to worsening hypercholesterolaemia, the IF regimen generated an obesity profile showing augmented visceral adipose mass, fat carcass content, adipocyte size and plasma leptin levels.
In addition to the increased availability of plasma TAG-derived NEFA to the adipose tissue, lipid synthesis rates in the adipose tissue may also be enhanced. Bruss et al. ( Reference Bruss, Khambatta and Ruby 29 ) showed that energy-restricted mice showed an increase in newly synthesised fatty acids stored in the epididymal and subcutaneous adipose tissues in the first hours after feeding( Reference Bruss, Khambatta and Ruby 29 ). Increased lipogenesis occurs in the tissues of LDL-receptor knockout mice( Reference Hau, Smelt and Bindels 20 ) because this animal model has an already activated state of sterol response element-binding proteins (SREBP), transcription factors that up-regulate the expression of genes for both cholesterol and fatty acid synthesis( Reference Oliveira, Cosso and Alberici 12 , Reference Martini and Pallottini 30 , Reference Brown and Goldstein 31 ). Therefore, cycles of energy depletion during the fasting day and energy refuelling after the feeding day may be fully compensated in a healthy context; however, cholesterol-deprived tissues found in this LDL-receptor knockout model plus the alternate energy intake may overactivate SREBP, exacerbating lipogenesis in liver and adipose tissues. Lean and obese C57BL6 control mice submitted to 30 % of ER per d respond with significant reductions in both fat mass (4 %) and lean mass (8–10 %)( Reference Kurki, Shi and Martonen 32 ). In another study using intermittent feeding, C57BL6 mice showed a reduction in visceral adipose tissue, but no overall change in body fat mass( Reference Varady, Hudak and Hellerstein 28 ). In the present study, control C57BL6 mice submitted to the IF regimen also lost fat mass. Conversely, LDL-receptor knockout mice under IF exhibited a differential substrate partitioning, leading to a reduction in lean mass but the preservation of adipose tissue mass. These findings may be explained by the genotype-dependent increased liver lipid synthesis and secretion that are exacerbated during the fed state and stored in the adipose tissue.
It has previously been demonstrated that ER decreases plasma glucose and insulin concentrations( Reference Masoro, McCarter and Katz 33 , Reference Argentino, Dominici and Munoz 34 ) and improves glucose tolerance and insulin sensitivity( Reference Park, Choi and Choi 35 ). Conversely, LDL-receptor knockout mice under IF became more intolerant to glucose and acquired insulin resistance, exhibiting a typical diabetic phenotype. It has consistently been shown that obesity and the insulin resistant state are deeply linked( Reference Kahn, Hull and Utzschneider 36 ). In the present study, the carcass fat content positively correlated with fasting glycaemia and with the degree of glucose intolerance, demonstrating the link between the disturbed glucose homeostasis and obesity in IF LDL-receptor knockout mice. In addition, it is possible that the reduced body lean mass contributes to lower glucose clearance, causing glucose intolerance and muscle insulin resistance. The higher plasma levels of insulin (approximately 50 %) would contribute to the preservation of adipose tissue mass. In fact, lean body mass was negatively correlated with fasting glucose and with the degree of glucose intolerance.
Previous studies have demonstrated that ER in rodents minimises oxidative stress by reducing mitochondrial ROS production( Reference Gredilla, Sanz and Lopez-Torres 4 – Reference Sanz, Caro and Ibanez 6 , Reference Caro, Gomez and Lopez-Torres 10 ). The present study shows that IF ameliorates at least two oxidative stress markers, the susceptibility of VLDL to oxidation and the rate of mitochondrial ROS generation. The higher resistance of VLDL to oxidation may indicate that this lipoprotein is secreted with fewer oxidised components, or that it may have acquired more antioxidants during the hepatic assembly process.
The levels of systemic markers of inflammation, TNF-α and PCR, were determined since inflammation is always present in obesity and atherosclerosis( Reference Rocha and Libby 37 – Reference Gustafson 39 ), and ER has been shown to be anti-inflammatory( Reference Spaulding, Walford and Effros 40 , Reference Park, Park and Valacchi 41 ). However, these markers were instead increased in the plasma of KO-IF mice. The presence of hypercholesterolaemia, obesity, insulin resistance and systemic inflammation led to severe advancement of atherosclerosis in KO-IF mice. All of these disturbances have been shown to be directly involved in all phases of disease development( Reference Rocha and Libby 37 , Reference Bornfeldt and Tabas 42 , Reference Kleemann, Zadelaar and Kooistra 43 ). Importantly, the atherosclerosis lesion size in KO-IF mice is positively correlated with carcass fat content, plasma leptin and TNF-α level, even after adjusting for plasma cholesterol levels, which reinforces the close association between inflammation, obesity and atherosclerosis.
The IF regimen in rodents typically leads to increased food consumption above the energy needs on the days when food is provided, in contrast to humans who only slightly consume in excess of energy needs on the ‘feed’ days of IF( Reference Klempel, Bhutani and Fitzgibbon 44 ). It is unclear from the present study as to what the respective contributions of fasting and hyperphagia were to the cardiometabolic derangements observed in the LDL-receptor knockout mice. If hyperphagia is important, then, in humans who do not exhibit it( Reference Klempel, Bhutani and Fitzgibbon 44 ), these disturbances may not occur. We believe that hyperphagia is particularly noxious in the case of LDL-receptor deficiency.
In conclusion, ER promoted by the IF regimen induced obesity and diabetes and worsened the development of spontaneous atherosclerosis in LDL-receptor knockout mice. Therefore, the reported benefits of food restriction are not applicable in the context of genetic hypercholesterolaemia due to the defective LDL receptor.
Acknowledgements
H. C. F. O. and A. E. V. obtained grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). G. G. D. was supported by CNPq fellowship, and J. C. R., H. F. R. and B. A. P. were supported by FAPESP fellowships. FAPESP and CNPq had no role in the design, analysis or writing of this article.
The authors' contributions are as follows: G. G. D. designed and conducted the research, analysed the data, wrote the paper and had primary responsibility for the final content; J. C. R., C. J. F. L., B. A. P. and H. F. R. conducted the research and analysed the data; A. E. V. analysed the data and reviewed the paper; H. C. F. O. was involved in the project conception, designed the research, analysed the data, wrote the paper and had primary responsibility for the final content. All authors read and approved the final manuscript.
None of the authors has any conflict of interest.







