1. Introduction
Previously, schizophrenia was regarded as a chronic illness with a deteriorating course [Reference Lieberman1, Reference Harding2], but today the illness outcome is conceived as more diverse [Reference Kahn, Sommer, Murray, Meyer-Lindenberg, Weinberger and Cannon3, Reference Joseph, Kremen, Franz, Glatt, van de Leemput and Chandler4]. Long-term studies (7–20 years) show that about 32% of first-episode schizophrenia patients achieve full and steady symptomatic remission after first hospitalization [Reference Ceskova, Prikryl and Kasparek5], whereas about 59% of the patients experience intermittent or continuing (chronic) symptoms of schizophrenia with few or no periods of recovery over the following 20 years [Reference Strauss, Harrow, Grossman and Rosen6, Reference Harrow, Grossman, Jobe and Herbener7].
The concept of schizophrenia has gradually developed in parallel with changes in the diagnostic systems. According to the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) the diagnosis can be set after symptoms have been present for one month [8]. A recent meta-analysis found high (90%) diagnostic stability of schizophrenia in first-episode studies, but also reported a lower diagnostic stability in the more recently published studies [Reference Fusar-Poli, Cappucciati, Rutigliano, Heslin, Stahl and Brittenden9]. In line with this, two recent long-term studies of participants diagnosed with schizophrenia according to ICD-10 found prospective diagnostic stabilities of 70% [Reference Baca-Garcia, Perez-Rodriguez, Basurte-Villamor, Fernandez, Moral and Jimenez-Arriero10] and 75% [Reference Heslin, Lomas, Lappin, Donoghue, Reininghaus and Onyejiaka11].
Symptomatic remission was defined by the Andreasen criteria as a state of mild severity of symptoms described in absolute terms for all patients rather than an indicator of the individual symptomatic improvement [Reference Andreasen, Carpenter, Kane, Lasser, Marder and Weinberger12]. Long-term studies (5–10 years) of participants with first-episode schizophrenia [Reference Ceskova, Prikryl and Kasparek5, Reference Marchesi, Affaticati, Monici, De Panfilis, Ossola and Tonna13–Reference Bodén, Sundström, Lindström and Lindström15] or first-episode psychosis [Reference Weibell, Hegelstad, Auestad, Bramness, Evensen and Haahr16, Reference de Haan, van Nimwegen, van Amelsvoort, Dingemans and Linszen17] find symptom remission rates of 29–53%. A systematic review of psychosis studies showed associations between symptomatic remission and better premorbid function, milder symptoms at baseline (especially negative symptoms), early response to treatment, and shorter duration of untreated psychosis [Reference Alaqeel and Margolese18]. Likewise, higher premorbid intelligence has been associated with symptomatic remission indicating an important role for the general cognitive abilities in the symptomatic course of illness [Reference Andreou, Roesch-Ely, Veckenstedt, Bohn, Aghotor and Köther19]. Despite symptomatic improvements, relatively poor long-term functioning has consistently been found, even decades after illness onset [Reference Weibell, Hegelstad, Auestad, Bramness, Evensen and Haahr16, Reference Santesteban-Echarri, Paino, Rice, González-Blanch, McGorry and Gleeson20–Reference Jobe and Harrow22].
Functional remission is often defined by specific criteria [Reference Simonsen, Andreassen, Sundet, Romm, Berg and Bjella23] such as living independently and having a job/studying and may also include a specific minimum level of functioning on a rating scale [Reference Bodén, Sundström, Lindström and Lindström15, Reference Simonsen, Andreassen, Sundet, Romm, Berg and Bjella23–Reference Austin, Mors, Secher, Hjorthøj, Albert and Bertelsen26]. A five year first-episode schizophrenia study found 46% to be in functional remission, when defined as working or studying ≥50% the past year, living independently, and meeting friends ≥ once a month, and showed that symptomatic remission was not equal to a good functional outcome: 14% did not have a good functioning in spite of symptomatic remission, and 8% had good functioning without symptomatic remission [Reference Bodén, Sundström, Lindström and Lindström15]. A meta-analysis of first-episode psychosis studies showed associations between shorter duration of untreated psychosis and better cognitive ability, and functional remission [Reference Santesteban-Echarri, Paino, Rice, González-Blanch, McGorry and Gleeson20].
Full recovery can be defined as a state of both symptomatic and functional remission [Reference Simonsen, Andreassen, Sundet, Romm, Berg and Bjella23, Reference Lieberman, Kopelowicz, Ventura and Gutkind27–Reference Menezes, Arenovich and Zipursky30]. Around 15% of patients with first-episode schizophrenia [Reference Henry, Amminger, Harris, Yuen, Harrigan and Prosser14, Reference Albert, Bertelsen, Thorup, Petersen, Jeppesen and Le Quack24] fulfill the criteria for full recovery after 5–7 years. Long-term full recovery has been associated with higher functional outcome at baseline, higher age at illness onset, growing up with both parents, higher level of social skills, lower severity of negative symptoms, and female sex [Reference Albert, Bertelsen, Thorup, Petersen, Jeppesen and Le Quack24]. The combined concepts of diagnostic stability, symptomatic and functional remission, and full recovery have not previously been studied in initially antipsychotic-naïve schizophrenia spectrum cohorts.
2. Aims of the study
In this prospective cohort study, we assessed several outcome measures 4–18 years after the first-episode antipsychotic-naïve state. We aimed to: i) determine the diagnostic stability of the initial ICD-10 schizophrenia diagnosis, ii) asses outcome measures of symptom severity and symptomatic remission, iii) estimate outcome rates of functional remission and full recovery, and investigate associations between outcome status of remission and recovery; and baseline variables of age at diagnosis, premorbid intelligence, and sex. We expected rates of symptomatic and functional remission and recovery to increase with higher levels of premorbid intelligence, higher age at diagnosis, and female sex.
3. Methods
3.1 Participants
Patients with symptoms of schizophrenia were referred to the research department where the ICD-10 diagnoses of schizophrenia (F20.X) or schizoaffective disorder (F25.X) were confirmed and comorbid drug abuse was assessed based on the Schedules for Clinical Assessment in Neuropsychiatry, version 2.0 and 2.1 (SCAN) [Reference Wing, Babor, Brugha, Burke, Cooper and Giel31] (Fig. 1). Participants were originally recruited in the Copenhagen catchment area into three different cohorts with similar baseline examinations in 1998–2002; 2003–2007; and 2008–2014 [Reference Andersen, Fagerlund, Rasmussen, Ebdrup, Aggernaes and Gade32–Reference Nielsen, Rostrup, Wulff, Glenthøj and Ebdrup34], and were antipsychotic-naïve (they had never received treatment with antipsychotic medication).
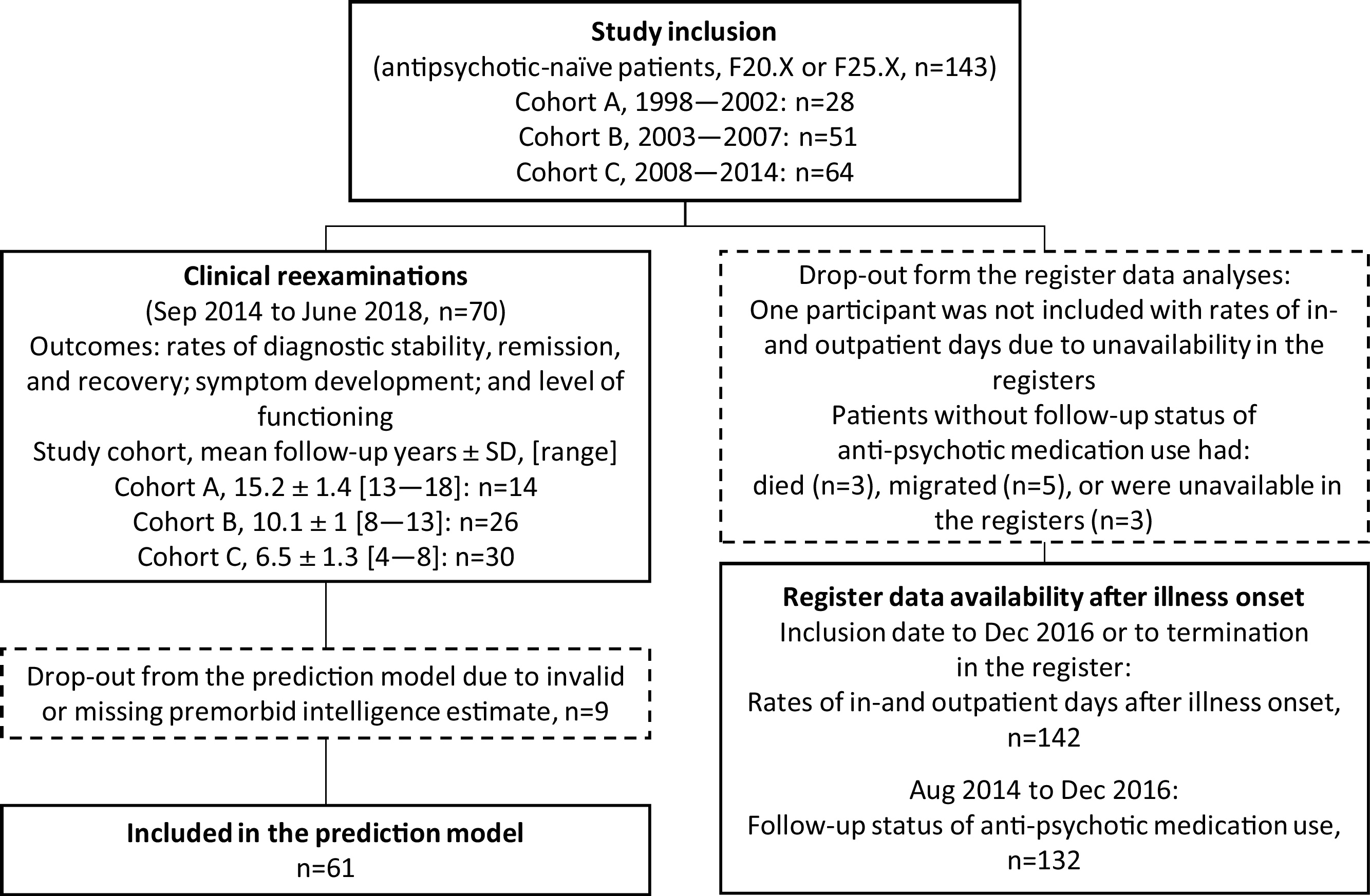
Fig. 1. Study flow chart of clinical examinations and use of register data.
Exclusion criteria were prior or current use of antipsychotic medication, current compulsory hospitalization (due to Danish legislation), a previous diagnosis of mental retardation, and an acute need of antipsychotic medication hindering un-medicated baseline examinations.
At follow-up, we obtained current addresses for the participants via the Danish nationwide registers and invited participants for re-examination between September 2014 to June 2018. The length of the follow-up period was the time between baseline inclusion and re-examination (mean 9.6 years, SD ±3.5, range [4.3–8.9]). All participants provided informed consent at baseline and follow-up. The project was approved at baseline by the Ethics Committee of Copenhagen and Frederiksberg and at follow-up by The Regional Scientific Ethical Committee (H-6-2014-014 and H-15017062) and the Danish Data Protection Agency (CSU-FCFS-2017-012).
3.2 Assessments
We clinically re-evaluated the diagnosis at follow-up (including a retrospective assessment of the presence of psychiatric symptoms in the follow-up period) using the Present State Examination interview [Reference Wing, Babor, Brugha, Burke, Cooper and Giel31, Reference Wing and Sturt35]. At baseline and follow-up we assessed symptom levels during the past week with the Positive and Negative Syndrome Scale (PANSS) [Reference Kay, Fiszbein and Opler36] and segmented the scores according to the Wallworks five-factor model [Reference Wallwork, Fortgang, Hashimoto, Weinberger and Dickinson37]. We assessed functional outcome at follow-up with the Personal and Social Performance scale (PSP) [Reference Morosini, Magliano, Brambilla, Ugolini and Pioli38] and the functioning scale of the General Assessment of Functioning (GAF-F) [Reference Jones, Thornicroft, Coffey and Dunn39]. The use of illicit drugs at follow-up was assessed with WHO assist version 3.0 [Reference Humeniuk, Victoria, Ali and Group WAPIS40] and a urinary drug test (Rapid Response, BTNX Inc., Canada). We assessed premorbid intelligence at inclusion with the Danish version of the National Adult Reading Test (DART) [Reference McGurn, Starr, Topfer, Pattie, Whiteman and Lemmon41, Reference Nelson and O’Connell42]. Premorbid intelligence scores were considered valid if participants had Danish as their primary language, had test scores >5, and did not have known dyslexia.
We collected data from central population-based registers for all participants included at baseline. Inpatient days and use of outpatient facilities were available from the baseline time of inclusion to December 2016 or the termination date in the registers. Because of variability in follow-up length in the registers we standardized in- and outpatient days into percentage of the total follow-up period. Follow-up status of being medicated with antipsychotic medication was estimated from register data as having redeemed ≥3 prescriptions from the pharmacy in a fixed period from August 2014 to December 2016.
3.3 Definitions of remission and recovery
Symptomatic remission was defined on the basis of the Andreasen criteria [Reference Andreasen, Carpenter, Kane, Lasser, Marder and Weinberger12] by which participants are considered in remission when they have PANSS scores of ≤3(mild) on the following items: delusions, conceptual disorganization, hallucinatory behavior, blunted affect, social withdrawal, lack of spontaneity, mannerisms/posturing, and unusual thought content.
We adapted the definition of functional remission from previous studies as living independently and having a GAF-F score ≥60 during the last month [Reference Bodén, Sundström, Lindström and Lindström15, Reference Albert, Bertelsen, Thorup, Petersen, Jeppesen and Le Quack24, Reference Lambert, Naber, Schacht, Wagner, Hundemer and Karow25]. We report on the vocational status of the participants but do not include vocational status in the remission definition as regulations of disability pension assessments changed markedly in Denmark during the follow-up period; occupational status would not be a valid measure of employability.
Full recovery was adapted from the recommendations of Lieberman et al. as being both in symptomatic and functional remission [Reference Lieberman, Kopelowicz, Ventura and Gutkind27].
3.4 Statistical analysis
We performed statistical analyses in the Statistical Package for Social Science for Windows version 25 (SPSS Inc., Chicago, IL, USA). We assessed normality of variables with skewness (-1 < mean<1 and mean<3*SD) and kurtosis (-3 > mean<3). The drop-out analysis was conducted with independent t-test for continuous normally distributed variables, Mann-Whitney U test for non-normally distributed continuous variables, and χ2-test for categorical variables. We examined effects of time on symptom severity and the level of functioning with paired t-tests.
To determine the impact of baseline variables on outcome at follow-up, we performed binominal logistic regressions with: 1) symptomatic remission; 2) functional remission; and 3) full recovery at follow-up as outcome and baseline independent predictors of: a) age at schizophrenia diagnosis; b) sex; and c) premorbid intelligence. We subsequently added the following variables individually: d) baseline PANSSpositive; e) PANSSnegative f) PANSSgeneral; and g) cohort of origin: 1998–2002; 2003–2007; or 2008–2014. By substituting age at schizophrenia diagnosis with h) time between the inclusion of the first participant (January 19th, 1998) and study inclusion at baseline we investigated effects of drift in referrals and/or diagnostic practice over time on outcome status.
4. Results
4.1 Drop-out analyses
At baseline, we recruited 143 antipsychotic-naïve participants with first-episode schizophrenia or schizoaffective disorder [Reference Andersen, Fagerlund, Rasmussen, Ebdrup, Aggernaes and Gade32, Reference Fagerlund, Søholm, Fink-Jensen, Lublin and Glenthøj43] and 70 participants (49%) attended the clinical follow-up examinations (mean follow-up years: 9.6 ± 3.5, range years[4.6–18.1]) (Fig. 1). Register data covering: 1) the use of mental health care services after illness onset was available for 142 participants (99%) (follow-up years: 9.4 ± 3.5 [0.8–19]) and 2) medication status at follow-up was available for 132 participants (92%).
The drop-out group from the clinical follow-up examinations displayed no significant differences on demographic variables; baseline variables of: symptom levels, levels of functioning, sex, or premorbid intelligence; or follow-up register data on: hospitalization rates, use of outpatient facilities, or medication status. The 1998–2002 cohort was significantly older at baseline (28.5 ± 6.6) when compared with the 2008–2014 cohort (mean 24.7 ± SD 5.8) (t(90) = 2.7, p = 0.008).
4.2 Diagnosis and symptom severity
Baseline and follow-up data regarding clinical and demographic data are summarized in Table 1 and clinical diagnoses are listed in Table 2. Of the 70 participants, who were initially diagnosed with schizophrenia or schizoaffective disorder, 56 participants (80%) met the criteria for schizophrenia or schizoaffective disorder at follow-up, whereas 14 participants did not. At follow-up, 12 of these participants met the diagnostic criteria of dependency syndrome (n = 2), other non-organic psychosis (n = 1), or affective disorders (n = 9) including bipolar affective disorder and recurrent depressive disorder. Two participants no longer met the criteria of any psychiatric diagnosis (within F00-F59); i.e. they reported no psychiatric symptoms, had no use of psychiatric medication since the baseline episode, and reported no previous symptoms to fulfil the diagnostic criteria for schizophrenia at follow-up. The diagnostic prospective consistency for schizophrenia alone (F20.X) (excluding schizoaffective disorder) was 81%; 16% met other psychiatric diagnostic categories including schizoaffective disorder (2%) and other non-organic psychosis (2%).
Table 1 Demographic and clinical characteristics at inclusion and follow-up (only participants with both baseline and follow-up participation). DART: Danish Adult Reading Test (the Danish version of the NART), PANSS: Positive and Negative Syndrome Scale, GAF-F: General Assessment of Functioning - functioning scale, PSP: Personal and Social Performance Scale. †Marked severity was operationalized as difficulties that interfere heavily with role performance in the area, §Medication status at follow-up was defined as having redeemed ≥3 prescriptions from the pharmacy in the fixed follow-up inclusion period. NA: Not applicable as it was an inclusion criterion that participants were antipsychotic-naïve at baseline.

Table 2 Primary ICD-10 diagnosis at baseline and follow-up for participants in the clinical re-examinations (n = 70).

At baseline, 8 participants (11%) had comorbid abuse of alcohol, cannabinoids, or multiple substances. At follow-up, 14 participants (20%) had comorbid abuse of alcohol, opioids, cannabinoids, cocaine, central stimulants, or multiple substances.
Symptom scores improved within all the Wallworks symptom factors and PANSStotal-scores reduced from moderate to mild illness severity [Reference Leucht, Kane, Kissling, Hamann, Etschel and Engel44].
4.3 Symptomatic remission
Twenty-four (34%) out of 70 participants were in symptomatic remission at follow-up. A subsample of 8 participants (11%) achieved symptomatic remission in the absence of functional remission (Fig. 2). Symptomatic remission was associated with age at diagnosis, premorbid intelligence, and sex in a logistic regression model (n = 61, χ2(3) = 18.159, p < 0.001). We found a decreased likelihood of remission with higher age at diagnosis: OR = 0.873; confidence interval, CI 0.766-0.966 and increased likelihood with higher premorbid intelligence: OR = 1.121; CI 1.027–1.244 and female sex: OR = 7.873; CI 1.980-31.309. The model explained 35.3% (Nagelkerke R2) of the variance in symptomatic remission status. Secondary analyses showed no significant predictive value of baseline symptom severity, cohort of origin, or time between study start-up and study inclusion date (all p≥0.089).

Fig. 2. Outcome status at 4–18-year follow-up (n = 70). Functional remission only: n = 13 (19%), symptomatic remission only: n = 8 (11%), full recovery (simultaneous symptomatic and functional remission): n = 16 (23%), and neither symptomatic nor functional remission: n = 8 (47%). A total of n = 32 (41%) were in functional remission (GAF-F ≥ 60, and living independently), a total of n = 24 (34%) were in symptomatic remission (Nancy Andreasen criteria [Reference Andreasen, Carpenter, Kane, Lasser, Marder and Weinberger12]: PANSS scores of 3≤ (mild) on the following items: delusions, conceptual disorganization, hallucinatory behavior, blunted affect, social withdrawal, lack of spontaneity, mannerisms/posturing, and unusual thought content), and a total of n = 16 (23%) were in full recovery.
4.4 Level of functioning
At follow-up, the participants had a mean GAF-F of 57.0 ± 13.6 and PSP of 60.0 ± 12.0 corresponding to moderate difficulties in functioning [Reference Morosini, Magliano, Brambilla, Ugolini and Pioli38] (Table 1). GAF-F scores at baseline were available for the most recently included cohort (n = 29, follow-up 4–8 years) who showed improvements from baseline: 40.1 ± 10 to follow-up: 61.5 ± 14.9, (t(28) = 7, p < 0.001). The functional improvement corresponded to a change from major deficits within several areas to some difficulties within the domains of social or vocational functioning [Reference Morosini, Magliano, Brambilla, Ugolini and Pioli38].
4.5 Functional remission and full recovery
We summarized rates of functional remission and full recovery in Table 3. At follow-up, 29 (41%) out of 70 patients were in functional remission and 16 patients (23%) were in full recovery. Including vocational status (having a job or studying) as a criterion reduced the functional remission rate to 30% and the full recovery rate to 17%. A subsample of 13 patients (19%) achieved functional remission in the absence of symptomatic remission (Fig. 1).
Table 3 Functional remission, symptomatic remission and full recovery at follow-up. GAF-F: General Assessment of Functioning - functioning scale. †Simultaneous symptomatic and functional outcome required. ‡ Simultaneously being in symptomatic and functional remission corresponds to the study definition of recovery.

The binary logistic regression models depending on age, sex, and premorbid intelligence were significant for functional remission (n = 61, χ2(3) = 13.876, p = 0.003) and full recovery (n = 61, χ2(3) = 17.444, p ≤ 0.001). However, in both models only female sex was significantly associated with a higher likelihood of functional remission: OR = 8.1; CI 2.19–29.93 and full recovery: OR = 14.452; CI 3—69.72. The models explained outcome variance with 27.3% for functional remission and 37.7% for full recovery (Nagelkerke R2). Secondary analyses showed no significant predictive value of baseline symptom severity. Participants included in the earliest cohort (1998–2002) had a decreased likelihood of achieving functional remission by OR = 0.111, p = 0.028. More recent inclusion in the study significantly increased the likelihood for functional remission by OR = 1.254, p = 0.016 and for full recovery by OR = 1.254, p = 0.024 as investigated by substituting age at schizophrenia diagnosis with time between study start-up and study inclusion at baseline.
4.6 Relationship between antipsychotic medication and outcomes
Register data regarding the antipsychotic medication status in the follow-up inclusion period was available for 69 (99%) out of 70 participants with clinical re-examinations and 35 (51%) of these were medicated with antipsychotics in the follow-up inclusion period. Ten (42%) of the 24 symptomatic remitters and 25 (56%) of the 45 non-remitters were medicated with antipsychotics and the difference was non-significant (p = 0.272). Eleven (36%) of the 29 functional remitters and 24 (60%) of the 40 non-remitters were medicated with antipsychotics trending an association between being in functional remission and being un-medicated (p = 0.070). Six (38%) out of the 16 participants in recovery and 29 (55%) of the 53 non-recovered participants were medicated with antipsychotics, and the difference was non-significant (p = 0.227).
Register-based data on the antipsychotic medication status in the follow-up inclusion period was available for 132 (92%) out of 143 baseline participants. Fifty-eight (43%) out of 132 baseline participants, were medicated with antipsychotics in the follow-up inclusion period. Additional Mann-Whitney U test showed significantly lower hospitalization rates in the follow-up period for the un-medicated participants (mean rank = 59) compared with the medicated participants (mean rank = 76) (U = 1591, z=-2.569, p = 0.010).
5. Discussion
In this group of patients with schizophrenia or schizoaffective disorder, who were antipsychotic-naïve at baseline, 80% continued to meet these diagnostic criteria at follow-up, however, 53% were in symptomatic and/or functional remission. Seven-teen percent met the criteria for other psychiatric diagnoses than schizophrenia whereas 3% no longer met the criteria for any psychiatric diagnosis (F00-F59) after clinical assessment. There were significant overall improvements in both symptom severity and level of functioning from baseline to follow-up. We found that 34% of patients were in symptomatic remission, 41% were in functional remission, and 23% were fully recovered. Symptomatic remission was significantly associated with higher premorbid intelligence, lower age at diagnosis, and female sex. Status of functional remission and recovery was only significantly associated with female sex, with an effect also observed for later year of study-inclusion, that is, participants in the more recent cohorts had a higher probability of being in functional remission and full recovery.
We found a diagnostic stability of the schizophrenia diagnosis of 81%. This is in line with the 70 and 75% prospective diagnostic stability found in studies with similar years of inclusion [Reference Baca-Garcia, Perez-Rodriguez, Basurte-Villamor, Fernandez, Moral and Jimenez-Arriero10, Reference Heslin, Lomas, Lappin, Donoghue, Reininghaus and Onyejiaka11] and supports the emerging trend towards lower stability in more recently published studies compared with the otherwise high (90%) stability of the schizophrenia diagnosis found in a first-episode meta-analytic study [Reference Fusar-Poli, Cappucciati, Rutigliano, Heslin, Stahl and Brittenden9]. Less emphasis on chronicity when assessing schizophrenia based on the ICD-10 compared with previous versions may contribute to the decreasing diagnostic stability. Together with previous recent long-term studies [Reference Fusar-Poli, Cappucciati, Rutigliano, Heslin, Stahl and Brittenden9–Reference Heslin, Lomas, Lappin, Donoghue, Reininghaus and Onyejiaka11], our results support the importance of continuously evaluating the diagnosis after the first psychotic episode. Steps towards such a continuous diagnostic evaluation and specification of the variability of symptomatic outcome have already been taken; the recently launched ICD-11 emphasizes classification of illness course (first-episode, multiple episode, or chronic) and omits the subtypes (e.g. paranoid schizophrenia) [45]. Additionally, ICD-11 specifies that disturbances must be present in multiple mental modalities, including thinking, perception, self-experience, cognition, affect, and behavior. Our results support this inclusion of cognitive disturbances in the ICD-11 criteria for schizophrenia: In line with other studies [Reference Woodberry, Giuliano and Seidman46], we have previously found reductions of premorbid intelligence in the included patient cohorts corresponding to approximately half a standard deviation [Reference Andersen, Fagerlund, Rasmussen, Ebdrup, Aggernaes and Gade32, Reference Fagerlund, Søholm, Fink-Jensen, Lublin and Glenthøj43, Reference Jensen, Bak, Rostrup, Nielsen, Pantelis and Glenthøj47] and in accordance with other studies [Reference Andreou, Roesch-Ely, Veckenstedt, Bohn, Aghotor and Köther19], we find that lower levels of premorbid intelligence decrease the chances of long-term symptomatic remission.
Our finding of a 34% symptomatic remission rate at follow-up is in line with the 29–53% found in previous first-episode psychosis long-term studies [Reference Ceskova, Prikryl and Kasparek5, Reference Marchesi, Affaticati, Monici, De Panfilis, Ossola and Tonna13–Reference de Haan, van Nimwegen, van Amelsvoort, Dingemans and Linszen17]. Baseline differences between cohorts may cause some of the variation in symptomatic remission rates, e.g. high symptomatic remission rates (52%) were found in a study with high remission rates already after index hospitalization (73%) [Reference Ceskova, Prikryl and Kasparek5].
The observed associations between female sex and better symptomatic and functional outcome are in line with previous studies [Reference Castle, Wessely and Murray48–Reference Angermeyer, Kühn and Goldstein50]. Possible explanations are a less severe symptomatic expression in females [Reference Castle, Wessely and Murray48, Reference Roy, Maziade, Labbé and Mérette49, Reference van Os and Kapur51] combined with the presence of protective factors, e.g. better premorbid social and work adjustment [Reference Angermeyer, Kühn and Goldstein50], better neurocognitive functioning [Reference Castle, Wessely and Murray48, Reference Vaskinn, Sundet, Simonsen, Hellvin, Melle and Andreassen52], and higher estrogen levels reducing symptoms levels and enhancing treatment response [Reference Castle, Wessely and Murray48, Reference van Os and Kapur51, Reference Kulkarni, Riedel, De Castella, Fitzgerald, Rolfe and Taffe53–Reference Kulkarni, Riedel, De Castella, Fitzgerald, Rolfe and Taffe55].
Our finding of decreased likelihood of symptomatic remission with higher age is somewhat surprising, since early onset has previously been associated with worse outcome [Reference Albert, Bertelsen, Thorup, Petersen, Jeppesen and Le Quack24]. However, the analysis showed that the participants included in the earliest cohort were the most functionally impaired but also significantly older at first-episode. The Danish OPUS long-term study (10 years) also found that younger age at diagnosis increased the chances of recovery and suggested that younger age at diagnosis may signify earlier detection of illness and better chances of a good outcome [Reference Austin, Mors, Secher, Hjorthøj, Albert and Bertelsen26]. Furthermore, as suggested by the decreasing diagnostic stability, the diagnosis of schizophrenia may be increasingly used for milder clinical cases in young adults.
Rates of employment/full time studying (41%) and rates of independent living (81%) were within rates found in previous studies (employment: 14–50% [Reference Harrow, Grossman, Jobe and Herbener7, Reference Bodén, Sundström, Lindström and Lindström15, Reference Helgason56] and independent living 25–90% [Reference Bodén, Sundström, Lindström and Lindström15, Reference Weibell, Hegelstad, Auestad, Bramness, Evensen and Haahr16, Reference Crumlish, Whitty, Clarke, Browne, Kamali and Gervin57–Reference Stirling, White, Lewis, Hopkins, Tantam and Huddy59]). Local factors such as freely available education and standard financial stipends for students in Denmark may affect vocational rates in the high end of the spectrum. The slightly higher rates of full recovery in our study (23% compared with 16% found in a meta analytic-study [Reference Jääskeläinen, Juola, Hirvonen, McGrath, Saha and Isohanni60]) may also be attributable to differences in the recovery definition (current state in our study versus two-year recovery demand in the meta-analysis).
Receiving antipsychotic treatment showed no associations to any of the outcome status definitions in our study. Previous studies have shown that about 20–35% of patients with schizophrenia recover without medication and that more un-medicated than medicated patients recover or show higher functional levels [Reference Jobe and Harrow22, Reference Harrow and Jobe61]. Meanwhile, lack of adherence to the treatment in spite of repeated psychotic symptoms has been associated with an increased risk of relapse, hospitalization, and a range of poorer long-term outcomes [Reference Ascher-Svanum, Faries, Zhu, Ernst, Swartz and Swanson62, Reference Novick, Haro, Suarez, Perez, Dittmann and Haddad63]. Lower hospitalization rates in our un-medicated participants indicate less severe illness trajectories in patients who discontinue antipsychotic treatment. However, we must consider that long-term medication rates in our initially antipsychotic-naïve cohorts may not be representative of the full patient population.
Some limitations should be considered. The drop-out rate of 51% for the long-term clinical assessments introduces a potential bias. However, information from nationwide registers on the complete cohort ascertained that the clinically re-evaluated participants did not differ from the drop-outs. Our inclusion criteria of participants being in an antipsychotic-naïve state at baseline offers novelty to the literature on the prediction of long-term outcome in schizophrenia. Meanwhile, some patients may not have been referred to the study because they were unable to go through the examinations before receiving treatment. Likewise, patients who were compulsory admitted were excluded at baseline due to Danish legislation possibly resulting in inclusion of a less severely affected patient group. In the early cohorts, we did not assess level of functioning at baseline, which would have added strength to the prediction of the long-term outcome. Likewise, data on duration of illness/untreated psychosis were collected slightly differently across cohorts, which prohibited using this as an independent variable in the analyses. Medical records or the use of a structured interview with mapping of the course of illness would have provided valuable knowledge regarding the course of illness.
This is a unique extended long-term schizophrenia study assessing both diagnostic stability, remission status, functional outcome, and register data from an initially antipsychotic-naïve schizophrenia/schizoaffective patient group. Our results confirm the emerging evidence of decreasing long-term diagnostic stability of schizophrenia, underscores the importance of continued re-evaluation of psychiatric symptoms after illness onset, and highlights the pertinence of including cognitive disturbances in the diagnostic classification. We confirm the associations of female sex and higher premorbid intelligence with symptomatic remission. Functional outcome remains impaired for most participants, especially for men, and future research should target the improvement of functioning as well as symptoms. Increased likelihood of symptomatic remission with lower age at schizophrenia diagnosis may indicate the importance of early illness detection or be a consequence of diagnostic practice including milder and more transient psychoses into the diagnoses of schizophrenia.
Funding source declaration
The study was financially supported by the Lundbeck Foundation [ID: R25-A2701 and R155-2013-16337, 2013], the Mental Health Service in the Capital Region of Denmark, and Læge Sofus Carl Emil Friis og Hustru Olga Doris Friis' Legat. CP was supported by a Senior Principal Research Fellow ship from the Australian National Health and Medical Research Council [NHMRC ID: 1105825, 2016] and by a grant from the Lundbeck Foundation [ID: R246-2016-3237, 2016]. The funding sources had no role in the design and conduct of the study; the collection, management, analysis and interpretation of the data; the preparation, the review or approval of the manuscript; or in the decision to submit the manuscript for publication.
Declaration of Competing Interest
BHE has received lecture fees and/or is part of Advisory Boards of Bristol-Myers Squibb, Eli Lilly and Company, Janssen-Cilag, Otsuka Pharma Scandinavia AB, Takeda Pharmaceutical Company and Lundbeck Pharma A/S. CP has participated on Advisory Boards for Janssen-Cilag, Astra-Zeneca, Lundbeck, and Servier. He has received honoraria for talks presented at educational meetings organised by Astra-Zeneca, Janssen-Cilag, Eli-Lilly, Pfizer, Lundbeck and Shire.
Acknowledgements
We thank all patients who participated in the study, the research staff, Research Secretary Lisbeth Jensen, and Data Manager Mikkel Erlang Sørensen at the Centre for Neuropsychiatric Schizophrenia Research, Glostrup, Denmark.








Comments
No Comments have been published for this article.