Improving the health of racial minorities remains a public health priority that has long been sidelined. Minority patient inclusion of Blacks in multiple sclerosis (MS) and neuromyelitis spectrum disorder (NMOSD) pivotal trials continues to be trivial. Unless policy changes are made at the funding level or the rules of publication in major journals undergo a comprehensive overhaul to mandate inclusion of minority participation a requirement, there is little hope that fundamental change will occur. As it stands today, the impact of disease modifying therapies (DMT) in racial minority populations is unknown; no systematic, prospective studies are published or are in the pipeline that describe the effect of DMTs in minorities. At the individual patient–physician level, the choice of a DMT or optimization of therapy for patients of color with either MS or NMOSD remains speculative. Additionally, minority data are not typically available in most pivotal trial data publications making it a challenge to extrapolate a drug’s efficacy or side-effect profile when treating such patients.
Mismatch between the demographics of the clinical trial population and the real-world numbers who may require treatment remains a roadblock in choosing the optimal treatment option for minorities with MS. As demographics of the U.S. population shift and non-Caucasian numbers increase, ignoring disease data among burgeoning minority groups has negative consequences for the health system. At the local and national levels, regulatory interventions, and policy changes that are driven by patient and community advocacy groups might play a role in fostering access to minority populations to clinical trials.Reference Chen, Lara and Dang 1
As opposed to pivotal trials in MS, minority participation in NMOSD pivotal studies is better and relatable to the general population. However, inclusion of Blacks even for pivotal studies of NMOSD remains poor. Since demographic data based on race are not made available for most published pivotal trials in MS, we scoured a recently created (2015) Food and Drug Administration (FDA) website and Drug Trials Snapshots (DTS) for answers. The FDA’s Center for Drug Evaluation and Research (CDER) created DTS to share information on the racial diversity of patients in pivotal clinical trials. Consumers and healthcare professionals are provided with concise information by DTS about who participated in clinical trials that supported the FDA approval of new drugs and it is part of an overall FDA effort to make demographic data more available and transparent. The information in the Snapshots also highlights where the trials were conducted and whether there were any differences in the benefits and side effects among different demographic groups. While the intent and goals of the FDA in developing DTS are laudable, side-effect profiles among different demographic groups are non-sequitur since data cannot be collected from minority groups who are not included in the trials. For our analysis, we chose Caucasian, Black, Asian, or Hispanic as distinct groups that comprised patient demographics, as advocated previously by the FDA. (https://www.fda.gov/downloads/regulatoryinformation/guidances/ucm126396.pdf). All drugs listed in our study had FDA approvals between 2017 and 2020.
The problem of poor minority representation in clinical trials is probably pervasive across the medical landscape—minority enrollment in trials sponsored by the National Cancer Institute (NCI) showed that only a meager 2% of a total of 10 000 clinical trials had met criteria outlined by NCI 20 years prior to include minorities.Reference Chen, Lara and Dang 1 Similar to the paucity of enrollment in clinical trials outlined by the NCI, pivotal trial data in MS/NMOSD are scarce on the treatment responsiveness, drug tolerability or side-effects to disease-modifying drugs as inclusion rates for minorities, particularly Blacks, remains low.
We obtained baseline data from MS pivotal studies done for ocrelizumab,Reference Hauser, Bar-Or and Comi 2 siponimod,Reference Kappos, Bar-Or and Cree 3 and ozanimodReference Cohen, Comi and Selmaj 4 and from two studies for NMOSD—inebilizumabReference Cree, Bennett and Kim 5 and satrilizumab.Reference Yamamura, Kleiter and Fujihara 6 In our analyses, we compared baseline patient demographic data of each of the three MS drugs and two NMOSD drugs against published demographic data in DTS. We found that Black patients enlisted in the pivotal trials ranged from 0.6% (siponimod), 5.3% for OPERA study,Reference Hauser, Bar-Or and Comi 2 1.9% for ORATORIO,Reference Montalban, Hauser and Kappos 7 and 0.5% for RADIANCE.Reference Cohen, Comi and Selmaj 4 With the exception of OPERA 1 and 2 studies, the percentage of Black patients included in the pivotal clinical trials in MS was <2%.
Another recent MS drug pivotal trial data bungle involving race and inequity involves siponimodReference Liu and Obeng 8 and its metabolism. While CYPC29 gene alleles *1, *2, and *3 are more common in Caucasians, *5, *6, *8, and *11 are more prevalent in Black and Hispanic ancestry.Reference Liu and Obeng 8 Siponimod is contraindicated in individuals with a *3/*3 genotype owing to decreased/lack of function of the enzyme that metabolizes the drug. However, the genotypic variants for CYPC29 in siponimod metabolism among Blacks/Hispanics was not studied as they were not included in the pivotal study. Hence, the makers of siponimod may need to consider collecting additional postmarketing data in Blacks and Hispanics, specifically the CYPC29 gene allele distributions that are prevalent in these populations or issue a black box warning that pending more data collection, that the drug needs to used primarily in the Caucasian populations. An updated warning to providers should be given on CYP2C9 *5, *6, *8, and *11 alleles that may warrant a dose reduction and close monitoring for adverse effects.Reference Liu and Obeng 8
In the phase 3 study on satralizumabReference Yamamura, Kleiter and Fujihara 6 for NMOSD (# NCT02028884), 16/41 (39%) patients who received the drug were from Asia and 25/41 (61%) were from Europe. In the inebilizumabReference Cree, Bennett and Kim 5 study (# NCT02200770), Asians comprised 37/161 (23%), American Indian or Alaskan Natives comprised 11/161 (6.8%), whereas Blacks made up 14/161 (9%) indicating that efforts to enlist patients from diverse ethnic backgrounds was made. The epidemiology of NMOSD reveals that there is varying prevalence observed in different racial groups. The prevalence of NMOSD among Caucasians is ~1/100 000 population, with an annual incidence of <1/million population.
The population-based metrics of various racial backgrounds in MS/NMOSD are listed in Figure 1 and Table 1. We did not include percentages of “miscellaneous groups” given the paucity of information and inconclusive inferences that can be drawn from such low numbers. Data from NMOSD clinical trials offer a better representation of the racial diversity but enrolment among Blacks is poor. As for pivotal clinical trials in MS, linking funding or publication policies that link minority inclusion a priority is a first step in bridging the gap between clinical trial demographics and changing population statistics.

Figure 1. Racial distribution of multiple sclerosis (MS) trial participants who received investigational drug.
Table 1. Panels A and B Showing Distribution of Patients in MS and NMOSD Pivotal Clinical Trials, Respectively
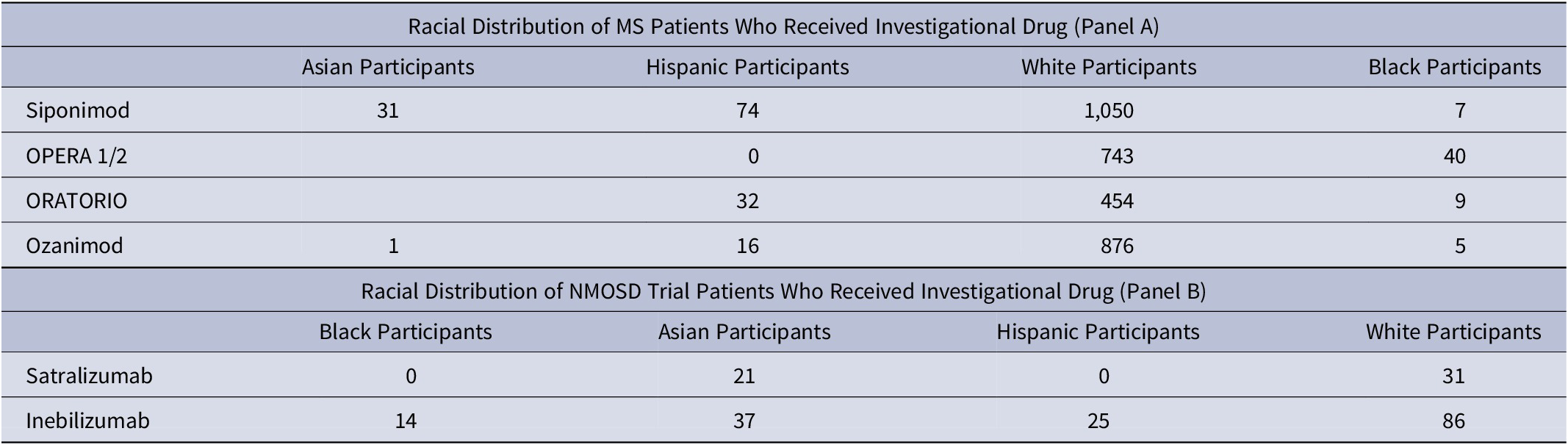
Raw numbers are shown.
Abbreviations: MS, multiple sclerosis; NMOSD, neuromyelitis spectrum disorder.
Disclosures
The authors declare that they have no conflict of interest in the submission of this manuscript to CNS Spectrums, and hereby declare that they have not received any third-party funding or payments for this work, and have no financial relationships with any entities that would preclude/influence submission of their work to the journal and have no patents or intellectual property relevant to the work submitted.




