Duchenne muscular dystrophy (DMD) is an X-linked, recessive, degenerative muscle disease, and the mdx mouse is an animal model of DMD( Reference Bulfield, Siller and Wight 1 ). Approximately one in 3500 boys are afflicted with the disease( Reference Blake, Weir and Newey 2 ). Compared with those in their wild-type counterparts on the same diet, skeletal muscle phospholipid (PL) in mdx mice exhibit increased oleic acid (18 : 1) and linoleic acid (18 : 2) abundances and reduced DHA (22 : 6) abundance( Reference Tuazon and Henderson 3 ). Results from similar assessments of fatty acid (FA) composition in the muscle of human subjects with DMD are unclear due to a lack of dietary control in these studies( Reference Kunze, Reichmann and Egger 4 – Reference Takagi, Muto and Takahashi 6 ) as well as issues related to age matching and sample handling( Reference Pearce, Johnsen and Wysocki 5 ). In DMD and the mdx mouse, the skeletal muscle sarcolemma is destabilised as a result of a mutation in the dystrophin gene( Reference Blake, Weir and Newey 2 ). Corticosteroid treatment is useful but not sufficiently effective( Reference Wong and Christopher 7 ), and lifestyle changes could be additionally useful to favourably alter the trajectory of disease progression. Little is known regarding appropriate dietary recommendations for patients with DMD. However, based on known dysfunction in dystrophic muscle, hypotheses can be generated. The skeletal muscle of DMD patients( Reference Tews and Goebel 8 , Reference Monici, Aguennouz and Mazzeo 9 ) and mdx mice( Reference Acharyya, Villalta and Bakkar 10 – Reference Messina, Bitto and Aguennouz 14 ) exhibits high levels of inflammation and membrane permeability or leakiness. Therefore, dietary treatments that could alter these aspects of cellular function might be useful. The importance of various dietary factors, including the dietary FA composition, has been investigated in great depth for other pathologies such as atherosclerosis, but in DMD, nutrition has received little attention. For a pathology as serious as DMD, nutrition alone is not expected to be the sole treatment approach, but sound dietary recommendations could assist in the management of the disease and could potentially slow the progression along with other treatments. As pathology is severe in dystrophin deficiency, subtle changes in diet might not overcome any meaningful portion of the cellular insult, but it may be that sizable changes in dietary components could alter disease severity.
α-Linolenic acid (ALA) is a major component of flaxseed oil and other oils such as rapeseed oil. ALA is the dietary essential n-3 FA and is a type of PUFA. ALA probably has direct effects on physiology and metabolism and is also converted to other bioactive n-3 FA. ALA is converted to EPA and DHA. These n-3 FA (ALA, EPA and DHA), and probably others such as docosapentaenoic acid, are expected to increase membrane fluidity and have anti-inflammatory properties( Reference Singer, Shapiro and Theilla 15 , Reference Treen, Uauy and Jameson 16 ). Along with these potential effects, increased membrane permeability may also be intrinsic to increases in n-3 FA abundance( Reference Ehringer, Belcher and Wassall 17 , Reference Stillwell, Ehringer and Jenski 18 ). Importantly, when increasing any dietary constituent, others may decrease in relative abundance. For example, when increasing PUFA intake while controlling for total unsaturated FA, MUFA intake could be reduced proportionally. However, similar to that of PUFA, intake of appreciable amounts of MUFA may lead to health benefits. So, it is unknown whether replacing a portion of the dietary oleic acid content (the predominant MUFA) with ALA (a dietary essential PUFA) would be beneficial. High MUFA intake has been linked to a reduced risk of heart disease in humans( Reference Kris-Etherton, Pearson and Wan 19 , Reference Schwingshackl, Strasser and Hoffmann 20 ). It is possible that MUFA have useful physiological effects on skeletal muscle, as well. So, an important and unanswered question would be whether enrichment of the dietary FA profile with oleate or with ALA is better for skeletal muscle health. Herein, we report effects of altering the balance between this MUFA and n-3 PUFA in the diet on dystrophic muscle.
Inflammation appears to play a role in the progression of DMD. The current therapeutic intervention to reduce inflammation is corticosteroid treatment; however, this causes adverse side effects( Reference Wong and Christopher 7 ). The primary reason for n-3 PUFA dietary treatments being considered for DMD is probably their anti-inflammatory properties. Generally, n-3 FA (ALA, EPA and DHA) are considered anti-inflammatory and n-6 FA (linoleic acid and arachidonic acid (ARA)) are considered pro-inflammatory. n-3 FA suppress the abundance of inflammatory cytokines and reduce the activity of NF-κB( Reference Ren and Chung 21 , Reference Zhao, Etherton and Martin 22 ). NF-κB exists as homodimers and heterodimers with p65 as a commonly abundant subunit. NF-κB regulates the transcription of genes involved in inflammatory responses and is highly activated in mdx muscle( Reference Acharyya, Villalta and Bakkar 10 – Reference Messina, Bitto and Aguennouz 14 ) and in DMD( Reference Monici, Aguennouz and Mazzeo 9 ). When NF-κB is quiescent, it is located in the cytoplasm in an inactive form bound to its inhibitor, IκB( Reference Baeuerle and Baltimore 23 ), with the p65 subunit in its unphosphorylated form( Reference Viatour, Merville and Bours 24 ). The phosphorylation of p65 at serine 536 (phospho-p65 (Ser536)) enhances translocation to the nucleus and transcriptional activity and so is closely associated with the activation of this inflammatory factor( Reference Viatour, Merville and Bours 24 ). Indeed, mdx mice exhibit enhanced abundance of phospho-p65 (Ser536) in skeletal muscle( Reference Singh, Millman and Turin 25 ). Therefore, we decided to assess the abundance of phospho-p65 (Ser536) as an index of NF-κB activity and inflammation in the skeletal muscle of mdx mice in response to dietary treatment.
Using the mdx mouse model, in the present study, we tested two different dietary FA compositions and the resulting impact on the selected markers of pathology in dystrophic mice. A high-MUFA diet was compared with a high-PUFA diet in which ALA was the predominant form of PUFA enrichment that was substituted for oleate. Skeletal muscle histopathology as well as markers of membrane leakiness and inflammation was assessed following consumption of the assigned diets. The results depict an impact of dietary FA composition on dystrophin-deficient animals and add critical data relevant to emerging concepts in dietary prescription for muscular dystrophy.
Experimental methods
Animals
Institutional and national guidelines for the care and use of animals were followed, and all experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee (IACUC) of Rutgers University (protocol no. 11-040). C57BL/10ScSn-Dmdmdx/J mice (‘mdx’) and C57BL/10ScSnJ control mice (‘C57’) were ordered for the purpose of breeding from Jackson Laboratories. Mdx breeders were fed a high-MUFA diet (high 18 : 1 FA abundance) or a high-PUFA diet (reduced 18 : 1 and high 18 : 3 abundances) (Research Diets), starting at least 4 weeks before breeding. In the high-PUFA diet, oleate level was reduced, and oleate was replaced with ALA such that the total unsaturated FA content was similar between the diets (Table 1). The study question was related to the dietary treatment of mdx mice. However, additionally C57 mice were studied on a single diet to determine how any diet-related reductions in pathology observed in mdx mice may compare in magnitude to pathological marker levels in healthy control mice. C57 breeders were fed only the high-MUFA diet to act as a control group and were started on the diet at least 4 weeks before breeding. We generated twenty-four male mdx mice (twelve in each dietary group) for the proposed experiments and nine C57 male control mice. Mdx mouse offspring were generated by breeding female homozygous mdx mice (mdx/mdx) with male hemizygous mdx mice (mdx/Y). From weaning to the conclusion of the study, male offspring were fed the same diet that their mothers had consumed. Mice were maintained under a 12 h light–12 h dark photoperiod, housed individually, and allowed ad libitum access to food and water. From weaning onwards, mice were weighed weekly, and their food was weighed weekly to calculate food intake. In using mdx mice as a model for human DMD, we only studied males in the present study, because dystrophin deficiency is X-linked and recessive, and thus it is primarily the male sex that is afflicted with DMD( Reference Blake, Weir and Newey 2 ).
Table 1 Dietary fatty acid (FA) composition*
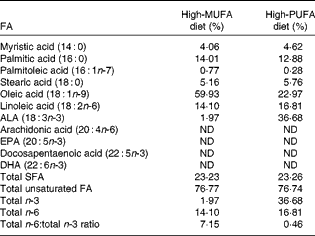
ALA, α-linolenic acid; ND, not detected.
* Values are molar percentages of FA in total lipids in the high-MUFA diet and high-PUFA diet. Total SFA (myristic acid, palmitic acid and stearic acid); total unsaturated FA (palmitoleic acid, oleic acid, linoleic acid, ALA, arachidonic acid, EPA, and docosapentaenoic acid, DHA); total n-3, (ALA, EPA and docosapentaenoic acid, DHA); total n-6, (linoleic acid and arachidonic acid).
Diet
Mice were fed the assigned diet until the time of testing and tissue collection at 8 weeks of age. The macronutrient distribution (expressed as percentage of energy) was 16 % fat, 62 % carbohydrate and 22 % protein. The dietary FA profiles, following the extraction and saponification of dietary lipids by our procedure, described previously( Reference Tuazon and Henderson 3 , Reference Liou, Tuazon and Burdzy 26 ), were analysed by liquid chromatography/MS (LC/MS) as described below. These dietary FA profile results are given in Table 1. Both diets contained similar amounts of saturated fat and unsaturated fat. Fat in the high-MUFA diet was comprised of differing amounts of refined olive oil, anhydrous butter, rapeseed oil, safflower oil and soyabean oil, with olive oil being the predominant fat source (65 % of total fat). Fat in the high-PUFA diet included small amounts of these other oils, but the predominant fat source was flaxseed oil (lignan free), making up approximately 75 % of the fat in the diet and generating an 18·6-fold difference in ALA content between the two diets and a 2·6-fold difference in oleate level.
Grip strength tests
Strength was measured with a grip strength meter (Columbus Instruments). Grip strength measurements were taken during the 8th week of life, 2 d before killing the mice. During grip strength assessments, front-limb strength was tested first with five tests in immediate succession, and then mice were allowed to rest for 1 h before testing total-limb strength. Total-limb strength assessments similarly consisted of five sequential tests. To measure front-limb strength, mice were briefly placed on a metal grid attached to the force transducer and pulled gently by the base of the tail until they released their grip. Peak tension (grams force) was recorded. To measure total-limb grip strength, an angled mesh grid was attached to the same force transducer (i.e. the grip strength meter). Mice were held by the tail and lowered onto the grid and allowed to grasp with all their four limbs, and then mice were pulled towards the force transducer until they released their grip, and peak compression (grams force) was recorded. Grip strength was recorded as the average of the three strongest efforts divided by the mouse's fat-free mass. Fatigue index was measured as a percentage of decline in force from the sum of the first two attempts to the sum of the last two attempts and was calculated as follows:
The investigator carrying out the grip strength tests was blinded to the group assignments of each mouse.
Body composition assessment
Body mass was measured once per week and immediately before body composition assessment. Body composition was assessed using an EchoMRI magnetic resonance system (Echo Medical Systems) during the 8th week of life, 2 d before killing the mice. From fat mass and body mass, fat-free mass and body fat percentage were calculated.
Tissue collection
All mice were killed by CO2 inhalation, and blood was collected by cardiac puncture and then tissues were rapidly collected subsequently. Tibialis anterior (TA) muscle from one limb and quadriceps muscle tissues were immediately frozen in liquid N2 and then stored at − 80°C until the time of biochemical analyses. TA muscle from the other limb was fixed in 10 % buffered formalin. Blood was allowed to clot, centrifuged to obtain serum and then stored at − 80°C until analysis.
Histology
Skeletal muscle samples were processed for histological analysis by the Cancer Institute of New Jersey and the Center for Molecular Medicine and Infectious Diseases at the Virginia–Maryland Regional College of Veterinary Medicine. TA muscle samples (fixed in 10 % buffered formalin) were dehydrated through an 8 h graded series of alcohols (70 %/80 %/95 %/100 %) followed by treatment with a clearing agent (xylene) before infiltration in Paraplast for 3 h. After embedding procedures, 4 μm sections were cut, and cross sections were mounted on glass slides. Haematoxylin and eosin staining was performed starting with heat-drying of the slides and multiple xylene deparaffinisation baths, followed by a series of alcohol steps down to water before dipping in a regressive haematoxylin solution for 10 min followed by that in water and then a dilute acid rinse and water wash with commercial bluing agent before dipping in an eosin counterstain for 2 min. The slides were subjected to multiple absolute alcohol dips ending in xylene (clearing step) before being coverslipped. Muscle degeneration was identified by swollen eosinophilic, hyalinised and pyknotic muscle fibres. Muscle regeneration was classified into two distinct morphological categories: immature muscle fibres containing centralised nuclei, typically small in diameter and with basophilic cytoplasm, and mature fibres with centralised nuclei, consistent in size, shape and staining with normal fibre morphology. Necrotic fibres were identified as swollen muscle fibres with disrupted cell membranes, invaded by inflammatory cells. Image analysis software (Image-Pro Plus; Media Cybernetics, Inc.) was used to analyse tissue sections for pathological markers in fields selected with battlement technique from the entire cross section of a TA muscle sample. A grid was superimposed over each selected field, and the number of intersections that overlay pathological markers was reported as a percentage of the total intersections that overlay muscle tissue. The investigator analysing the slides was blinded to the group assignments of each tissue.
Isolation of phospholipids from skeletal muscle
PL were isolated and recovered from TLC plates by the method reported by our group previously( Reference Tuazon and Henderson 3 , Reference Henderson 27 ) as follows. To extract lipids with the solvent mixture of Folch et al. ( Reference Folch, Lees and Sloane Stanley 28 ), samples of approximately 30 mg of TA muscle were homogenised using a Potter S Homogenizer (Sartorius-Stedim) in 5 ml of chloroform–methanol (2:1, v/v) containing 0·05 % butylated hydroxytoluene in 5 ml vessels housed in an ice water-bath. Next, 1 ml water was added followed by vigorous mixing, and then the solution was centrifuged at 750 g for 5 min to separate phases and to pellet any undissolved compounds. After centrifugation, the chloroform phase was transferred to a new tube and dried under a stream of N2. Lipids were reconstituted with 40 μl chloroform and spotted onto TLC plates (20 × 20 cm, Silica Gel G; Analtech) along with the standard in separate lanes (dioleoyl l-α-phosphatidylcholine). The TLC plates were developed for approximately 16 cm with hexane–diethyl ether–acetic acid (160:40:1). After drying, the plates were developed for approximately 10 min with chloroform–methanol–water (13:6:1). PL spots in standard lanes were visualised with iodine vapour, and PL of samples were scraped from the TLC plates and then re-extracted with 3 ml of chloroform–methanol (2:1, v/v). After centrifugation (750 g for 5 min), the supernatant was dried under N2, followed by the addition of 40 μg of heptadecanoic acid as an internal standard and then saponification to NEFA as described in detail previously( Reference Henderson 27 ). FA profiles were analysed by LS/MS as described below.
Liquid chromatography–MS
Using an Agilent 1200 HPLC system, an Ascentis C18 2·1 × 150 mm column (Sigma-Aldrich) and a Varian 1200L quadrupole mass spectrometer with electrospray ionisation, FA abundances were determined for total dietary lipids and for skeletal muscle PL following saponification. Mobile phase A was 80 % acetonitrile with 0·5 mm-ammonium acetate and mobile phase B was 100 % acetonitrile with 0·5 mm-ammonium acetate. The flow rate was 0·4 ml/min, and FA were eluted isocratically with 45 % mobile phase A and 55 % mobile phase B for chromatographic separation as described previously( Reference Henderson 27 ), followed by a column wash at higher organic strength. FA were identified by retention time and mass:charge ratio (m/z). The concentration of each FA was calculated based on external standard curves. The relative abundance of eleven FA in PL was calculated as molar percentage based on the following FA external standards purchased from Sigma-Aldrich or Nu-Check Prep: myristic acid (14 : 0); palmitic acid (16 : 0); palmitoleic acid (16 : 1n-7); stearic acid (18 : 0); oleic acid (18 : 1n-9); linoleic acid (18 : 2n-6); ALA (18 : 3n-3); ARA (20 : 4n-6); EPA (20 : 5n-3); docosapentaenoic acid (22 : 5n-3); DHA (22 : 6n-3).
Serum creatine kinase activity assay
Serum creatine kinase (CK) activity was assessed spectrophotometrically using a commercially available kit (StanBio Laboratory). The reaction was carried out at 37°C in a ninety-six-well plate format as a kinetic assay.
Skeletal muscle TAG content measurement
Approximately 5–10 mg of TA tissue were collected from a mid-belly slice of the muscle. Lipids were then extracted by the method of Folch et al. ( Reference Folch, Lees and Sloane Stanley 28 ) by homogenising in 2 ml of chloroform–methanol (2:1, v/v) for 2 min at 300 rpm using Potter S Homogenizer (Sartorius-Stedim). Next, 0·4 ml deionised water was added, and the homogenate was thoroughly mixed followed by centrifugation at 750 g for 5 min. The lower phase was isolated and dried under N2, and then TAG content was measured with a commercially available kit (Sigma-Aldrich) and was normalised to muscle wet tissue weight expressed as a percentage (w/w).
Phosphorylated p65 NF-κB subunit assay
Cell Lysis Buffer (Cell Signaling Technology) containing protease inhibitor (Complete Mini; Roche Applied Science), phosphatase inhibitor (Halt Phosphatase Inhibitor Cocktail; Thermo Scientific) and 1·5 % β-mercaptoethanol was used as the homogenisation buffer. Approximately 50 mg of quadriceps muscle tissue were added to a 20 × w/v homogenising buffer (i.e. approximately 1000 μl). The samples were homogenised for 2 min at 150 rpm on Potter S Homogenizer (Sartorius-Stedim) and then centrifuged at 10 000 rpm in a microcentrifuge for 15 min at 4°C to pellet insoluble proteins. The supernatant was stored at − 80°C. The protein concentration of homogenates was determined by the Bradford method( Reference Bradford 29 ) (BioRad) and diluted in Laemmli buffer with 5 % β-mercaptoethanol, followed by denaturation at 95°C for 5 min. BioRad Criterion precast gels (4–15 % Tris–HCl, 1·0 mm) were used for SDS-PAGE. The running buffer was Tris/glycine/SDS buffer (BioRad). The samples were loaded (30 μg protein) and then the gels were run at 100 V for approximately 10 min and then at 200 V for approximately 30 min. The transfer buffer was prepared with 2·96 g Tris, 1·46 g glycine, 450 ml water, 50 ml methanol and 2 ml of 10 % SDS. We carried out semi-dry transfer of samples onto nitrocellulose membranes with a BioRad Trans-Blot sd transfer cell at 17 V for 60 min. The membranes were then dried and blocked with 5 % milk in Tris-buffered saline for 1 h. After blocking, the membranes were incubated overnight at 4°C with rabbit anti-phospho-p65 (Ser536) antibodies (Cell Signaling Technology) at a 1:1000 dilution in 1 % milk in Tris-buffered saline with 0·1 % Tween 20 (TBST). The membranes were then washed with TBST and incubated for 1 h at room temperature with goat anti-rabbit secondary antibodies (Licor Biotechnology) at a 1:20 000 dilution in 1 % milk in TBST with 0·02 % SDS. The membranes were then washed with TBST and imaged. Subsequently, probing was carried out with rabbit anti-β-actin antibodies (1:000 dilution) (Cell Signaling Technology) in a similar fashion using the same secondary antibody as above. Imaging was performed with a Licor Odyssey fluorescence image scanner. Band intensities of phospho-p65 were normalised to those of β-actin.
Statistical analyses
Results are presented as means with their standard errors. Data were analysed by one-way ANOVA, as a 1 × 3 design for parameters in which data for the three groups are presented (C57 high-MUFA, mdx high-MUFA and mdx high-PUFA) and as a 1 × 2 design for parameters in which quantitative data are presented only for the two mdx groups. Fisher's protected least significant difference post hoc test was used following ANOVA as appropriate. Significance level was α = 0·05, and statistical analyses were conducted using JMP 10.0 software (SAS Institute).
Results
Skeletal muscle phospholipid acyl chain profile
Table 2 summarises the molar percentages of eleven FA in the skeletal muscle PL of mdx mice. The muscle PL of mdx mice on the high-PUFA diet contained a higher abundance of the n-3 FA ALA, EPA and docosapentaenoic acid (P< 0·05 for each) and displayed a trend for DHA to also be elevated (P= 0·09). ALA content in muscle PL was 20·7-fold higher in mdx mice on the high-ALA diet (i.e. high-PUFA diet) than in those on the high-MUFA diet. The abundance of EPA was 6·9-fold higher and that of docosapentaenoic acid was 2·4-fold higher in mdx mice on the high-PUFA diet than in those fed the high-MUFA diet. Overall, there was a higher abundance (2-fold difference) of total n-3 FA in the muscle PL of mdx mice on the high-PUFA diet than in that of mdx mice on the high-MUFA diet (P< 0·05). Oleate content in muscle PL was higher (1·8-fold) in mdx mice on the high-MUFA diet than in those on the high-PUFA diet (P< 0·05). Mdx mice on the high-PUFA diet had less ARA content in their muscle PL than those on the high-MUFA diet (6·4-fold difference; P< 0·05). Both mdx groups had similar abundances of linoleic acid. The total amount of n-6 FA was greater in mdx mice on the high-MUFA diet than in those on the high-PUFA diet (P< 0·05). Compared with mdx mice on the high-MUFA diet, those on the high-PUFA exhibited a lower ratio of total n-6:total n-3 FA in their muscle PL (4·4-fold difference; P< 0·05).
Table 2 Fatty acid (FA) composition in muscle phospholipids (PL)† (Mean values with their standard errors)
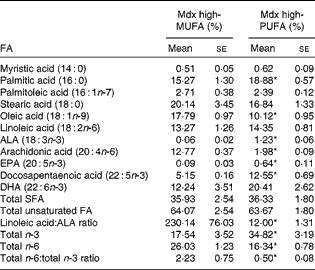
Mdx high-MUFA, mdx mice on the high-MUFA diet; mdx high-PUFA, mdx mice on the high-PUFA diet; ALA, α-linolenic acid. Sample sizes were 6 per group.
* Mean values were significantly different from those of the high-MUFA group (P< 0·05; ANOVA 1 × 2 design).
† Composition of FA in PL in the tibialis anterior muscle of mdx mice. PL compositions of C57 control mice were not analysed.
Body composition and assessments in vivo
Table 3 summarises group characteristics including body composition, grip strength and food intake. Mdx mice on the high-MUFA diet displayed lower body fat percentage than C57 mice on the same diet (P< 0·05). Body fat percentage was higher in mdx mice on the high-PUFA diet than in those on the high-MUFA diet (P< 0·05), but it was still lower than that in C57 mice (P< 0·05). Fat mass exhibited the same pattern of significant group differences as body fat percentage. Body mass and fat-free mass were higher in both mdx groups than in C57 mice (P< 0·05), with no effects of diet being observed in mdx mice. Despite differences in total body fat, skeletal muscle TAG content was not significantly different between the groups (C57: 0·18 (se 0·03) %; mdx high-MUFA: 0·18 (se 0·02) %; and mdx high-PUFA: 0·16 (se 0·03) %). Mdx mice compared with C57 mice exhibited less front-limb and total-limb grip strength (each normalised to fat-free mass) (P< 0·05), independent of the diet. There was no effect of diet on grip strength in mdx mice. Effects of dystrophin deficiency and diet on fatigue index generally followed a pattern similar to that of the effects on strength. Food intake was higher in mdx mice than in control mice (P< 0·05), with no effect of diet being observed in mdx mice.
Table 3 Group characteristics‡ (Mean values with their standard errors)
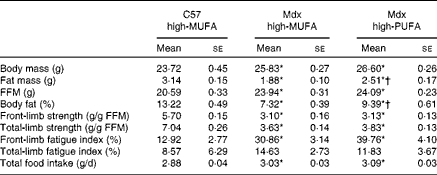
C57 high-MUFA, C57 control mice on the high-MUFA diet; mdx high-MUFA, mdx mice on the high-MUFA diet; mdx high-PUFA, mdx mice on the high-PUFA diet; FFM, fat-free mass. Sample sizes were 9 for C57 and 12 per group for Mdx.
* Mean values were significantly different from those of the C57 high-MUFA group (P< 0·05; ANOVA 1 × 3 design).
† Mean values were significantly different from those of the mdx high-MUFA group (P< 0·05; ANOVA 1 × 3 design).
‡ All data were obtained from measurements at 8 weeks of age.
Serum creatine kinase activity
Serum CK activity, as a marker of muscle membrane leakiness, is shown in Fig. 1. As expected, mdx mice exhibited higher serum CK activity than C57 mice on the same diet (P< 0·05). Mdx mice on the high-PUFA diet exhibited higher serum CK activity than those on the high-MUFA diet (approximately a 2-fold difference; P< 0·05).

Fig. 1 Serum creatine kinase (CK) activity. C57 high-MUFA, C57 control mice on the high-MUFA diet; mdx high-MUFA, mdx mice on the high-MUFA diet; mdx high-PUFA, mdx mice on the high-PUFA diet. Sample sizes were 9 for C57 and 12 per group for Mdx. Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different from those of the C57 high-MUFA group (P< 0·05; ANOVA as 1 × 3 design). † Mean values were significantly different from those of the mdx high-MUFA group (P< 0·05; ANOVA as 1 × 3 design).
Histology
Quantitative results obtained from the analysis of TA muscle following haematoxylin and eosin staining are shown in Fig. 2. There were no statistically significant differences between the mdx mice on different dietary treatments.

Fig. 2 Histopathology results for tibialis anterior following haematoxylin and eosin staining. Values are means, with their standard errors represented by vertical bars (sample sizes were 12 per group). MF-CN, mature fibres containing centralised nuclei; IMF-CN, immature muscle fibres containing centralised nuclei. No statistically significant differences were observed. Statistical analysis was carried out using an ANOVA 1 × 2 design. ![]() , Mdx mice on the high-MUFA diet; □, mdx mice on the high-PUFA diet.
, Mdx mice on the high-MUFA diet; □, mdx mice on the high-PUFA diet.
NF-κB phosphorylation
Phospho-p65 (Ser536) was assayed as a marker of NF-κB activity and inflammation in quadriceps muscle homogenates by Western blotting (Fig. 3). Mdx mice on the high-MUFA diet and those on the high-PUFA diet exhibited similar abundances of phospho-p65 (Ser536). Phospho-p65 was consistently detected in mdx mice, but was not detected in C57 mice, indicating higher inflammation in mdx mice than in C57 mice.

Fig. 3 Results obtained for phosphorylated p65 (phospho-p65) of NF-κB. Values are means, with their standard errors represented by vertical bars (sample size of 8 per group). C57 high-MUFA, C57 control mice on the high-MUFA diet; mdx high-MUFA, mdx mice on the high-MUFA diet; mdx high-PUFA, mdx mice on high n-3 PUFA diet. Bands were clearly visible for all mdx tissues, but were not visible for C57 tissues. Phospho-p65 was undetectable in the C57 high-MUFA group, and values were not significantly different between the mdx high-MUFA and mdx high-PUFA groups. Statistical analysis was carried out using an ANOVA 1 × 2 design.
Discussion
The major finding of the present study is that a high-MUFA diet in mdx mice is associated with approximately 50 % lower serum CK activity than that observed when MUFA are partially replaced with n-3 PUFA. This favourable effect of the high-MUFA diet was presumably due to decreased membrane leakiness. The primary treatment for DMD is that with corticosteroids (e.g. prednisone), which demonstrate some benefits but do not adequately ameliorate pathology in DMD and have undesirable side effects (e.g. increased appetite leading to obesity). Effective nutritional strategies may also help reduce dystrophic disease severity, but at present knowledge is limited. Using controlled diets to alter the FA composition of cellular membranes, our overarching goal was to determine an optimal dietary oil composition for boys with DMD. Consumption of a high-MUFA diet appears to be preferable to that of a high-PUFA diet for dystrophin deficiency. Despite no effect of the tested diets being observed on skeletal muscle inflammation or histopathology, based on the serum CK data, it appears that dietary FA composition may be a modulator of pathology in dystrophic animals.
Our expectation was that increasing ALA content and related n-3 PUFA content would lead to beneficial effects on the skeletal muscle of mdx mice and would rectify differences in tissue PL profile observed previously between C57 and mdx mice( Reference Tuazon and Henderson 3 ). We anticipated increased strength, reduced inflammation and improved muscle histology on high-PUFA diet consumption. Based on the anti-inflammatory properties of ALA, we expected to see a reduction in skeletal muscle inflammation. However, these hypotheses were unsupported by the present results. Actually, histopathology was not changed by the high-PUFA diet, and serum CK activity was more favourable with the high-MUFA diet. Though both dietary groups in the present study exhibited lower serum CK activity than typically observed on consumption of standard diets used in previous work( Reference Tuazon and Henderson 3 , Reference Evans, Call and Bassaganya-Riera 11 , Reference Payne, Yasuda and Bourgeois 30 ), serum CK activity observed on high-MUFA diet consumption was even lower than that on high-PUFA diet consumption. Importantly, to accommodate elevated ALA content in the high-PUFA diet, oleic acid content was reduced. Oleic acid (or MUFA overall) may be important for muscle health, and previously we observed approximately 2-fold elevation of oleate content in the PL of mdx muscle over that of C57 muscle( Reference Tuazon and Henderson 3 ). It is possible that this increase in oleate content is compensatory to cellular stresses imposed by dystrophic pathology and that a high-MUFA diet, providing ample oleate, assists dystrophic muscle to cope with the stress of the pathology. High oleate intake (i.e. high-MUFA diet compared with the high-PUFA diet) was associated with lower serum CK activity without any effect on grip strength. Thus, to understand the possible utility of a high-MUFA diet, we should address what the benefit of reducing muscle membrane permeability could be, even in the absence of changes in strength or inflammation. Future work with Evans Blue Dye staining or intracellular Ca concentration assay could confirm this inference of changes in membrane permeability. Over time, if a high-MUFA diet reduced sarcolemmal leakiness, there would be a lessening of the insult associated with the influx of Ca and other extracellular compounds. This may favourably alter the age-related trajectory of fibre loss and accumulation of fibrosis and other non-contractile components such as lipids in muscle. So, the high-MUFA diet, consumed lifelong, could be useful and improve muscle health even more at older ages, despite no difference being observed in strength at young ages. It may be appropriate in combination with other treatments, such as corticosteroids and other compounds that can reduce inflammation and improve strength. It is unknown whether the diets used in the present study (high-MUFA diet v. high-PUFA diet) affect serum CK activity even in non-dystrophic animals; this could be an important future area of research that would potentially have implications for numerous aspects of muscle health and physiology. Based on the results obtained for body composition in the present study (body fat percentage lower in mdx mice on the high-MUFA than in those on the high-PUFA diet), a high-MUFA diet may also be useful for preventing the accumulation of body fat over time in dystrophin deficiency. Of note is that muscle TAG content was not affected by the dietary FA profile, while total body fat was altered, so it appears that excess lipids were not deposited ectopically in muscle, but rather might have been stored in adipose tissue. It is unknown why the high-PUFA diet increased body fat percentage in mdx mice. However, it has been reported that muscle protein turnover rates are elevated in mdx mice( Reference MacLennan and Edwards 31 ) and in DMD( Reference Balagopal, Olney and Darmaun 32 ), while work in rats has shown that a high intake of n-3 FA can reduce muscle protein turnover( Reference Sohal, Baracos and Clandinin 33 ). If the high intake of n-3 FA in the high-PUFA group blunted muscle protein turnover rates in mdx mice in the present study, the reduction in this energetically demanding process could explain increased total body fat storage.
Altered membrane properties, including increased fluidity, may exacerbate stress in dystrophic skeletal muscle. MUFA may be more desirable dietary constituents than PUFA, because they are more saturated and so could allow for a more dense packing of PL molecules in the muscle membranes, reducing the leakiness. Further elevation of MUFA levels and further reduction of PUFA levels in mdx mice might be even more advantageous than the degree of dietary manipulation that we tested, and it is possible (but currently untested) that SFA are even more beneficial than MUFA. It is conceivable that in DMD a higher degree of saturation of FA in membrane PL is protective against pathological severity through reduction in leakiness or some other unidentified aspect of membrane properties or muscle physiology. Previously, we observed a reduced content of highly unsaturated n-3 FA in mdx skeletal muscle PL compared with C57 skeletal muscle PL( Reference Tuazon and Henderson 3 ), and this alteration may be protective. When designing diets for individuals with DMD, it may be best to focus on oils with high MUFA content and reasonably low PUFA content (e.g. olive oil). Again, it should be noted that it would be worth testing in the future whether saturated fat (or even trans-fat) is better than cis-MUFA. Indeed, along these lines, it has been shown that SFA intake is superior to n-3 FA intake at improving survival in a hamster model of limb-girdle muscular dystrophy( Reference Galvao, Brown and Hecker 34 ).
Nutritional supplementation using n-3 PUFA exerts therapeutic effects on several inflammatory disorders. DMD is a disease characterised by high levels of inflammation in skeletal muscle, so one could reasonably expect utility of n-3 PUFA intake for dystrophic muscle. In the present study, the ALA-enriched diet successfully increased EPA content in the skeletal muscle of mdx mice on the high-PUFA diet and also tended to increase DHA content and significantly reduce ARA content. As DMD is characterised by elevated levels of inflammation, it is surprising that this change in tissue FA profile was not advantageous. However, membrane FA composition per se, through effects on membrane characteristics, may be more critical than inflammation, and possibly oleate may be more important than the balance of n-3 v. n-6 FA abundance. Inflammation was not reduced in the mdx high-PUFA group as indicated by the phospho-p65 results. The expected reduction in inflammation due to reduced ARA content and increased n-3 FA content may have been countered by an equal increase in inflammation from an alternative pathway. Finally, we acknowledge that the ALA dose was high in the high-PUFA diet. We chose this dose because ALA content in muscle PL is expected to be relatively lower than the representation of this FA in dietary lipids( Reference Tuazon and Henderson 3 ), and the present results (Tables 1 and 2) confirmed this expectation. It appears that there is a strong propensity towards rapid elongation or oxidation of dietary ALA rather than a high affinity for its direct incorporation into muscle membranes, so a high intake of ALA was needed to even achieve a modest increase in ALA content in muscle. Nonetheless, we concede that lower doses could be tested; it remains possible that a more modest degree of supplementation with ALA could be less detrimental or possibly even beneficial in dystrophin deficiency.
Recent studies( Reference Machado, Mauricio and Taniguti 35 , Reference Fogagnolo Mauricio, Minatel and Santo Neto 36 ) have suggested that n-3 PUFA supplementation with pure EPA or with fish oil decreases muscle degeneration and inflammation in mdx mice. Although we used ALA as the n-3 FA source in the present study, we even achieved an elevation of EPA content in muscle PL in addition to the expected elevation of ALA content. However, despite enhancing total n-3 FA and EPA availability, we could not confirm the previously reported benefits of EPA( Reference Machado, Mauricio and Taniguti 35 ) or EPA/DHA mix from fish oil( Reference Fogagnolo Mauricio, Minatel and Santo Neto 36 ), and instead we observed a detriment. It may be that, even despite the effects of ALA ingestion on tissue EPA levels, the effects of ALA intake on muscle are still qualitatively different from those of marine n-3 FA (EPA and DHA) intake. However, there are also other possible explanations for the disagreement between the results of the present study and those of previous work in which EPA or fish oil was supplemented to mdx mice. Reasons for the disparity between the findings of the present study and those of the previous work( Reference Machado, Mauricio and Taniguti 35 , Reference Fogagnolo Mauricio, Minatel and Santo Neto 36 ) could be related to the age of the animals used, as very young mice were investigated previously, as well as the duration of treatment. Mice in the previous work were supplemented with pure EPA( Reference Machado, Mauricio and Taniguti 35 ) or fish oil( Reference Fogagnolo Mauricio, Minatel and Santo Neto 36 ) for a 16 d period beginning 14 d after their birth. Those mice were approximately 4 weeks old at the time of assessments, which yielded interesting results, but may be too early to show the prolonged effects. If benefits of n-3 FA are transient, then 2 weeks of treatment may result in benefits that would not persist to later time points. The mice in the present study were fed their specified diets during prenatal development and throughout the course of their 8-week postnatal lives, presumably through milk in the first 3 weeks until weaning and then certainly through the pelleted diets in the subsequent 5 weeks of life. The mice in the present study were assessed at 8 weeks of age with lifelong treatment (instead of at 4 weeks of age with 2 weeks of treatment as in the previous work). This aspect of the present study design may be preferable for observing longer-term dietary effects, and future work could examine dietary effects in a more detailed and extended time course.
In a hamster model of limb-girdle muscular dystrophy, investigators found that an ALA-enriched diet led to improved muscle histological appearance when animals were fed this diet from weaning to killing 150 d later( Reference Fiaccavento, Carotenuto and Vecchini 37 ). The results of this study are at odds with those of the present study regarding the role of ALA as a dietary constituent for the treatment of dystrophic muscle, and we consider the results of the previous study( Reference Fiaccavento, Carotenuto and Vecchini 37 ) to be weakened by the absence of a well-controlled diet design. The hamsters were fed either a control diet consisting of a standard pelleted diet or an ALA-enriched diet consisting of flaxseeds, apples and carrots. Although the investigators did observe an association between the n-3 PUFA-enriched diet and muscle health in dystrophic hamsters, there would have been numerous differences in macronutrient and micronutrient contents between those diets in addition to the FA composition, and any of these differences could have been the driver of the improvements in muscle health. In the present study, we controlled the diets such that the contents were identical aside from their FA compositions, and we held total SFA and total unsaturated FA contents constant between the diets. Using a carefully controlled diet design allowed us to confidently infer that a high intake of n-3 PUFA may be harmful for dystrophic muscle and that MUFA may be relatively advantageous. It is also possible that the disparity between the conclusions of the present study and those of the previous study( Reference Fiaccavento, Carotenuto and Vecchini 37 ) are related to differences in the disease model (mice v. hamsters and dystrophin v. δ-sarcoglycan deficiency). However, other work in this δ-sarcoglycan-deficient hamster model has shown a superiority of saturated fat over n-3 PUFA for survival( Reference Galvao, Brown and Hecker 34 ), and this finding is congruent with the findings of the present study.
In summary, in the present study, mdx mice fed high amounts of MUFA exhibited reduced serum CK activity, which may indicate reduced sarcolemmal leakiness in comparison with that in mdx mice consuming high amounts of n-3 PUFA. Future work in the mdx model may also address other FA classes in addition to MUFA and n-3 PUFA as dietary constituents, as well as different ages and treatment durations. The present results, and those of future work on the topic, can be considered when formulating dietary recommendations for DMD. In conclusion, caution is needed while considering flaxseed oil-derived n-3 FA as a possible treatment for dystrophic muscle. Based on our preclinical testing, it is not necessarily advisable to proceed to clinical trials in DMD to test n-3 FA supplementation without further preclinical testing of efficacy and safety, and such dietary supplementation should not be recommended for individuals with DMD at this point. The present results reveal that n-3 FA supplementation could actually do harm; in the present study, results were obtained for ALA, but they might also have implications for the longer-chain n-3 FA. Generally, MUFA intake may be preferable over PUFA intake in the mouse model of DMD. The results indicate that manipulation of dietary FA composition can have an effect in dystrophin deficiency and this dietary manoeuvre needs to be subsequently refined to optimise the potential clinical impact.
Acknowledgements
The authors thank the laboratory staff at the Histopathology and Imaging Laboratory of the Cancer Institute of New Jersey and the Center for Molecular Medicine and Infectious Diseases at the Virginia–Maryland Regional College of Veterinary Medicine. They also thank Laboratory Animal Services staff at Rutgers University for assistance with animal husbandry and D. Weinstock for contributions to data collection and manuscript preparation.
The present study was supported by the Division of Life Sciences at Rutgers University.
The authors' contributions are as follows: G. C. H. conceived and designed the experiment, performed sample collection and data acquisition, analysed the results, interpreted the findings and wrote the manuscript; N. P. E. performed data acquisition, analysed the results, interpreted the findings and wrote the manuscript; R. W. G. interpreted the findings and wrote the manuscript; M. A. T. performed sample collection and data acquisition and wrote the manuscript.
None of the authors has any conflicts of interest.








