INTRODUCTION
Noroviruses, rotaviruses, and enteroviruses are among the most diverse and clinically important enteric viruses [Reference Wilhelmi, Roman and Sanchez-Fauquier1–Reference Hansman3]. Norovirus is the most frequent cause of non-bacterial gastroenteritis in both children and adults (in industrialized countries) [Reference Lopman4, Reference Kaplan5]. Usually, the infection is self-limiting and symptoms subside within 2–3 days [Reference Koopmans6]. However, severe forms of the disease may occur in young children, the elderly and immunocompromised individuals [Reference Mattner7, Reference Murata8]. Rotaviruses are considered to be the major cause of severe infantile diarrhoea worldwide, and are thought to be responsible for 60% of all diarrhoeal episodes in developing countries and 40% in developed countries [Reference Thapar and Sanderson9–Reference Parashar11]. Both rotavirus and norovirus show marked wintertime seasonality in temperate countries [Reference Koopmans and Van12, Reference Mounts13].
Enteroviruses are second only to rhinoviruses as the most common viral infectious agents in humans [Reference Pasquinelli14]. Most enterovirus infections are typically mild or subclinical. The presentation of symptomatic infection is very variable, including hand, foot and mouth disease, myocarditis, aseptic meningitis, encephalitis and acute flaccid paralysis [Reference Palacios and Oberste15–Reference Yamashita17]. A characteristic feature in the epidemiology of these viruses is their seasonality, with peaks in late summer or early autumn in temperate regions [Reference Fisman18].
Thus, these three enteric viruses all have seasonal epidemiology. The seasonality is less pronounced in Sub-Saharan Africa, and the climate variables influencing their transmission or other factors explaining this seasonal pattern are not well understood, in part due to the fact that laboratory capacity for their detection is often lacking.
These viruses are highly transmissible, primarily via the faecal–oral route, contaminated food or water or person-to-person transmission via airborne droplets [Reference Parashar and Monroe19, Reference Arvelo20]. Waterborne transmission may occur in the context of contamination of drinking or recreational water by waste water [Reference Nenonen21]. The waterborne route constitutes a significant mode of transmission of these viruses, especially of norovirus genogroup GI while norovirus GII is mostly foodborne and also a common cause of outbreaks in the hospital setting [Reference Lysen22]. As climate change in the form of increased precipitation sets in, it is estimated that the incidence of waterborne infectious diseases will increase [Reference Shuman23]. Therefore, knowledge of the seasonality and influence on weather condition on the transmission of these viruses in the community could provide the information necessary to alert healthcare providers on when a peak period of infection in the community is likely to occur. This will also be important for prioritization of public health actions and programme planning interventions.
Over the years, molecular diagnostic methods such as real-time polymerase chain reaction (PCR) have improved viral detection and have often replaced traditional diagnostics such as antigen detection by immunofluorescence or enzyme immunoassays [Reference Wolffs24]. The recent advances of multiplex real-time PCR based on primers and probes designed to bind and amplify specific but conserved genomic regions of target genes has grown in importance due to their higher sensitivity. These tests can also detect more than one pathogen in a single reaction, thereby reducing cost and time [Reference Brittain-Long25]. The aim of this prospective study was to determine the seasonal fluctuations of the prevalence of norovirus, rotavirus and enterovirus in children and adults in Cameroon, as assayed by a highly sensitive molecular diagnostic assay. We also analysed the potential impact of weather variables such as temperature, relative humidity and rainfall, as well as demographic and other risk factors for infection. Better understanding of factors enhancing transmission and seasonality may provide insights into the relationship between physical environment and risk of infection of these viruses.
METHODS
Study region
The study was conducted in Limbe, located at the foot of Mount Cameroon, close to the Atlantic Ocean, with a population of about 84 000 (Fig. 1). Detailed demographic data on this study population was not available. The Limbe equatorial climate experiences the dry season from December to May, and the rainy season with heavy rainfall between June and November, with the peak period in August. Temperatures range from 23°C to 33°C and relative humidity often reaches as high as 86%. Sources of drinking water are mainly tap water and borehole wells.

Fig. 1 [colour online]. Map of Cameroon with neighbouring countries showing study location. Top left panel is a map of Africa showing the location of Cameroon.
Participants and longitudinal sampling
We conducted a prospective longitudinal exploratory study over a period of 1 year (from September 2011 to August 2012). The age distribution of participants was children aged 1–17 years (mean age 6·4 years) and adults aged 18–69 years (mean age 32 years) (Table 1). Inclusion criteria included healthy participants without any signs or symptoms of acute gastroenteritis and not receiving chemotherapy at the time of recruitment. A sample size calculation based on the population size and 95% confidence interval (CI) was used to determine the sample size. A two-stage sampling strategy was used. First, there was random selection of the households and then random sampling of child and adult participants in the household.
Table 1. Characteristics of study participants in Limbe, Cameroon, September 2011–August 2012
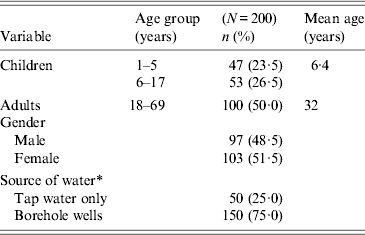
* Ten participants used both tap water and borehole wells and were classified under use of borehole wells.
A total of 154 children and 146 adults were randomly selected, of which 115 and 111 children and adults, respectively, met the inclusion criteria and were recruited. In the course of the study 13 children and 11 adults dropped out and two children died. Each participant received monetary remuneration of US$48 to discourage dropping out of the study. All participants visited the centre and were given containers to provide faecal samples. Samples were separated into 2-ml aliquots and stored at –20°C prior to analysis.
One year surveillance data of both children and adults was analysed (each adult participant was matched with a child from the same household). Surveillance included monthly collection of a faecal specimen. Each participant responded to questions in a questionnaire regarding age, gender, household size, and source of domestic water. It was observed that 50 participants used tap water only while 140 participants used borehole wells only. An additional 10 participants used both tap water and borehole wells.
The surveillance protocol was reviewed and approved by the South West Regional Delegation of Public Health in Cameroon, and participants (parents or guardians of children) provided written or oral informed consent.
Meteorological data collection
Weather information was recorded according to guidelines of the World Meteorological Organization [26]. Briefly, average daily temperature and relative humidity were recorded. Monthly mean values were calculated by adding the daily averages and dividing by the number of days in each calendar month. Total daily rainfall on the other hand was recorded and added up to give the monthly rainfall.
Stool preparation and nucleic acid extraction
Stool suspensions were prepared by adding 1 g stool sample to 5 ml phosphate-buffered saline containing 1 g glass beads (Corning Inc., USA). The mixture was shaken for 2 min, and centrifuged at 1500 g for 25 min at 4°C. Stool suspensions (supernatant) were aspirated and stored in aliquots. Nucleic acid (NA) from 130 μl faecal sample was extracted into an elution volume of 100 μl by the MagNA Pure LC robot (Roche Molecular Systems, Germany), using the Total Nucleic Acid external lysis protocol.
Real-time PCR
All assays were run on an ABI 7300 real-time PCR platform (Applied Biosystems, USA). The target viruses were detected in 25 μl reaction volumes containing 5 μl NA, 13 μl 2 × reaction mix with ROX (Invitrogen Ltd, UK), 0·5 μ m each primer and probe, 20 U RNase OUT and 0·5 μl Superscript III platinum one-step, following the manufacturer's instructions [Reference Nenonen27]. After a reverse transcription step at 48°C for 25 min and initial denaturation at 95°C for 10 min, 45 cycles of two-step (95°C for 15 s, 60°C for 60 s) PCR was performed in three parallel reactions, targeting four different viruses as described in Table 2. A cycle threshold (Ct) value <40 indicated that the specimen was positive. This method enables the semi-quantitative detection of target viruses, with the Ct value being inversely proportional to the amount of virus in each sample [Reference Brittain-Long25]. The limit of detection was about 50 copies per reaction as determined by serial dilution of the plasmid-containing inserts of the target region. The quality of the assays was ensured by performing parallel runs of positive and negative controls samples.
Table 2. Primers and probes used in the panel of real-time PCR tests
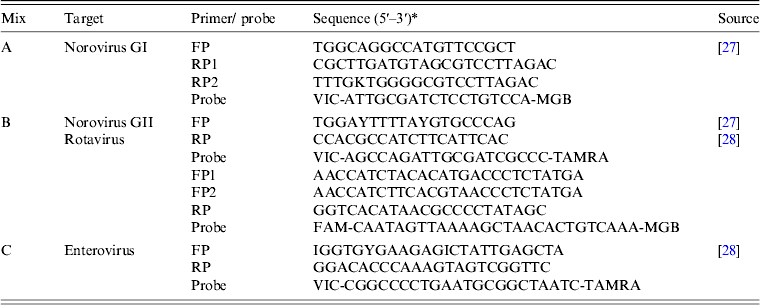
* FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine; MGB, minor groove-binding quencher; VIC, fluorescent label (Applied Biosystems, USA).
Statistical analysis
Monthly prevalence was calculated as the number of monthly cases of infection divided by the sample size multiplied by 100. Pearson regression analysis was performed to evaluate the effect of meteorological parameters and incidence of infection, and a bivariate analysis was performed to determine the relationship of demographic and risk factors for infection. Two-sided Fisher's test was used to compare proportions with alpha set at 0·05. Odd ratios (ORs) and 95% CIs were calculated using the SPSS software package v. 17.0 for Mac (SPSS Inc., USA).
RESULTS
Demographic characteristics of participants
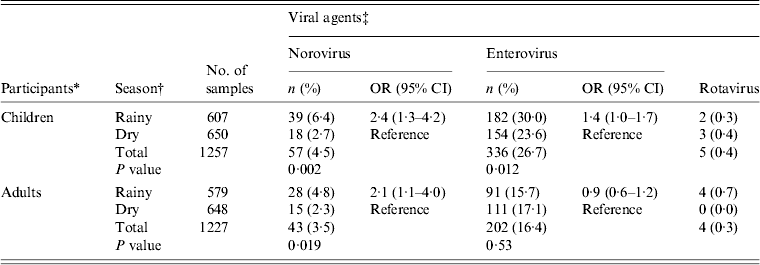
During the 12-month study period from September 2011 to August 2012, 200 participants were prospectively followed-up; 48·5% were males and 50% adults aged between 18 and 69 years. Children aged 1–17 years constituted 50% of the study population. The mean age of adults and children was 32 years and 6·4 years, respectively (Table 1). A total of 2484 faecal samples were collected; 1298 (52·2%) during the dry season and 1186 (47·8%) during the rainy season.
Virus detection and risk factors
Figure 2(a, b) shows the temporal pattern of virus detection. Enterovirus was the most frequently identified virus and was detected with a monthly prevalence ranging from 8% to 47% in children and 1–31% in adults. The prevalence of norovirus ranged from 1% to 16% in children and 1–12% in adults. In contrast, rotavirus detection was low with prevalence between 0% and 3% (Fig. 2 a, b). In a bivariate analysis, we observed that children aged <5 years were associated with an increased risk of norovirus infection. Risk of infection was also higher due to consumption of tap water by both children and adults (Table 3). Older children aged 6–17 years were also at higher risk of enterovirus infection. No significant differences in norovirus, rotavirus and enterovirus infection between males and females was observed.
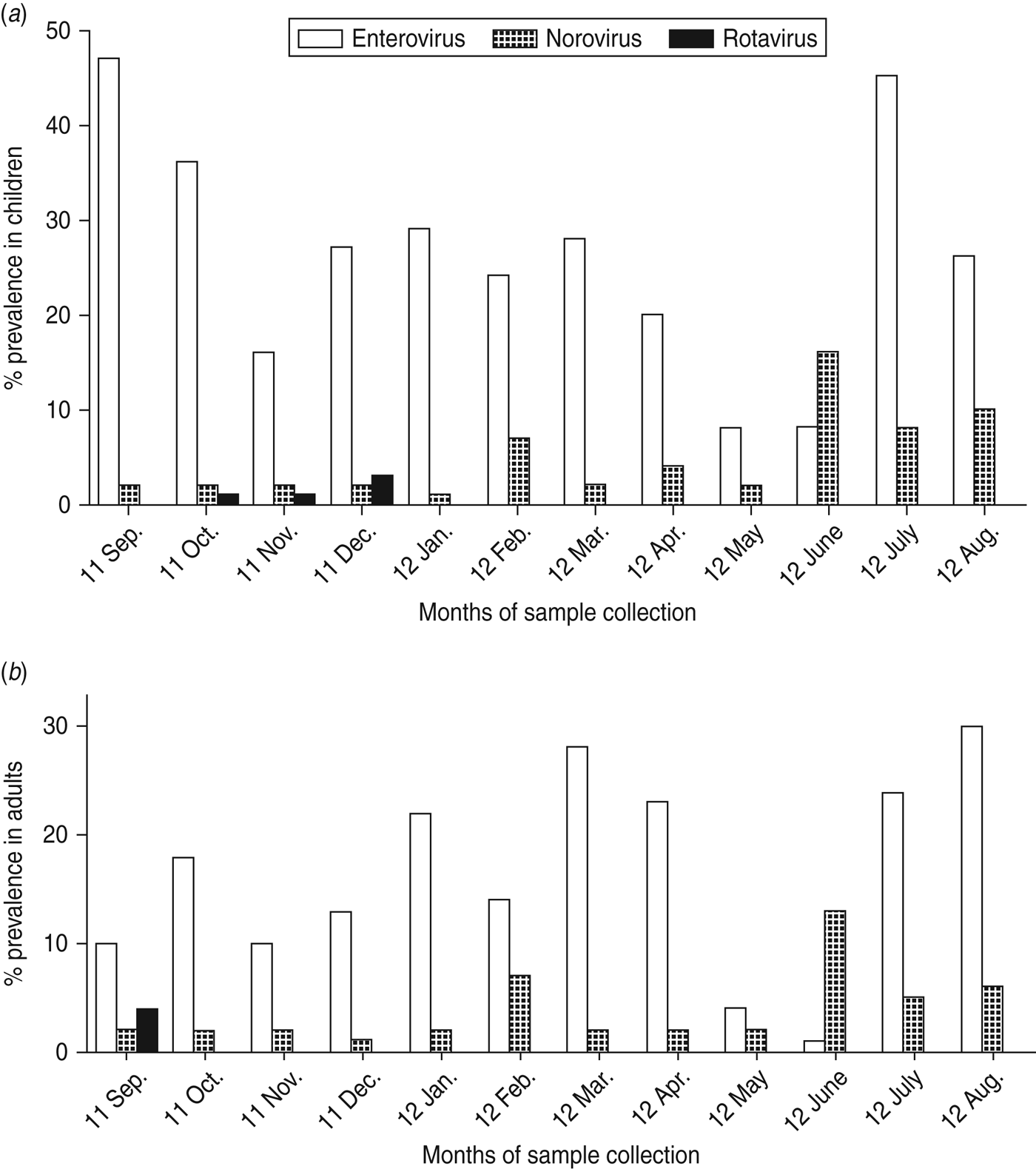
Fig. 2. Proportion (%) of samples positive for norovirus, rotavirus and enterovirus by real-time PCR in (a) children and (b) adults in Limbe, Cameroon, sampled monthly from September 2011 to August 2012.
Table 3. Risk factors for norovirus, rotavirus and enterovirus infection in Limbe Cameroon, September 2011-August 2012
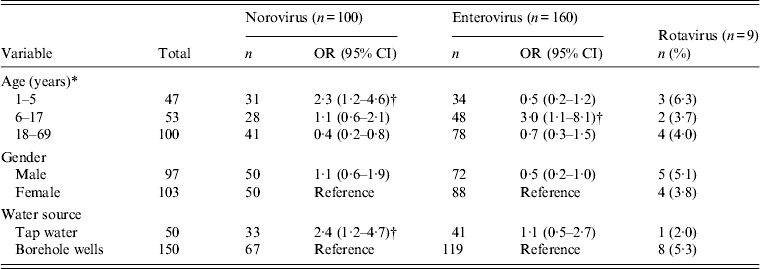
OR, Odds ratio; CI, confidence interval.
* To calculate the odds of infection for an age group, the rest of the other age groups combined were used as the reference. Both demographic and risk factors for infection were determined by bivariate analyses.
† Statistically significant data (P < 0·05), rotavirus data were sparse, and were thus excluded from analyses.
Seasonality
We observed norovirus and enterovirus circulation throughout the study period in both children and adults. However, a markedly low enterovirus activity was observed in May and June. Norovirus peak prevalences were observed from June to August (Fig. 3). This peak of norovirus circulation coincided with an increase in total rainfall (Fig. 4). Few rotavirus infections were detected during September–December with no further detection during January–August. Rotavirus was therefore excluded from the statistical analyses. In all, detection of norovirus infection was statistically higher in the rainy season than in the dry season for both children and adults (P = 0·02) (Table 4). A weak positive correlation of temperature and relative humidity with respect to norovirus, rotavirus and enterovirus infections was observed (r ⩽ 0·4, P > 0·05).
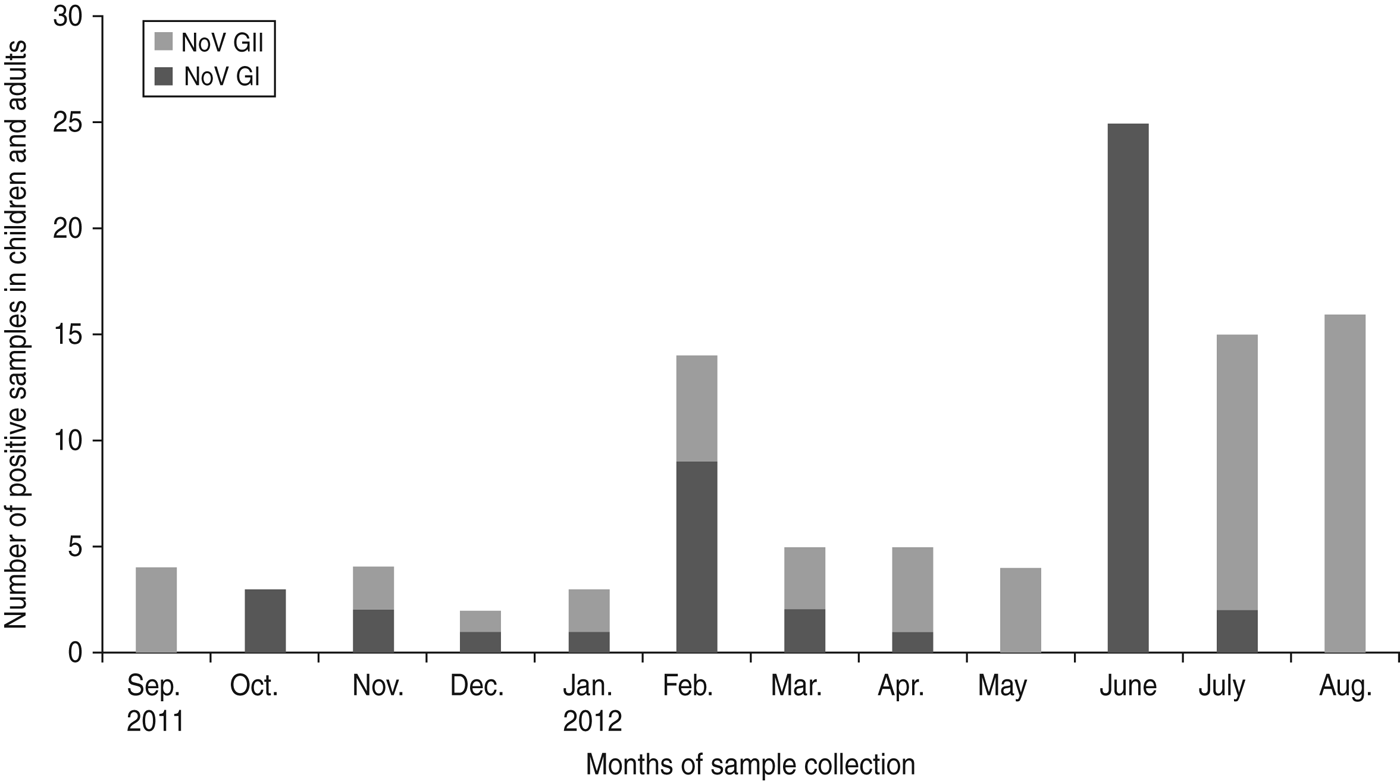
Fig. 3. Seasonal pattern of the prevalence of norovirus (NoV) genogroups GI and GII in children and adults in Limbe, Cameroon sampled from September 2011 to August 2012.

Fig. 4. Variation of weather factors and real-time PCR positivity (%) of norovirus, rotavirus and enterovirus in Limbe, Cameroon from September 2011 to August 2012. (a) Rainfall, (b) relative humidity, (c) temperature. Norovirus and enterovirus prevalence correlated positively with rainfall (r ⩾ 0·6, P < 0·05). Moreover, the correlation with relative humidity and temperature was positive although weak (r ⩽ 0·4, P > 0·05).
Table 4. Norovirus, rotavirus and enterovirus detection during the dry and rainy season in Limbe, Cameroon, September 2011–August 2012
OR, Odds ratio; CI, confidence interval.
* Children (1–17 years), adults (18–69 years).
† Dry season (December–May), rainy season (June–November).
‡ P < 0·05 was considered to be statistically significant (two-sided Fisher's test). Rotavirus data were sparse, and were thus excluded from analyses.
Demographic and seasonal pattern of norovirus genogroups GI and GII circulation
Out of the 100 cases of norovirus detected during the study period, 45 were of GI and 55 were of GII. Moreover, out of the 55 cases of GII, 30 were detected in children and 25 in adults. There was no significant difference in infection by norovirus genogroups GI and GII in children and adults (P = 0·7). Although the two genogroups of norovirus circulated throughout the year, each month was characterized by the predominance of a particular genogroup. In June a predominance of GI was observed while a shift to GII predominance was seen during the subsequent months from July to September and then replaced again by genogroup GI in October (Fig. 3).
DISCUSSION
In this prospective study, the seasonality of norovirus, rotavirus, and enterovirus in Cameroon was investigated. The high prevalence of enterovirus in this study is consistent with that of our previous report [Reference Ayukekbong28]; however, our enterovirus seasonality is in slight contrast with a report by Njouom et al. [Reference Njouom29] who found that enterovirus in Cameroon was mostly detected from January to June. We found an all-year-round circulation of enterovirus with relatively low activity in May and June. This difference in enterovirus seasonality between the two studies could be due to difference in specimen type. Njouom et al. investigated respiratory enterovirus infections (by throat and nasopharyngeal swab sampling), and these infections may well have a different epidemiology and seasonality than enteric enterovirus infections [Reference Solomon30].
Similarly norovirus circulation was observed throughout the year with a major peak during the beginning of the rainy season from June to August. This is the first comprehensive prospective study of norovirus seasonality in tropical Africa. Our observation that circulation of norovirus occurs throughout the year is in accord with other surveys [Reference Dedman31, Reference Lopman32]. However, unlike the cold weather peak demonstrated in most studies performed in temperate regions of the Northern Hemisphere [Reference Mounts13, Reference McSwiggan, Cubitt and Moore33], out data demonstrate a sharp increase in norovirus prevalence, dominated by GI, at the beginning of the rainy season. Since this virus is highly stable in the environment, abundant rainfall is likely to wash sewage and household waste into the environment, thereby favouring norovirus transmission [Reference Cannon34].
In contrast to our expectation, an increased risk of norovirus infection was associated with consumption of tap water. It should be noted that the municipality supplies tap water to the homes. The stream, which supplies the municipality water treatment plant, is likely to be contaminated upstream by human sewage containing viral particles. Moreover, considering that norovirus is fairly resistant to chlorine disinfection which is used for tap water [Reference Duizer35, Reference Estes, Prasad and Atmar36], it is plausible that water from borehole wells may be relatively safer for consumption with regard to norovirus infection in Limbe, Cameroon. It should be noted that human norovirus infections are caused by genogroups GI, GII and to a lesser extent by GIV [Reference Zheng37]. Genogroup GII is the most common cause of norovirus outbreaks worldwide and these infections peak during the winter season in temperate regions [Reference Mounts13], and are often designated as ‘winter vomiting disease’. On the other hand, GI viruses have been linked to outbreaks associated with contaminated water source [Reference Riera-Montes38]. In our study, the significant increase in norovirus GI at the beginning of the rainy season showed a different seasonality to that described in temperate regions, and supported the notion of water as the main vehicle of transmission of these viruses. The results of the current study failed to show a statistical difference in norovirus genogroup infection between children and adults, and between males and females. Average temperature and relative humidity also correlated positively with an increase in the prevalence of norovirus and enterovirus infection, although the correlation was weak.
Overall, rotavirus detection was low and restricted to the end of the rainy season and the beginning of the dry season (September–December). This finding is compatible with a report by Oluwatoyin Japhet et al. in Nigeria who found a peak of rotavirus infection from October to January [Reference Oluwatoyin39]. Considering the variability of incidence of enteric viruses within the same population during a period of 1 year, results of epidemiological studies at a single time point should be interpreted with caution as different periods of the year may show different patterns of infection.
Several limitations may be underscored in the current study. Perhaps the most compelling is that the study period of 1 year does not allow testing the consistency of seasonal trends of these viruses, which may change from one year to another. Second, the study does not distinguish between symptomatic and asymptomatic infections with these viruses because our focus was on seasonality and meteorological influence on transmission. Third, due to the small sample size and the very few cases of rotavirus detected, rigorous statistical analyses were lacking.
Despite these limitations, this study is the first of its kind in Sub-Saharan Africa and extends knowledge on the seasonality and risk factors of norovirus, rotavirus, and enterovirus infection in South West Cameroon and suggests that rainfall significantly increases the transmission of noroviruses and enteroviruses in Limbe, Cameroon. Taken together, we hypothesize that as climate change occurs with an increase in precipitation, high incidence of norovirus and enterovirus infection may occur and a corresponding increase in the burden of disease due to these infections is likely.
These data may provide valuable information necessary to alert healthcare providers on when a peak period of infection in the community is likely to occur. Further studies with a larger sample size and covering a greater area in Cameroon are needed to test the consistency of this seasonal trend and to evaluate the potential contribution of other risk factors for enteric virus infections.
ACKNOWLEDGEMENTS
We thank Anette Roth for skilful technical assistance.
DECLARATION OF INTEREST
None.










