Mother’s milk is considered optimal for all infants as its constituents provide benefits beyond the basic nutrition needed to achieve daily macronutrient and energy requirements(Reference Victora, Bahl and Barros1). Although the benefits related to the bioactive immunological proteins in human milk have been well established, a new emerging area of research is focused on the bioactive lipid metabolome. It is understood that the majority of lipids in human milk are found as TAG, containing a variety of unique and essential fatty acids(Reference Koletzko, Rodriguez-Palmero and Demmelmair2). During lipolysis, fatty acids from the glycerol backbone of the TAG are released and can be absorbed(Reference Schweiger, Eichmann and Taschler3). TAG containing PUFA, including DHA and arachidonic acid (ARA) are of particular importance as they are necessary for brain development and growth(Reference Krohn, Demmelmair, Koletzko, Duggan, Watkins, Koletzkc and Walker4).
Oxidised metabolites of PUFA, known as oxylipins, have been detected in human milk, and are produced from lipid auto-oxidation or enzymatic activity (via cyclo-oxygenase, lipoxygenase and cytochrome P450 pathway)(Reference Yang, Schmelzer and Georgi5). Oxylipins are often bioactive and may function as potential signalling mediators, involved with cell proliferation, apoptosis, tissue repair and immune cell behaviour(Reference Yang, Schmelzer and Georgi5). The functions of many oxylipins are still under investigation. In vitro studies have demonstrated that some species of oxylipins isolated from human milk, including resolvins, maresins, protectins and lipoxins, may enhance macrophage activity and resolve acute inflammation. In fact, one study assessing the in-hospital growth of preterm infants suggested that lower levels of certain oxylipins derived from dihomo-γ-linolenic acid in human milk (15S-HpEDE (15S-hydroperoxy-11Z,13E-eicosadienoic acid) and two deoxy-dimethyl PGE2) were associated with a faster infant growth rate(Reference Alexandre-Gouabau, Moyon and Cariou6).
Pasteurisation of human milk is primarily conducted at milk banks to produce donor milk (DM) for vulnerable preterm infants (Holder method: 62·5°C for 30 min), but, in low resource settings and online, flash heating (bring to boil) is also used to pasteurise milk(7, 8). A well-known consequence of human milk pasteurisation is the loss of heat-sensitive vitamins (including vitamin C and folate) and bioactive components (including bile salt-stimulated lipase and lactoferrin)(Reference O’Connor, Ewaschuk and Unger9, Reference Peila, Emmerik and Giribaldi10). Previous research has demonstrated that high hydrostatic pressure processing (HHP), a promising non-thermal method of pasteurisation, can improve retention of nutrients and bioactives compared with Holder pasteurisation, flash heating or UVC irradiation(Reference Pitino, Unger and Doyen11).
Although there has been extensive research characterising total lipid concentrations in human DM following Holder pasteurisation, few studies have investigated fatty acid composition(Reference Delgado, Cava and Delgado12, Reference Moltó-Puigmartí, Permanyer and Castellote13). Moreover, our understanding into potential effects of UVC and HHP treatment of DM on the fatty acid profile is limited(Reference Delgado, Cava and Delgado12, Reference Moltó-Puigmartí, Permanyer and Castellote13). Oxylipins have been detected in human milk; however, it is unknown whether pasteurisation (both thermal and non-thermal) can affect their concentration(Reference Arnardottir, Orr and Dalli14). Maintaining the concentrations of oxylipins in human milk may have important implications for infant health given their potential immunomodulary properties. The aim of the present study, then, was first, to compare changes in fatty acid composition (saturated, monounsaturated and polyunsaturated) between raw, unpasteurised milk, conventional thermal pasteurisation (Holder and flash heating), as well as non-thermal pasteurisation methods (UVC irradiation and HHP). The second goal was to assess changes in PUFA-derived oxylipins as a result of pasteurisation. We hypothesised that non-thermal methods would result in fewer changes to fatty acid composition and oxylipins concentrations relative to thermal methods.
Materials and methods
Milk collection and preparation
The present study is a subanalysis of a study described previously by Pitino et al. (Reference Pitino, Unger and Doyen11). Ethics approval was received from both Mount Sinai Hospital and The Hospital for Sick Children. The study was also approved by The Human Milk Banking Association of North America prior to collection of human DM. In brief, human milk from seventeen different donors known to contain a bacterial load of >5 × 107 colony-forming units/l was collected from the Rogers Hixon Ontario Human Milk Bank. This milk was used as its bacterial load disqualified it for infant consumption according to the local milk bank policy. For the purpose of the present study, milk from eight donors was chosen at random to undergo additional lipid analyses. Milk was pooled in a 2 litre glass beaker and gently warmed in a water bath (37°C) until a consistent temperature was maintained for 5 min. Aliquots for analysis were taken from each of the pools prior to pasteurisation and frozen at –80°C. The remainder of the pool was separated into four containers with approximately 400 ml of milk each and frozen at –20°C until pasteurisation. Prior to pasteurisation, frozen milk was thawed overnight at 4°C and warmed up to 37°C the next day to ensure homogeneity.
Pasteurisation techniques
Expressed human milk was pasteurised using four different techniques: Holder pasteurisation, flash heating, UVC and HHP. The Holder method (62·5°C for 30 min) was carried out using a tabletop pasteuriser (Sterifeed T30; Medicare Colgate Ltd). Flash heating was conducted as described previously with some modification(Reference Israel-Ballard, Abrams and Coutsoudis15). Samples of human milk were poured into heat-resistant glass media jars and heated on high heat in a beaker containing 1 litre of room-temperature water. UVC irradiation was carried out according to a previously published protocol by Christen et al. (Reference Christen, Lai and Hartmann16) with modification using a germicidal lamp (2·3 W; Phillips TUV-PL-L). Samples of human milk were poured into polypropylene graduated cylinders and the UV lamp was submerged. To ensure that the human milk was culture negative (<1 × 103 colony-forming units/l), 25 min of UV exposure was determined to be necessary. HHP was carried out in a commercial-scale system using water (4°C) as the transmission medium (model 135 Hiperbaric) at 500 MPa for 8 min.
GC analysis of total fatty acids
Lipids were extracted from human milk following methodologies adapted from Folch et al. (Reference Folch, Lees and Sloane Stanley17). In brief, 200 μl samples of milk were homogenised and extracted in 2:1:0·8 chloroform–methanol–potassium chloride (0·88 %) with a known amount of C22 : 3n-3 internal standard (NuChek-Prep) as described previously(Reference Chen, Liu and Bazinet18). Extracted samples were then dried down under nitrogen gas, and methylated by heating at 100°C in 0·3:1 hexane–boron trifluoride methanol (Sigma Aldrich). Deionised water was first added to stop the methylation reaction. Further, hexane was added to isolate the fatty acid methyl esters. Fatty acid methyl esters were quantified according to previously published methods using a Varian-430 gas chromatograph (Varian)(Reference Chen, Liu and Bazinet18, Reference Lin, Chen and Hildebrand19). A quantity of 1 μl of sample was injected (splitless mode) into the capillary column (Agilent DB-23; 30 m × 0·25 mm internal diameter, 0·25 µm film thickness) used to separate the fatty acid methyl esters. A temperature of 250°C was used for both the injector and detector. The oven temperature was initially set at 50°C for 2 min, and then increased at 20°C/min until a temperature of 170°C. After 1 min at 170°C, the temperature was increased by 3°C/min until a final temperature of 212°C was reached and was held for 5 min. Helium was used as the carrier gas (0·7 ml/min). Peak identification was conducted using certified fatty acid methyl esters standards (Nu-Chek Prep, Inc.). Fatty acid methyl esters peak analysis was conducted using Compass CDS software (version 3.0.0.68; Scion Instruments) by proportional comparisons with the C22 : 3n-3 internal standard.
Liquid chromatography-tandem MS analysis for oxylipins
Oxylipins were quantified by liquid chromatography-tandem MS using a method adapted from a previously published protocol(Reference Colas, Shinohara and Dalli20). All oxylipin standards were purchased from Cayman Chemicals Company, with the exception of thioredoxin B3 and thioredoxin A3, which were provided by Dr Cecil Pace-Asciak (The Hospital for Sick Children). External standards were diluted in ethanol to produce a nine-point calibration curve (0·01–10 ng). An internal standard mixture (in ethanol) was added to each 100 μL milk sample and calibration curve standards alike prior to extraction. Table 1 summarises the oxylipins detected and the deuterated internal standards used in quantification. Oxylipins that were measured but undetected in any sample are presented in the online Supplementary Table S1. To limit production of auto-oxidative products during extraction, samples were kept on ice in dim light conditions. Isolation of oxylipins was conducted using a solid-phase extraction system (Vac Elut SPS-24 Varian) as described previously by Alashmali et al. (Reference Henderson, Fay and Hamosh21). In brief, solid phase C18 cartridge (Sep-Pak, Waters) was equilibrated using 12 ml of methanol and water. Dilute HCl solution (pH 3·5) was added to each sample, which was immediately transferred to the C18 cartridge. Cartridges were washed with hexane and eluted with methyl formate. Residues were evaporated under nitrogen gas (Organomation), reconstituted in a 1:1 ratio of acetonitrile and water and transferred into amber vials for liquid chromatography-tandem MS analysis.
Table 1. Oxylipins detected in raw and pasteurised human milk, organised by parent fatty acid and deuterated internal standard (IS) for quantification

LA, linoleic acid; GLA, γ-linolenic acid; ALA, α-linolenic acid; DHET, dihydroxy eicosatrienoic acid; OxoETE, oxo-eicosatetraenoic acid; HETE, hydroxyl eicosatetraenoic acid; EET, epoxy eicosatrienoic acid; PGF2a; PG F2-α; DHy-TxB2, dehydroxy thromboxane B2; HODE, hydroxyl octadecadienoic acid; HOTrE, hydroxyl octadecatrienoic acid; OxoODE, oxo-octadecadienoic acid; DiHOME, dihydroxy octadeca(mono)enoic acid; EpOME, epoxy octadecenoic acid; EpETE, epoxy eicosatetraenoic acid; EpDPE, epoxy docosapentaenoic acid; DiHETE, dihydroxy eicosatetraenoic acid; DiHDPA, dihydroxy docosapentaenoic acid.
A 1290 UHPLC (Agilent Technologies) and a QTRAP5500 mass spectrometer (Sciex) were used for liquid chromatography-tandem MS analysis using a previously published protocol(Reference Alashmali, Kitson and Lin22). Quantitative analyses were conducted using Multiqant 3.0.1 software (Sciex). The area of each integrated peak was plotted against the calibration curve for quantification. The limit of quantification was determined to be 0·01 ng per sample.
Statistical analysis
Statistical analyses were conducted using SAS (version 9.4; SAS Institute). The normality of the outcome variables was verified (PROC UNIVARIATE). Mean oxylipin and fatty acid concentrations were compared across each group (raw, Holder, flash heat, UVC and HHP) by ANOVA, using mixed models (PROC MIXED). If a statistically significant difference was found, pairwise comparisons were conducted (LS-MEANS). For all statistical analyses, P values <0·05 were considered significant.
Results
Total lipid fatty acid composition
Composition of esterified fatty acids and NEFA was grouped by saturation: (1) SFA, (2) MUFA and (3) PUFA. The percentage composition of SFA is presented in Table 2. With the exception of C21 : 0, there were no statistically significant changes in the composition of SFA compared with raw milk and between all pasteurisation methods. Compared with raw milk, UVC had significantly lower C21 : 0 (all P < 0·05). SFA constitute approximately 41 % of total fatty acids, with C16 : 0 being the most abundant SFA (21 %). Similarly, MUFA constitute approximately 42 % of total lipids, with oleic acid (C18 : 1n-9) as the largest contributor (36 % of total lipids). Table 3 summarises the percentage composition of MUFA in raw and pasteurised milk. Overall, there were no statistically significant differences between raw milk compared with any pasteurisation method. Much like SFA, there were no detectable differences between methods. The percentage composition of PUFA is presented in Table 4. PUFA contributed approximately 17 % towards to total lipids extracted from DM, with the most common fatty acid being linoleic acid (LA; C18 : 2n-6). The n-6:n-3 ratio remained relatively constant between raw and pasteurised milk (range = 6·4–6·6). There were no significant changes observed in PUFA concentrations compared with raw concentrations and among pasteurisation groups.
Table 2. Composition of SFA in human donor milk before and after pasteurisation*
(Mean values with their standard errors)

HHP, high hydrostatic pressure processing.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* All values expressed as mean percentage of total fatty acids unless specified otherwise.
† Values expressed as mg/g of human milk.
Table 3. Composition of MUFA in human donor milk before and after pasteurisation*
(Mean values with their standard errors)

HHP, high hydrostatic pressure processing.
* All values expressed as mean percentage of total fatty acids unless specified otherwise.
† Values expressed as mg/g of human milk.
Table 4. Composition of PUFA in human donor milk before and after pasteurisation*
(Mean values with their standard errors)

HHP, high hydrostatic pressure processing.
* All values expressed as mean percentage of total fatty acids unless specified otherwise.
† Values expressed as the n-6:n-3 ratio.
‡ Values expressed as mg/g of human milk.
Arachidonic acid-derived oxylipins
Concentrations of ARA-derived (C20 : 4n-6) oxylipins are shown in Fig. 1. Statistically significant differences in pairwise comparisons were only found for the following oxylipins: (±) 5,6-DHET lactone (P < 0·003), 15-OxoETE (P < 0·03), 5-HETE (P < 0·02), 5,6-EET, P < 0·03), 11,12-EET (P < 0·001), 14,15-EET (P < 0·03) and (all P < 0·03). The ARA-derived oxylipins, 5,6-DHET lactone, 5,6-EET, PGF2α, were higher in milk pasteurised by UVC compared with raw milk and to the other pasteurisation techniques. The ARA-derived oxylipins, 15-OxoETE, 5-HETE, were highest overall following HHP. This is in contrast to 11,12-EET and 14,15-EET, where levels following HHP were lowest. In general, milk pasteurised by HHP was more variable in its ARA-derived oxylipin content. For expansions of oxylipin abbreviations, see Table 1 and Fig. 1.

Fig. 1. Changes in arachidonic acid-derived oxylipins following pasteurisation of human donor milk. Data are presented as medians (first and third quartiles), n 8 for each group. Whiskers were calculated to a maximum of 1·5 times the interquartile range. Mean differences were assessed using linear regression models (PROC MIXED) followed by pairwise comparisons (LSMEANS) if P value <0·05. a,b,c,d Median values with unlike letters were significantly different (P < 0·05). DHET, dihydroxy eicosatrienoic acid; OxoETE, oxo-eicosatetraenoic acid; DHy-TxB2, dehydroxy thromboxane B2; HETE, hydroxyl eicosatetraenoic acid; EET, epoxy eicosatrienoic acid; PGF2a, PG F2-α; HHP, high hydrostatic pressure processing.
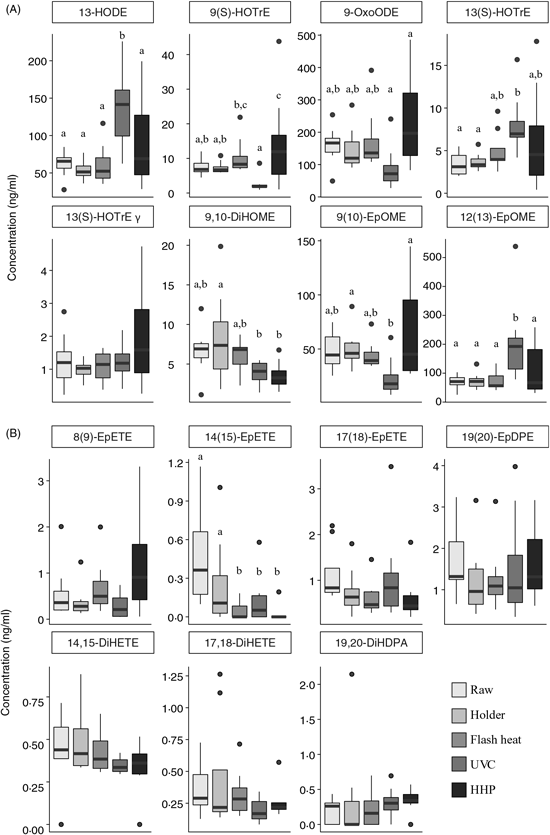
Oxylipins derived from 18-carbon fatty acids
Oxylipins derived from 18-carbon PUFA, including α-linolenic acid (18 : 3n-3), LA (18 : 2n-6) and γ-linolenic acid (18 : 3n-6) are shown in Fig. 2(A). In all measured oxylipins, with the exception of 13 (S)-HOTrE γ (as γ-linolenic acid oxylipin), there were significant changes as a result of pasteurisation. Oxylipins derived from 18-carbon PUFA after HHP are more variable. HHP-treated human DM resulted in higher concentrations of 9(S)-HOTrE (as α-linolenic acid oxylipin, P<0·04) compared with raw milk and other pasteurisation techniques. Holder pasteurisation and flash heating did not significantly alter the concentration of any oxylipins derived from 18-carbon fatty acids; however, UVC pasteurised milk had higher quantifiable levels of 13-HODE (as LA oxylipin, P = 0·0013) and 12(13)-EpOME (P = 0·0018) compared with raw milk.

Fig. 2. Changes in 18-carbon-, EPA- and DHA-derived oxylipins following pasteurisation of human donor milk. Data are presented as medians (first and third quartiles), n 8 for each group. Whiskers were calculated to a maximum of 1·5 times the interquartile range. Mean differences were assessed using linear regression models (PROC MIXED) followed by pairwise comparisons (LSMEANS) if P value <0·05. a,b,c Median values with unlike letters were significantly different (P < 0·05). (A) 18-Carbon-derived oxylipins; (B) EPA- and DHA-derived oxylipins. HODE, hydroxyl octadecadienoic acid; HOTrE, hydroxyl octadecatrienoic acid; OxoODE, oxo-octadecadienoic acid; DiHOME, dihydroxy octadeca(mono)enoic acid; EpOME, epoxy octadecenoic acid; EpETE, epoxy eicosatetraenoic acid; EpDPE, epoxy docosapentaenoic acid; DiHETE, dihydroxy eicosatetraenoic acid; DiHDPA, dihydroxy docosapentaenoic acid; HHP, high hydrostatic pressure processing.
EPA and DHA-derived oxylipins
Concentrations of EPA 20 : 5n-3 and DHA 22 : 6n-3-derived oxylipins are shown in Fig. 2(B). With the exception of 14(15)-EpETE (an EPA oxylipin), there were no statistically significant changes in concentration. Flash heat, UVC and HHP had significantly lower levels of 14(15)-EpETE as compared with raw or Holder pasteurised milk. Overall, there was no discernable trend in EPA/DHA-derived oxylipins.
Undetectable oxylipins in any sample
Many other oxylipins were measured in the analysis, presented in the online Supplementary Table S1, but were not detected.
Discussion
To our knowledge, this is the first study to concurrently assess fatty acid composition and oxylipin concentrations in human DM undergoing conventional thermal pasteurisation and non-thermal alternatives. Of the pasteurisation techniques studied (Holder, flash heating, UVC and HHP), there were few changes to the composition of fatty acids in human DM. Similar results have been found by others. In terms of Holder pasteurisation, out of four studies that previously investigated fatty acid composition in raw and pasteurised DM, three studies found no significant changes in fatty acid composition(Reference Delgado, Cava and Delgado12, Reference Moltó-Puigmartí, Permanyer and Castellote13, Reference Henderson, Fay and Hamosh21), whereas one study only found a 6 % reduction in total fatty acids as measured by GC(Reference Galvan, Maggini and Lepri23). This result is consistent with our previous observation whereby the concentration of total fat following all pasteurisations, as measured by mid-IR human milk analyser, was not significantly different from raw, unpasteurised milk, nor differed among the pasteurisation methods(Reference Pitino, Unger and Doyen11). It is important to note that the fatty acid composition in the DM used in the present study is comparably with previously reported studies(Reference Yuhas, Pramuk and Lien24).
To our knowledge, only one study has investigated fatty acid composition of UVC pasteurised milk and found an 18 % increase in C8 : 0, which was not detectable with our method. Although we found no differences in any MUFA or PUFA compared with raw milk and among pasteurisation methods, consistent with Molto-Puigmarti et al. (Reference Moltó-Puigmartí, Permanyer and Castellote13), some reductions in n-3/6 were observed by Delgado et al. (Reference Delgado, Cava and Delgado12) The present study provides additional evidence to suggest that both the concentration and composition of fat in human DM is neither affected by thermal nor non-thermal pasteurisation techniques. Understanding that the fatty acid composition does not change after both conventional Holder pasteurisation, in addition to non-thermal methods, including HHP, is important given the considerable interest in replacing the Holder method for pasteurising human DM.
Whereas the fatty acid composition of DM does not change as a result of pasteurisation, differences become apparent when assessing the concentration of oxylipins. Overall, it appeared that there were minimal changes in concentration of oxylipins following Holder and flash heating compared with raw milk, while significant differences were more consistent with UVC and HHP; however, it is important to note that it is difficult to disentangle whether the production of PUFA-derived oxylipins are mediated by enzymatic activity and/or auto-oxidation. Nonetheless, we report that the majority of differences arose in the ARA-derived- and the 18-carbon-derived oxylipins. Only 14(15)-epoxy eicosatetraenoic acid, an EPA-derived oxylipin was significantly different compared with raw milk after all methods of pasteurisation, with the exception of Holder, which resulted in lower concentrations.
Interestingly, our results indicated that following HHP, some ARA-derived oxylipins, including 15-OxoETE, 5-HETE and PGF2α were significantly higher than raw milk, whereas the levels of others, including 11,12-EET and 14,15-EET were significantly lower. We propose that the stabilisation of lipoxygenase and cyclo-oxygenase as a consequence of pressurisation may have in fact increased their enzymatic activity, thereby increasing levels of downstream metabolic intermediates(Reference Mozhaev, Lange and Kudryashova25). Some degree of auto-oxidation of the metabolic lipid intermediates may also be possible. Moreover, a potential denaturation of cytochrome P450 pathway epoxygenases, the enzyme responsible for the generation of 11,12-EET and 14,15-EET from ARA, may be a possible explanation as to why HHP resulted in lower levels of EET(Reference Capdevila, Falck and Harris26). The use of HHP technology has been previously shown to stabilise many enzymes(Reference Mozhaev, Lange and Kudryashova25); however, additional research is required to further elucidate a potential mechanism between pasteurisation methods and enzymes activity and whether cytochrome P450 pathway epoxygenases are affected.
A similar observation can be found in the results from the 18-carbon-derived oxylipins. Again, HHP resulted in higher levels of 9 (S)-HOTrE, produced from lipoxygenase degradation of α-linolenic acid, possibly attributable to enzymatic stabilisation(Reference Galliard and Phillips27). UVC-treated milk yielded higher levels of oxylipins derived from 18-carbon fatty acids, which reached statistical significance for 12(13)-EpOME, 13(S)-HOTrE, and 13-HODE. While 12(13)-EpOME and 13(S)-HOTrE are generated enzymatically by LOX, 13-HODE is a non-enzymatic product of LA oxidation(Reference Kumar, Gupta and Anilkumar28). We speculate that the generation of free radicals and reactive oxygen species while milk is exposed to UVC irradiation may be involved in the oxidation observed. Thus, while UVC may induce greater oxidation of LA, compared with the other pasteurisation methods, we observed no changes in the composition of LA.
Although our results show changes in oxylipins originating from PUFA, we do not see any corresponding changes in the composition, as assessed by GC-flame ionisation detection. We speculate that the higher sensitivity of the liquid chromatography-tandem MS used to quantify the oxylipins may account for this disparity; however, we cannot discount the possibility of oxylipin generation during freezer storage or during extraction for analyses(Reference Wu, Gouveia-Figueira and Domellöf29). It is worth mentioning that oxylipins are present in the samples in the order of ng/ml, whereas the parent fatty acids are present at mg/ml, which may be a million-fold higher. This could also explain why the increases in oxidative products did not affect the composition of fatty acids.
The present study is the first to assess oxylipins in pasteurised DM, which is primarily from mothers of term-born infants. Other studies have measured oxylipins in milk from mothers who delivered preterm, a potential reason why we did not detect any specialised proresolving mediators (e.g., resolvins, protectins, and maresins)(Reference Alexandre-Gouabau, Moyon and Cariou6, Reference Arnardottir, Orr and Dalli14, Reference Robinson, Palac and Baillif30). Studies which have detected these oxylipins, including resolvins, protectins and maresins, have been done so in preterm milk, not term milk which was used for the present study.
We acknowledge a few limitations of the present study. Although our paired study design permitted comparisons among groups, a strength of the present study, neither fatty acid composition nor oxylipin concentration was our primary outcome. The study was adequately powered for many of the oxylipins measured as we found significant differences; however, we may not have been adequately powered to detect differences in all outcome variables equally. A post hoc power calculation for some representative outcome variables of which we did not find any significant differences, including C16 : 0, C18 : 1, C18 : 2 and C22 : 6n-3, yielded a power of between 10 and 15 %.
In conclusion, there appeared to be no changes to the fatty acid composition following conventional thermal pasteurisation, or alternative (non-thermal) methods (UVC or HHP). Higher levels of some ARA-derived oxylipins following HHP may be a consequence of pressure-mediated quaternary structure stabilisation of cyclo-oxygenase and lipoxygenase. Higher concentrations of selected 18-carbon-derived oxylipins following UVC irradiation may be indicative of oxidation. Although the mechanistic properties of the bioactive lipid metabolome remain poorly understood, the present study provides some evidence of alterations as a result of processing. The biological relevance in altered oxylipin concentration is unclear, especially whether the concentrations in processed human milk are sufficiently high to affect downstream physiological processes. This warrants further exploration to better understand the potentially complex clinical implications of oxylipins. Understanding the lipid profile of human DM pasteurised by HHP may help facilitate its implementation in human milk banks.
Acknowledgements
The authors gratefully acknowledge all the women who generously donated their milk and all milk bank staff who facilitated donor screening and sample collection. The authors acknowledge Scott Lacombe and Dr Raphael Chouinard-Watkins. The authors also wish to thank Denis Reynaud of the analytical facility for bioactive molecules and the Hospital for Sick Children, Toronto, Canada, for the assistance with liquid chromatography-tandem MS.
The authors acknowledge funding from the Government of Canada through the Canadian Institutes of Health Research (CIHR funding no. 143233).
M. A. P., S. U., A. D., Y. P. and D. L. O. designed the research. M. A. P., S. M. A., A. D., and Y. P. conducted the research. M. A. P., S. M. A., K. H. analysed and interpreted the data. M. A. P. performed the statistical analyses. M. A. P. wrote the first draft of the article. R. P. B. had primary responsibility for the final content. All authors read and approved the final manuscript.
There were no conflicts of interest.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114519000916