Consumption of the Phaseolus vulgaris species of beans may be beneficial in the prevention and treatment of chronic diseases that are promoted by increased glycaemic stress (hyperglycaemia and hyperinsulinaemia). These conditions include diabetes and CVD, as well as cancer.
The importance of controlling postprandial blood glucose in the prevention and management of chronic disease has gained recognition in recent years(Reference Ceriello1–Reference Ceriello, Esposito and Piconi3). Glucose elevations cause oxidative stress that then alters the ability of the lining of blood vessels, or endothelium, to respond appropriately to blood flow. Some foods such as beans appear to stabilise or reduce postprandial glucose variability. Epidemiological studies show associations with increased legume consumption and decreased rates or prevalence of chronic diseases such as type 2 diabetes mellitus (T2DM)(Reference Bazzano, He and Ogden4–Reference Esposito, Ciotola and Giugliano6). Most beans such as the common bean (P. vulgaris sp., for example, pinto bean, black bean, navy bean) have a low glycaemic index (GI)(Reference Sievenpiper, Kendall and Esfahani7, Reference Winham, Hutchins and Melde8). In contrast, high-GI items such as white rice and white bread can elevate postprandial glucose and result in increased oxidative stress(Reference Atkinson, Foster-Powell and Brand-Miller9–Reference Lavi, Karasik and Koren-Morag11).
The low glycaemic response of beans alone has been documented(Reference Atkinson, Foster-Powell and Brand-Miller9, Reference Foster-Powell, Holt and Brand-Miller12), but few studies have looked at the acute effects of P. vulgaris or common beans on glycaemic response as part of a meal(Reference Winham, Hutchins and Melde8, Reference Bornet, Costagliola and Rizkalla13). In the limited number of studies that have looked at mixed meals, beans combined with a high-GI or refined carbohydrate food produced a glycaemic response that was in between the GI of the two foods when analysed alone(Reference Winham, Hutchins and Melde8, Reference Bornet, Costagliola and Rizkalla13, Reference Thompson, Winham and Hutchins14). It is not clear what kind of synergistic effects are produced or if an intermediate value always exists when the composition of the foods is varied. These findings are important for guiding recommendations to improve diabetes control and lower CVD and cancer risk(Reference O'Keefe and Bell10). It is possible that adding a low-GI food may reduce damage produced by other high-GI components of that meal. One important consideration is that we do not know the magnitude of the effect. It also appears that the glycaemic response attenuation is not necessarily linear.
The present paper discusses the relationship between glycaemic concentrations and glycaemic response produced by the consumption of P. vulgaris species, and the impact that relationship may have on the risk of developing diabetes, CVD and cancer. Glycaemic concentration refers to blood glucose measures at a particular point in time (for example, fasting) and glycaemic response is defined as blood glucose concentration following meal consumption, which is established by the rate at which glucose is released into and subsequently removed from circulation(Reference Sheard, Clark and Brand-Miller15).
Review methods
The electronic databases MEDLINE®, CINAHL® and the Cochrane Library were searched in November 2010, May 2011 and August 2011 with no date limitations. Keywords used for the search were ‘Phaseolus vulgaris’, ‘beans’, ‘legumes’ and ‘glycaemic response’. Abstracts of articles identified as potentially relevant based on the use of the terms Phaseolus vulgaris, beans, or legumes and glycaemic response in the abstract or keywords were obtained. The 1438 article abstracts were then reviewed to determine if the article investigated the impact of P. vulgaris on glycaemic response or the prevention or treatment of diabetes mellitus, heart disease, CVD, obesity, weight management or cancer. Relevant articles (n 118) were then collected in full text.
The full-text articles were screened for inclusion based on the following criteria: (1) published in a scientific peer-reviewed journal; (2) used P. vulgaris as a sole treatment or as part of a treatment; (3) published in English; (4) addressed the impact of P. vulgaris on glycaemic response or prevention or treatment of diabetes mellitus, CVD or cancer; (5) used human subjects; and (6) not an editorial, expert opinion, review or instructive article. The reference lists of included articles, review articles and meta-analyses were hand-searched for articles that met the inclusion criteria but that had not been identified during the electronic database search. Relevant articles from the reference lists that met the inclusion criteria were collected in full text. We found twenty-three articles meeting the criteria for inclusion in the tables in the present review.
Evidence for health outcomes
Evidence that beans induce low glycaemic response
Beans and other dry grain pulses typically reduce postprandial glucose elevations in short-term studies with non-diabetic and diabetic individuals compared with most starch foods(Reference Bornet, Costagliola and Rizkalla13, Reference Thompson, Winham and Hutchins14, Reference Jenkins, Wolever and Taylor16–Reference Tovar, Granfeldt and Bjorck23). Most studies that examined the impact of legumes on glycaemic control have utilised either normoglycaemic or T2DM participants.
The lower glycaemic response to beans has been attributed to their low GI or delayed digestion of the carbohydrate within and, therefore, delayed absorption of glucose(Reference Sievenpiper, Kendall and Esfahani7, Reference Winham, Hutchins and Melde8, Reference O'Keefe and Bell10, Reference Foster-Powell, Holt and Brand-Miller12–Reference Thompson, Winham and Hutchins14). One important question to address, however, is determination of the magnitude and nature of the effect of beans on the glycaemic response to meals containing high-GI foods. The few studies that have explored this question generally report that beans combined with a high-GI food produce a glycaemic response that is intermediate between the high- and low-GI foods, but this is not to say that the nature of the effect is additive or linear(Reference Winham, Hutchins and Melde8, Reference Bornet, Costagliola and Rizkalla13, Reference Thompson, Winham and Hutchins14, Reference Leathwood and Pollet17, Reference Potter, Coffman and Reid19–Reference Torsdottir, Alpsten and Andersson22).
The mixed-meal findings presented in Table 1 have important implications for chronic disease risk reduction(Reference O'Keefe and Bell10, Reference Eckel, Kahn and Robertson24). Individuals generally consume foods in combination, not in isolation, so determining the overall glycaemic response to the combination of foods has greater ‘real-life’ application to determining chronic disease risk reduction. Demonstrating that inclusion of beans in a meal results in a lower glycaemic response to the meal will provide a realistic, food-based mechanism for reducing the oxidative stress, endothelium-dependent vasodilation, and increased blood pressure associated with increased risk for some chronic diseases such as T2DM, complications of T2DM and CVD. Further research is required to examine the lower glycaemic response associated with bean meals along with changes in oxidative stress, endothelium-dependent vasodilation and blood pressure to confirm the validity and strength of this relationship. These studies should be adequately powered randomised controlled trials lasting at least 6 weeks in order to effectively assess the impact of beans' ability to lower the glycaemic response on these markers for chronic disease risk.
Table 1 Impact of Phaseolus vulgaris species on glycaemic response
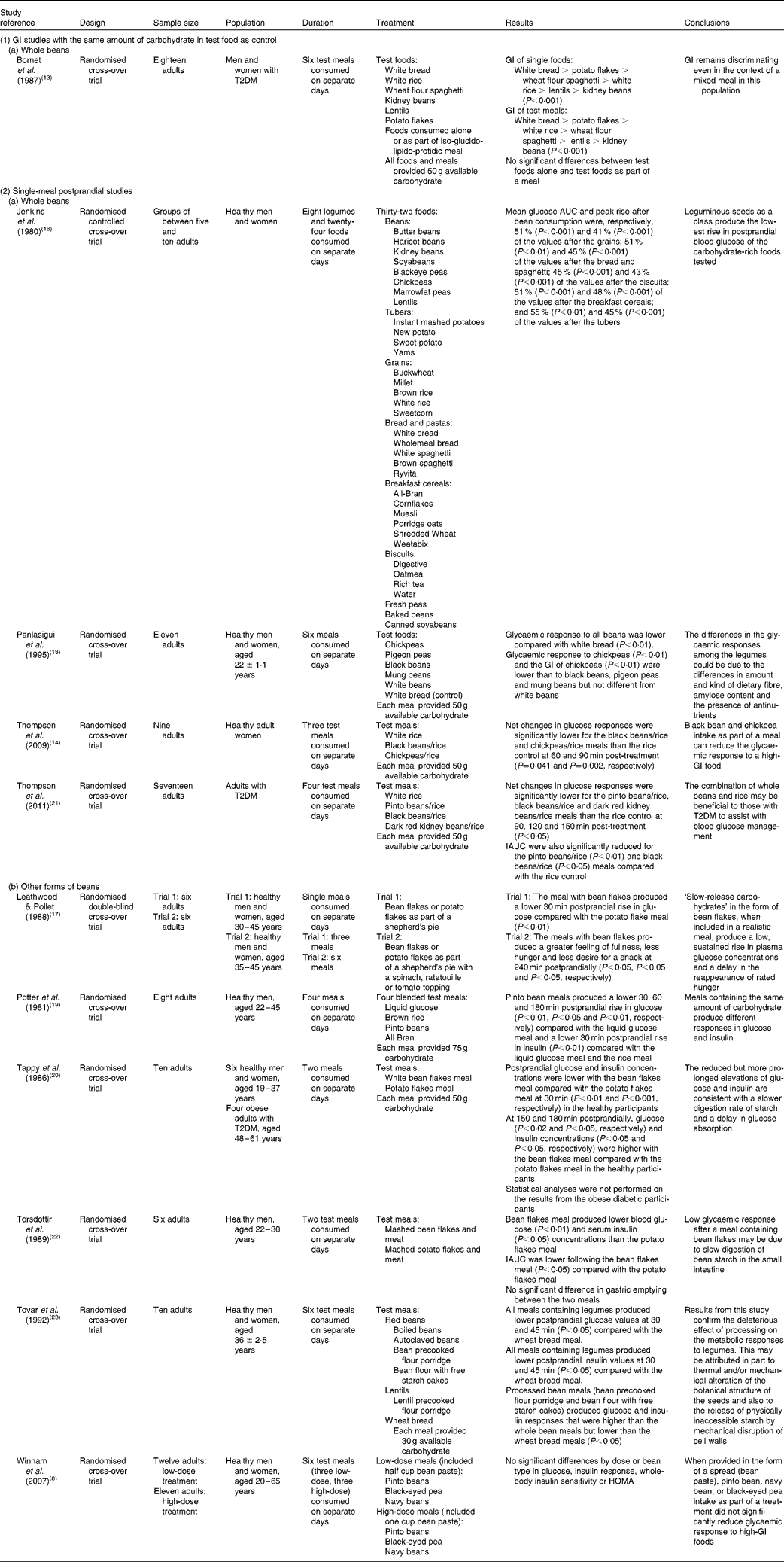
T2DM, type 2 diabetes mellitus; GI, glycaemic index; AUC, area under the curve; IAUC, incremental area under the curve; HOMA, homeostasis model assessment.
Impact of Phaseolus vulgaris species and glycaemic response on type 2 diabetes mellitus and risk factors for type 2 diabetes mellitus
Controlling postprandial glucose increases through incorporation of low-GI foods such as P. vulgaris sp. has a favourable impact on glucose control. Studies demonstrating that consumption of low-GI foods such as beans improve glucose control and T2DM control, as well as reduce risk for developing T2DM, have been analysed and summarised elsewhere and will be covered briefly in the present review(Reference Jenkins, Kendall and Augustin25–Reference Venn and Mann27). A recent Cochrane review assessed eleven randomised controlled trials and determined the effects of low-GI or low-glycaemic load (GL) diets and found that they improve glycaemic control in individuals with diabetes(Reference Thomas and Elliott28). Their positive conclusions are supported by other meta-analyses(Reference Brand-Miller, Thomas and Swan29, Reference Opperman, Venter and Oosthuizen30) which demonstrated reductions in HbA1c of 0·27 (95 % CI − 0·5, − 0·03)(Reference Opperman, Venter and Oosthuizen30) to 0·43 (95 % CI 0·13, 0·72)(Reference Brand-Miller, Thomas and Swan29) when low-GI diets were compared with high-GI diets. The reductions in HbA1c achieved with a low-GI diet are comparable with those produced by pharmacological interventions (for example, hypoglycaemic medications)(Reference Brand-Miller, Thomas and Swan29). However, the studies included in these reviews treated low-GI, or low-GL, diets in a more global fashion and did not focus solely on beans.
A meta-analysis(Reference Sievenpiper, Kendall and Esfahani7) examined forty-one studies that examined the effects of legume consumption alone, legume consumption as part of a low-GI diet, and legume consumption as part of a high-fibre diet. Pooled analyses demonstrated that legumes, alone or in low-GI or high-fibre diets, improve markers of longer-term glycaemic control (HbA1c and fructosamine). Of the reports from the meta-analysis(Reference Sievenpiper, Kendall and Esfahani7) that focused on P. vulgaris consumption, seven(Reference Anderson, Story and Sieling31–Reference Wursch, Acheson and Koellreutter37) are summarised in Table 2. These results are encouraging news for individuals with or at risk for T2DM since they indicate that simple diet changes, such as the inclusion of beans, can have a positive impact on glycaemic control. Nevertheless, these studies also illustrate the fact that few studies have focused on how bean intake influences risk factors for or the treatment of T2DM, let alone whether their effect is linked solely to the lower glycaemic response when consumed. Studies that explore how beans exert their influence on T2DM prevention and treatment and whether their effect on glycaemic response is related to that influence are required.
Table 2 Phaseolus vulgaris species, glycaemic response and type 2 diabetes mellitus and CVD risk

RR, relative risk; T2DM, type 2 diabetes mellitus; OGTT, oral glucose tolerance test; hs-CRP, high-sensitivity C-reactive protein.
Impact of Phaseolus vulgaris species and glycaemic response on CVD and CVD risk factors
Besides controlling postprandial glucose increases, numerous research studies indicate that a low-GI diet may also play a role in reducing the risk for or preventing CVD; however, these studies did not focus on beans as a low-GI food. These studies have been analysed and summarised elsewhere(Reference Jenkins, Kendall and Augustin25, Reference Barclay, Petoca and McMillan-Price26, Reference Rizkalla, Bellisle and Slama38–Reference Leeds40) and so will not be covered in detail in the present review. Despite the interest in the role of a low-GI diet in CVD risk reduction, the mechanisms behind this risk reduction have yet to be determined. A Cochrane review examined twenty-one randomised controlled trials that included a total of 713 participants. This review found no evidence that low-GI diets influenced changes in certain well-known risk factors for CVD including HDL-cholesterol, LDL-cholesterol, TAG or total cholesterol concentrations. The authors of the Cochrane review reported that many of the trials included in the review were ‘short-term, of poor quality and did not have sufficient power to detect clinical important differences’(Reference Kelly, Frost and Whittaker41).
Observational studies and a very limited number of randomised controlled trials indicate a beneficial effect of bean consumption on short-term satiety and weight loss when combined with energy restriction(Reference McCrory, Hamaker and Lovejoy42, Reference Williams, Grafenauer and O'Shea43), but these effects may not be related to the relationship between bean intake and glycaemic response. Few studies isolated and examined P. vulgaris species intake separately from other food groups(Reference McCrory, Hamaker and Lovejoy42, Reference Williams, Grafenauer and O'Shea43), and none of these studies directly addressed the relationship between bean intake, glycaemic response and short-term satiety and weight loss, so results in this area should be interpreted with caution.
Although P. vulgaris species are known to decrease LDL-cholesterol, a well-known risk factor for CVD, in normocholesterolaemic and hypercholesterolaemic participants(Reference Winham and Hutchins35, Reference Winham, Hutchins and Johnston36, Reference Anderson, Gustafson and Spencer44–Reference Nestel, Cehun and Chronopoulos47), this effect is most probably attributed to the soluble fibre found in the beans and not to the impact the beans have on glycaemic response. Nevertheless, other risk factors for CVD, such as oxidative stress, have been identified and low-GI foods such as beans and low-GI diets may favourably influence these risk factors.
In addition to chronic hyperglycaemia, elevated postprandial glucose can increase oxidative stress, worsen endothelium-dependent vasodilation and raise blood pressure(Reference O'Keefe and Bell10, Reference Davignon and Ganz48–Reference Ceriello, Esposito and Piconi50). Dysmetabolic changes after eating are significant contributors to CVD risk and individuals with T2DM are already at increased risk of CVD. On the other hand, controlling the postprandial glucose response by the inclusion of low-GI/GL foods in the diet can decrease CVD risk. Dietary patterns that include low-GI/GL foods, such as beans, were associated with a lower risk for CVD, even after accounting for other known risk factors such as cigarette smoking, obesity and family history in a prospective cohort study of 44 875 men aged 40–75 years(Reference Hu, Rimm and Stampfer51).
Impact of Phaseolus vulgaris species and glycaemic response on cancer and cancer risk factors
Researchers hypothesise that glycaemic response may increase cancer risk through the modulation of hormone concentrations (for example, insulin-like growth factor) by insulin and that hyperinsulinaemia may increase cancer risk(Reference Stoll52, Reference Kaaks53). Studies examining the effect of GI and GL on the risk for various cancers (breast, colorectal, endometrial, gastric, ovarian, pancreatic, prostate, renal) report mixed results(Reference Augustin, Dal Maso and La Vecchia54–Reference Strayer, Jacobs and Schairer81). Most studies utilised either a cohort or case–control design, relying on FFQ to determine the average daily GI and GL of participants(Reference Augustin, Dal Maso and La Vecchia54–Reference Cho, Spiegelman and Hunter59, Reference Flood, Peters and Jenkins61–Reference Galeone, Pelucchi and Maso64, Reference Higginbotham, Zhang and Lee67–Reference Meinhold, Dodd and Jiao70, Reference Potischman, Swanson and Coates74–Reference Strayer, Jacobs and Schairer81). Inaccurate memory of foods consumed over the recall period (typically 1–2 years) and recall bias are potential confounding factors with FFQ and retrospective studies in general. Researchers also acknowledge that the reliability and validity of estimating average daily GI and GL from FFQ is questionable. Some GI and GL values have been obtained from small samples and the variability of the values is undetermined(Reference Augustin, Dal Maso and La Vecchia54–Reference Augustin, Polesel and Bosetti58).
Few studies have reported the relationship of P. vulgaris species and glycaemic response on cancer risk (Table 3) (Reference Cho, Spiegelman and Hunter59, Reference Hartman, Albert and Zhang66, Reference Potischman, Swanson and Coates74). We were unable to find any studies that had the examination of bean intake, glycaemic response and cancer risk or incidence as a primary objective. One cohort and one case–control study found weak associations between legume intake and a reduction in cancer risk related to a decrease in glycaemic response(Reference Cho, Spiegelman and Hunter59, Reference Potischman, Swanson and Coates74). A randomised controlled trial found that a high-legume diet and a healthy American diet both favourably influenced biomarkers for cancer risk(Reference Hartman, Albert and Zhang66). If the hypothesis that glycaemic response makes an impact on cancer risk via insulin actions and interactions, then incorporation of beans into the diet to modulate the glycaemic response could have a favourable impact on the risk for a variety of cancers. However, determining the impact of glycaemic response and beans on cancer risk in a human population will require more accurate and reliable methods of tracking diet intake over long periods of time.
Table 3 Phaseolus vulgaris species, glycaemic response and cancer risk

GI, glycaemic index; GL, glycaemic load; RR, relative risk; CRP, C-reactive protein; sTNFRI/II, soluble tumour necrosis factor-α receptors I and II; NCI, National Cancer Institute.
Composition of Phaseolus vulgaris species of beans
According to the Dietary Guidelines for Americans and the United States Department of Agriculture, beans are classified as both a protein and a starchy vegetable source(82). Beans contain a high amount of protein, with one serving of most bean types (half cup) providing 7–8 g. Beans are also an excellent source of fibre, providing 3–9 g of soluble and insoluble fibre per half-cup serving(Reference Messina83).
Beans contain very little fat, generally accounting for less than 3 % of the energy content, and have a very low saturated fat content(Reference Aykroyd, Doughty and Walker84). Beans are also high in folate, Fe, Mg, Zn, n-3 fatty acids and antioxidants(Reference Sievenpiper, Kendall and Esfahani7, Reference Halvorsen, Holte and Myhrstad85–Reference Mitchell, Lawrence and Hartman88). They contain phytate and phenolic compounds that may function similarly to glucose-lowering α-glucosidase or α-amylase inhibitor T2DM medications such as metformin and acarbose(Reference Sievenpiper, Kendall and Esfahani7, Reference Kalogeropoulos, Chiou and Ioannou86).
The predominant macronutrient in beans is carbohydrate, contributing 60–65 % of the energy content. Starch, the primary digestible carbohydrate in beans, can be categorised as readily digestible, slowly digestible and resistant starch(Reference Bednar, Patil and Murray89). All bean varieties including the P. vulgaris species contain a higher ratio of slowly digestible:readily digestible starch compared with other starchy foods. In general, most beans contain 30–40 % amylose, a linear polymer of glucose units (α1–4 linkages), whereas most other starches contain 20–30 % amylose. Starches with more than 30 % amylose are readily digestible or resistant starch depending on the amylose content and hydrothermal treatment applied to the food. Beans also contain a substantial amount of resistant starch, considered as a dietary fibre. Resistant starch is defined as any starch that resists digestion by amylase in the small intestine and progresses to the large intestine for fermentation by the gut bacteria(Reference Thorne, Thompson and Jenkins90, 91). Slowly digestible starch is associated with reduced glycaemic responses and lower postprandial glucose levels compared with readily digestible starch. This attenuated glycaemic response can benefit both insulin-resistant individuals and individuals with diabetes.
Proposed mechanisms of action
The mechanism of action responsible for the low glycaemic response to beans is multifaceted. Possible explanations include a high content of viscous fibre, protein, relatively high amylose starch and antinutrients. In addition, processing methods affecting the physical form of the beans may alter their glycaemic response.
Beans are commonly consumed in their whole form, or as a minimally processed food with little or no grinding. Eating the intact bean maintains the integrity of the cell wall, slowing digestion of the bean in the upper small intestine. Whole beans also have cell walls that are more resistant to digestion than the cell walls of cereal grains. Minimal or no processing of the bean combined with the resistance of the bean cell wall to digestion provides a likely primary mechanism of action that explains the low glycaemic response to beans(Reference Noah, Guillon and Bouchet92).
Viscous fibres form a gel-like substance along the digestive tract, which may slow the rate of gastric emptying and absorption rate of nutrients. Inclusion of a viscous fibre with a test meal may reduce the blood glucose response by an average of 44 %(Reference Wolever, Jenkins and Spiller93). Purified viscous fibres also reduce postprandial gastric inhibitory polypeptide and insulin levels more effectively than non-viscous fibres(Reference Jenkins and Jenkins94, Reference Jenkins, Kendall and McKeown-Eyssen95). Beans are particularly high in soluble fibres that increase viscosity of the intestinal lumen or the unstirred water layer(Reference Brownlee, Havler and Dettmar96–Reference Fuse, Bamba and Hosoda98). However, Tappy et al. (Reference Tappy, Wursch and Randin20) found significantly lower glucose and insulin responses to a bean meal alone compared with a potato meal with added bean fibre. Therefore, the attenuated glycaemic response seen as a result of bean consumption cannot be explained solely by the beans' fibre content.
The protein fraction of beans may interact with starch to reduce the digestibility and glycaemic response of that starch. Alli & Baker(Reference Alli and Baker99) found carbohydrates tightly bound to proteins isolated from uncooked beans using citric acid and sodium hydroxide extracts, providing evidence for a starch–protein interaction.
The ratio of amylose:amylopectin starch found in beans may also alter the glycaemic response. The higher molecular weight, greater surface area and branching structure of amylopectin make it subject to faster digestion than amylose. High-amylose meals (70 % amylose) compared with high-amylopectin meals (70 % amylopectin) result in significantly lower plasma glucose in healthy normoglycaemic adults at 30 and 60 min after meal consumption(Reference Behall, Scholfield and Canary100). Among natural sources of carbohydrates, beans have the highest percentage of starch as amylose (30–40 %), which is 5–10 % more amylose than is found in most cereals(Reference Thorne, Thompson and Jenkins90).
In addition to protein–starch interactions and the nature of the starch in beans, the phytic acid content of beans may influence the glycaemic response after bean consumption. The phytic acid content of beans is high compared with non-bean foods. There is a negative correlation between phytic acid concentrations and glycaemic indices for non-diabetic adults (r − 0·78, P < 0·001)(Reference Yoon, Thompson and Jenkins101). A study using unleavened bread made from navy bean flour (containing phytic acid) demonstrated that consuming the navy bean bread significantly reduced blood glucose area under the curve by 64 % compared with that of unleavened bread made from white wheat flour(Reference Thompson, Button and Jenkins102). Removing the phytic acid from the navy bean flour significantly increased the glycaemic area under the curve by 141 %. Phytic acid is believed to inhibit starch digestion both directly and indirectly. Structurally, phytic acid binds directly with starch through phosphate bonds and reduces starch digestibility(Reference Thompson, Button and Jenkins102). Indirectly, phytic acid may bind to cations such as Ca. Since the stability of α-amylases, including pancreatic α-amylase, is dependent on Ca(Reference Yoon, Thompson and Jenkins101), the lack of available Ca can decrease the effectiveness of α-amylases, slowing the rate of starch digestion. Phytic acid also binds to negatively charged groups on proteins, such as α-amylases, at neutral and alkaline pH(Reference Thompson, Button and Jenkins102), rendering them useless and reducing the digestion of starch by amylase.
Future directions
All beans are not created equal – nor do they elicit identical biological responses when consumed. Even though the GI values for beans are typically very low, studies examining the glycaemic effects of assorted beans from P. vulgaris species have demonstrated that the glycaemic response differs based on the bean used. Researchers should continue to study different beans from the P. vulgaris species to determine the individual glycaemic effects associated with each bean type.
Definitively determining if the form of the bean consumed changes the glycaemic response, or other positive biological effects associated with bean consumption, should be a priority. As interest in the P. vulgaris species increases, the food industry will probably formulate functional or manufactured foods that contain ground beans, bean powder, bean paste, etc. to address the marketing potential in this area. Research is needed to ensure that the various forms in which the bean can be utilised impart the same beneficial properties associated with the consumption of whole beans, including maintaining the low-GI/GL qualities.
Studies such as the one conducted by Kallio et al. (Reference Kallio, Kolehmalnen and Laaksonen103) demonstrate that foods can make an impact and act via molecular pathways by affecting signal transduction and gene function. The constituents of foods that act on these pathways go beyond the traditional macro- and micronutrient content typically reported for such foods. Research that identifies the phytochemical components of foods, including beans, is desperately needed to allow research in this area to progress. We are just beginning to explore the mechanisms of action that are responsible for the chronic disease risk-reduction benefits conferred by whole bean consumption. More research is required to define the pathways involved, including those related to changes in oxidative stress, endothelium-dependent vasodilation, and blood pressure, in order to determine the full extent of the influence that beans have on the prevention of chronic disease.
Summary
Traditional foods such as beans should be retained in the diet because of their many health benefits, including a positive impact on postprandial glycaemic response. Hyperglycaemia, whether it occurs following a meal or due to poorly controlled T2DM, is known to increase oxidative stress, contribute to hypertension and increase the risk for CVD. Examination of the glycaemic response to meals, especially culturally important food combinations such as beans and rice, is important for the prevention and control of hyperglycaemia-induced diseases.
Understanding the glycaemic responses elicited by the beans of the P. vulgaris market classification and how these responses vary depending on the bean consumed is essential. Since not all beans are equal in response, these findings will allow provision of accurate nutrition education to individuals who have, or are at risk for, T2DM.
Acknowledgements
The preparation of the present review article received no specific grant funding from any funding agency in the public, commercial or not-for-profit sectors. A. M. H. and S. V. T. were responsible for retrieving references. A. M. H. and D. M. W. were responsible for writing the article. S. V. T. was responsible for the design of the tables. A. M. H., S. V. T. and D. M. W. were responsible for critical reading and evaluation, presentation of the data and article editing. The article has been read and approved by all authors. A. M. H. and D. M. W. have previously received research funding from Bush Brothers & Company and the US Dry Bean Council. A. M. H. is a member of the editorial advisory board for the Dry Bean Quarterly, published by the Northarvest Bean Growers Association, serves as an editor for Bean Briefs, published by the US Dry Bean Council, and has written a white paper supporting the promotion of bean consumption for the US Dry Bean Council. D. M. W. has served as a research advisor for Bush Brothers & Company and Pulse Canada. S. V. T. has no conflicts of interest to report related to the content of the present review article.





