INTRODUCTION
Recent evidence describing the 2009–2010 pandemic A(H1N1) experience in pregnant females has been consistent with historical pandemic and seasonal influenza experiences, suggesting that pregnant women are at increased risk of influenza complications, which may require hospitalization including critical care [Reference Lapinsky1]. Findings specific to the pandemic illustrated that pregnant women were not only disproportionately admitted to hospital; in many cases, they also represented an above-average proportion of intensive-care unit (ICU) admissions and deaths. In a pooled systematic review of Australia and the USA, pregnant women accounted for about 1% of the population, but constituted 6·3% of all hospitalizations, 5·9% of all ICU admissions, and 5·7% of deaths directly attributed to A(H1N1) infection [Reference Mosby, Rasmussen and Jamieson2]. However, no evidence exists comparing the burden of illness in pregnant women during the pandemic compared to the burden of illness in inter-pandemic years.
The literature also suggests that interventions such as immunization [Reference Tamma, Steinhoff and Omer3] or antiviral prescription [Reference Creanga4–Reference Gérardin8] positively prevented and/or decreased the risk of severe disease in pregnant women. In Canada, their increased use during the second wave may have also contributed to decreasing the burden of severe illness relative to the first wave [Reference Helferty9].
We describe the epidemiology of hospital admission for all pregnant women with pandemic A(H1N1) 2009 influenza [pandemic A(H1N1)] in Canada during the 2009–2010 pandemic. We also compare waves 1 and 2 of pandemic activity to identify any differences in patient characteristics and clinical features between the two waves. Finally, we also compare pandemic pregnant influenza admission to pregnant influenza admissions during non-pandemic years to qualify the magnitude of the outbreak in pregnant women.
METHODS
During the 2009–2010 A(H1N1) pandemic, all 13 Canadian provinces and territories (P/T) were legally required to provide weekly hospitalization and death counts of laboratory-confirmed pandemic A(H1N1) influenza cases to the Public Health Agency of Canada's (PHAC) national influenza surveillance programme. Probable or suspected cases were not reported to the PHAC.
The case definition that was used to classify cases as confirmed was as follows (and remained consistent during the study period): a person with or without clinical symptoms, whose infection was confirmed by either RT–PCR, viral culture, and/or a fourfold rise in pandemic A(H1N1) influenza virus-specific neutralizing antibodies. No testing protocol was provided to the P/T by the PHAC; however, patients admitted to hospital were prioritized for laboratory testing. Consequently, case ascertainment was deemed relatively consistent during the pandemic period [Reference Campbell10].
Mandatory data elements reported by the P/T to the PHAC included: P/T-assigned unique identifier; P/T reporting case; P/T of residence; name; date of birth or age; sex; reporting, onset, testing, hospitalization and, if applicable, discharge or death dates; admission type (hospitalization, ICU, post-mortem); Aboriginal status (yes/no) and ethnicity (Inuit, First Nations, Metis, other); pregnancy status and, if available, trimester; underlying conditions; and an open text field to capture potentially relevant clinical/outcome information not captured elsewhere.
Aboriginal status was self-reported by the patient and provided to the PHAC, whenever available. Ontario and Nova Scotia informed the PHAC that they did not collect this information and were therefore omitted from any analysis of this variable. Pregnancy status was reported as an underlying condition and was therefore provided by the medical institutions to the appropriate P/T who, in turn, reported this back to the PHAC.
Provinces and territories were required to report on a specific list of underlying conditions (Table 1) but were also provided with an ‘other’ option and an open text field to record both additionally clinically relevant underlying conditions or more information on the selected conditions. All underlying conditions reported for pregnant cases were double-checked by two medical specialists (L. P. and R. R.) and, when appropriate, underlying causes reported as ‘other’ were reclassified into the list of underlying conditions provided for reporting purposes.
Table 1. Underlying condition data collected by Canadian provinces and territories reported to the Public Health Agency of Canada's national pandemic influenza surveillance system, 12 April 2009 to 3 April 2010
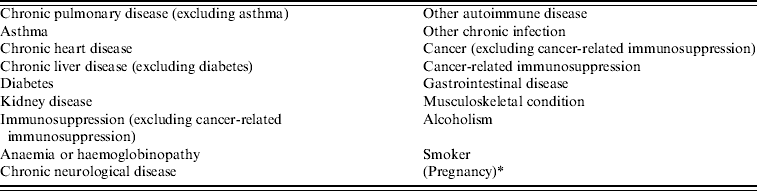
* Not included in counts of underlying conditions.
From this surveillance dataset, laboratory-confirmed pregnant pandemic A(H1N1) cases hospitalized between 12 April 2009 (week 15) and 3 April 2010 (week 13) were extracted, representing the Canadian pandemic experience for pregnant women. Open-ended fields were inspected closely to extract additional information on the pregnant cases. In the event of incomplete or illogical (e.g. male case reported as being pregnant) findings, the authors (E. R-H. and J. V.) contacted the P/T to complete the missing and/or correct the data elements.
No sensitivity analyses were conducted on these data. However, in an effort to ensure that the pregnancy dataset was as representative as possible, the authors (E. R-H. and J. V.) actively contacted the P/T to ensure that all pregnant cases had been reported to the PHAC.
Using this enhanced dataset (i.e. pregnant cases exclusively), we conducted a descriptive analysis of mutually exclusive case types [hospitalized only (i.e. not admitted to ICU or resulting in death), admitted to ICU, death], age, trimester, ethnicity, underlying medical conditions which predispose to influenza complications, month of hospital admission, case severity, time to care and time to death. Pregnant case admission patterns in wave 1 (12 April–29 August 2009) vs. wave 2 (30 August–3 April 2010) were also compared. In the event that the date of symptom onset was missing, the date of admission was used to classify a pregnant case as occurring in wave 1 or in wave 2.
The distribution of pregnant cases was compared to: (1) all women of reproductive age (14–44 years) who were admitted with confirmed pandemic A(H1N1) and were reported to the PHAC; (2) all cases (male and female) admitted with confirmed pandemic A(H1N1) and reported to the PHAC during the same study period. Both of these comparisons were conducted using the general PHAC surveillance dataset. A third comparison between the pregnant cases and a national 3-year historical baseline mean (2006–2007, 2007–2008, 2008–2009) of seasonal influenza admissions and pneumonia and influenza (P&I) admissions for pregnant women in Canada was also conducted. These data were provided by the Canadian Institute for Health Information (CIHI).
Legally, all P/T are also supposed to annually report all of their hospitalizations to the CIHI where the data are reviewed for accuracy and completeness before being used to populate the Fichier des hospitalisations ‘MedÉcho’ (for Quebec) and the Discharge Abstract Database (for all other P/T). Using these two CIHI datasets for the 2006–2007, 2007–2008 and 2008–2009 fiscal calendar years (April–March), CIHI used a case selection approach used by Thompson et al. [Reference Thompson11] and by Crighton et al. [Reference Crighton12] to identify and extract inpatient records where influenza or pneumonia was defined as the most responsible diagnosis (i.e. main reason for admission) [13].
Four subgroups of cases were identified:
(1) Cases where influenza was explicitly documented in the inpatient chart [International Classification of Disease Version 10.0 Canadian modification (ICD-10-CA)] codes J09, J10.-, J11.- (defined in Table 2) (for seasonal influenza cases only).
(2) Cases coded as pneumonia from various underlying organisms (ICD-10-CA codes J12.-, J13, J14, J15.-, J16.-, J18.-, U04, J85.1).
(3) Cases with both chronic obstructive pulmonary disease (COPD) and influenza/pneumonia (ICD-10-CA code J44.0).
(4) Cases with pneumonia as a proxy main diagnosis (ICD-10-CA code J17.-). CIHI data uses a diagnosis type 6 to identify proxy most responsible diagnoses when a diagnosis is ‘the manifestation of an underlying condition’ [13] (e.g. septicaemia due to underlying pneumonia). An exception to this was in Quebec where diagnosis typing does not occur; given that most non-Quebec cases with a diagnosis type 6 reported septicaemia as the most commonly reported diagnosis, Quebec cases were included in the data abstraction if the main diagnosis was septicaemia (ICD-10-CA codes A40.– or A41.-) combined with a J17.- code in any other position.
Seasonal influenza cases only included cases where influenza was explicitly documented in the inpatient chart (scenario 1), whereas P&I cases included cases from any of the four above-ementioned coding scenarios.
Table 2. International Classification of Disease Version 10 (ICD-10) diagnosis codes used by the Canadian Institute for Health Information to extract seasonal influenza and pneumonia and influenza hospitalizations for pregnant women in Canada

COPD, Chronic obstructive pulmonary disease.
* Condition A exclusive of conditions B, C, D=seasonal influenza; conditions A, B, C or D=pneumonia and influenza.
From these data, only pregnant cases were extracted. These were further distilled by either (1) selecting cases where the main diagnosis code was O98.8–: or O99.5–: (further excluding cases where the sixth digit was reported as ‘4,’ which identifies postpartum cases), (2) or by selecting cases where one of the following pregnancy codes was used in any position: Z32, Z33, Z34, Z35, Z36 and Z37.
These CIHI data were used to calculate 3-year historical baseline averages for influenza-related hospitalizations in pregnant women in Canada. From there, CIHI quarter 1/quarter 2 (‘Q1/Q2’; April–September) 3-year historical baseline averages were compared to wave 1 PHAC pregnant cases, while quarter 3/quarter 4 (‘Q3/Q4’; October–March) 3-year historical baseline averages were compared to wave 2 PHAC pregnant cases.
RESULTS
Descriptive analyses
During the two waves of the A(H1N1) pandemic in Canada, all provinces and territories, with the exception of Saskatchewan (which did not report any pregnant cases), reported that a combined total of 263 pregnant women were admitted for or developed laboratory-confirmed pandemic A(H1N1) while seeking medical treatment. Of these, 29 (11·0%) were admitted to ICUs and survived. There were four (1·5%) fatalities over the study period, all were women in their third trimester. The first pregnant case was admitted to hospital on 20 May 2009, and the last pregnant case was admitted on 24 December 2009 (Fig. 1). No additional pregnant cases were reported in 2010.

Fig. 1. H1N1 2009 Epidemiological curve for Canadian pregnant cases, 12 April 2009 to 31 December 2009, reported to the Public Health Agency of Canada national influenza surveillance programme. (Based on date of symptom onset. If symptom onset date was missing, the date of admission was used.)
Table 3 provides a breakdown of these cases by trimester, age group, ethnicity and underlying conditions. Trimester information was missing for about half of the cases. For cases where trimester was reported, the over-representation of third trimester pregnant cases was consistent across all mutually exclusive case types (hospitalized, admitted to ICU, death).
Table 3. Characteristics of hospitalized pregnant women reported to the Public Health Agency of Canada's national influenza surveillance programme, 12 April 2009 to 3 April 2010
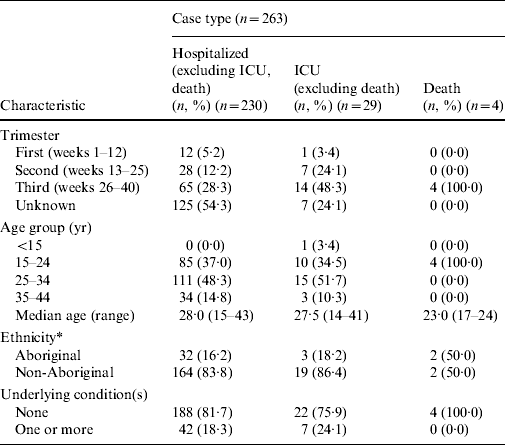
ICU, Intensive care unit.
* Excluding Ontario and Nova Scotia; hospitalized (n=196), ICU (n=22), Death (n=4).
The age of hospitalized (i.e. non-ICU, non-death) pregnant cases ranged between 15 and 43 years (median 28·0 years). The age range and median age of pregnant ICU cases were similar (range 14–41 years, median 27·5 years). The pregnant fatalities were substantially younger (median 23·0 years, range 17–24 years) than the hospitalized or ICU pregnant cases. However, the majority of hospitalized and ICU pregnant cases were aged between 25 and 34 years (Table 3). Ages were available for all cases.
For the descriptive analyses of ethnicity by pregnant case type, cases from two of the 13 jurisdictions (Ontario and Nova Scotia) were excluded as they did not report on ethnicity (hospitalized, n=34; ICU, n=7; death, n=0). A total of 32 pregnant hospitalized cases (16·2%) were of Aboriginal ethnicity, while three ICU cases (18·2%) and two fatalities (50·0%) were pregnant self-reported Aboriginal women (Table 3). Overall, 16·7% of cases where ethnicity was reported were pregnant women of self-reported Aboriginal ethnicity.
Underlying medical condition status (excluding pregnancy) was assigned based on the presence of one more conditions known to predispose to influenza-related complications (excluding pregnancy) [14, 15]. The majority of pregnant cases, regardless of the case type, reported no underlying conditions (Table 3). Of particular note was the fact that the P/T where pregnant fatalities occurred reported no underlying conditions for any of these cases. Of those who reported one or more underlying conditions, 12·6% of hospitalized cases and 17·2% of ICU cases reported a chronic pulmonary condition.
The median time between symptom onset and death was 23·0 days (range 6–72 days; ~1–10 weeks). However, the median time was based on a small number of events and should therefore be interpreted with caution.
Admission of pregnant cases occurred during every month under study here, up to and including December 2009 (Fig. 1). The cumulative majority of pregnant hospitalizations and ICU admissions occurred in November (n=83, 36·1% and n=7, 24·1% respectively), followed by October (n=59, 25·7% and n=8, 27·6%, respectively); the third highest number of pregnant hospitalizations and ICU admissions were during June (n=29, 12·6% and n=9, 31·0%, respectively). Pregnant ICU admissions were only reported in May and June, and from October to December. All pregnant deaths were admitted in May and June.
Wave 1 vs. wave 2 activity
Table 4 compares the case distribution patterns for wave 1 vs. wave 2 admissions of pregnant women. Seventy-five (28·5%) pregnant cases were admitted in wave 1, while 188 (71·5%) pregnant cases were admitted during the second wave. During wave 2, the proportion of pregnant cases who had a severe outcome (ICU or death), who reported an underlying condition, or who were of Aboriginal origin declined compared to wave 1 (P=0·01, P=0·026 and P<0·0001, respectively). Distribution of cases by trimester (excluding unknowns) did not differ significantly between the waves. It should be noted that the frequency of unknown values for trimester was substantially larger in wave 2 than in wave 1; this was due to an increased reporting burden on the participating P/T and resulted in a decreased number of variables being reported.
Table 4. Comparison of characteristics of hospitalized pregnant women reported to the Public Health Agency of Canada's national pandemic influenza surveillance system, pandemic wave 1 (12 April to 29 August 2009) vs. pandemic wave 2 (20 August 2009 to 3 April 2010)

ICU, Intensive care unit; n.a., not available.
* Two cases with no onset date.
† Sixty-one cases with no onset date.
‡ Excluding Ontario and Nova Scotia; wave 1 (n=59, 78·7%); wave 2 (n=164, 87·2%).
Comparison with other populations
The pregnant cases for whom we received detailed case information represented 3·2% of all admitted pandemic A(H1N1) cases reported to the PHAC and 19·6% of all reported admitted pandemic A(H1N1) cases in women of reproductive age (Table 5). Comparatively, it is estimated that Canadian pregnant women represent about 3·8% of the Canadian population [Reference Campbell10]. Of the 8301 admitted cases, 2331 (28·1%) occurred in individuals aged between 14 and 44 years of age (i.e. equivalent to the age range of pregnant and women of reproductive age cases). Severe outcomes (ICU or death) were less common in hospitalized pregnant women than in hospitalized women of reproductive age (P=0·005) and all admitted cases (P=0·006).
Table 5. Comparison of characteristics of hospitalized pregnant women relative to women of reproductive age (WRA) and all cases reported to the Public Health Agency of Canada's pandemic influenza surveillance system (12 April 2009 to 3 April 2010)
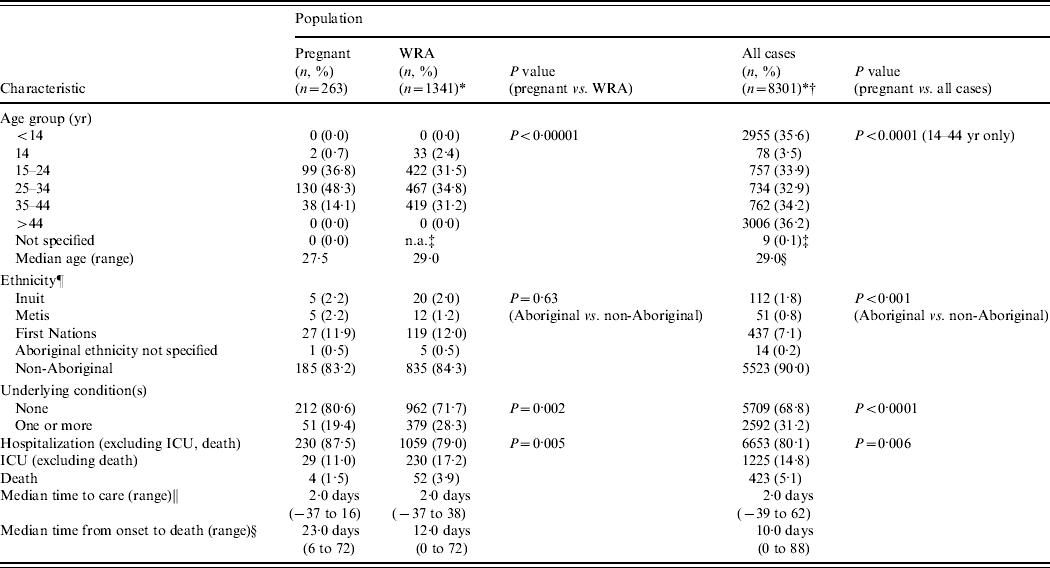
There is case overlap between categories (i.e. pregnant women are included in the WRA and all-case categories).
* Includes Saskatchewan.
† All cases between ages of 14–44 years (n=2331).
‡ 3/9 cases of unspecified age were female; these cases may have been of reproductive age, but are excluded from the WRA total count.
§ Based on 4 (100·0%) pregnant case deaths; 44 (84·6%) WRA case deaths; 373 (88·2%) all-case deaths.
¶ Excluding Ontario and Nova Scotia; pregnant (n=223, 84·8%); WRA (n=991, 73·9%); all cases (n=6137, 73·9%).
∥ Based on 218 (82·9%) pregnant cases; 991 (74·0%) WRA cases; 6137 (73·9%) of all cases.
Women of reproductive age
In comparing pregnant women to all women of reproductive age (including Saskatchewan), there was a significant difference in the age group distribution (P<0·0001) and underlying conditions (P=0·002). In general, it appeared that pregnant women were younger than women of reproductive age who were also admitted for A(H1N1) (median age 27·5 vs. 29·0 years).
All admitted cases
In further comparing admitted pregnant women to all admitted cases (also including Saskatchewan), there was a significant difference in the reproductive age-specific age group proportions, the ethnicity of cases, and frequency of underlying conditions. With a median age of 29·0 years for all admitted cases (i.e. all age groups and both sexes), compared to a median age of 27·5 years for all pregnant women who were admitted for pandemic A(H1N1), a larger proportion of all admitted cases were aged between 35 and 44 years than in pregnant cases. With respect to ethnicity, the proportion of pregnant cases of Aboriginal ethnicity was greater than in the all-cases group (P=0·001). Finally, the frequency of one or more underlying conditions was significantly larger in all admitted cases than in admitted pregnant cases (P<0·0001).
The differences in median time from onset to death for the three case populations was marked. Pregnant cases reported the largest median time between symptom onset and death (23·0 days), followed by women of reproductive age (12·0 days), and then by all cases, whose median time to death was less than half of that reported for pregnant women (10·0 days).
Comparison between the PHAC and the CIHI influenza data
The number of pregnant hospitalized cases reported to the PHAC as part of its pandemic influenza surveillance system was more than twice the annual average observed over a 3-year historical baseline for pregnant seasonal influenza cases, and 42·2% of average historical seasonal P&I hospital admissions reported to CIHI.
There was no significant difference in age distribution between the PHAC surveillance data and CIHI historical hospitalizations for both seasonal and P&I historical baselines. For ICU admissions, age distribution during the pandemic did not vary from the historical P&I baseline. These findings did not change by time period (i.e. wave 1 vs. wave 2). The proportion of deaths reported to the PHAC during the first wave of the pandemic was significantly higher than the proportion of P&I admissions resulting in death as reported to CIHI during the 3-year average of Q1/Q2 (P=0·03).
Most statistically significant differences between datasets and time periods were related to trimester of pregnancy (Table 6). More specifically, a comparison of A(H1N1) cases to CIHI's seasonal influenza admissions was significant for the full year (P=0·03). A comparison of the same PHAC data to CIHI's P&I values was significant for all three periods. Overall, first and second trimester cases appeared to be proportionally more common during the pandemic than is usually observed for average seasonal (P=0·03) and P&I (P<0·0001) admissions.
Table 6. Comparison of case volumes reported to Public Health Agency of Canada's pandemic influenza surveillance system (12 April 2009 to 3 April 2010) vs. the Canadian Institute for Health Information's (CIHI) 3-year historical baseline* by trimester, pandemic wave and dataset
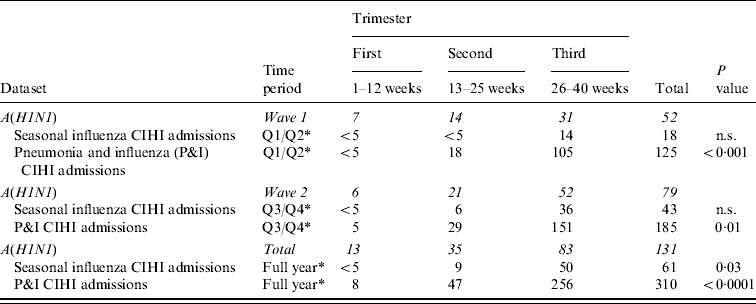
n.s., Not significant.
* Average value based on corresponding 2006–2007 , 2007–2008 , 2008–2009 cases reported to CIHI.
DISCUSSION
This paper provides a description of the full pandemic experience for all pregnant women admitted for A(H1N1) during the 2009–2010 pandemic. To the best of our knowledge, no other work describes a national-level pandemic experience for admitted pregnant A(H1N1) cases. The existing literature either (1) describes only part of the outbreak, (2) describes cases in a specified geographical location/medical facility (i.e. non-national coverage), or (3) describes severe cases (ICU and death) exclusively. Furthermore, by comparing pandemic and inter-pandemic activity, our study provides evidence suggesting that pandemic surge capacity to manage pregnant cases, ideally early on in the disease's clinical history, may positively influence clinical outcomes.
Overall, the Canadian pandemic experience for the 263 hospitalized pregnant women appeared to be similar to the experiences described elsewhere. Similarly to the Australian [Reference Hewagama16], Argentine [Reference Raffo17], and American [Reference Creanga4–Reference Siston6, Reference Jamieson18–Reference Miller20] experiences as well as pre-pandemic evidence from Canada and the USA, the largest proportion of Canadian pregnant hospital and ICU admissions were reported in women in their third trimester [Reference Dodds21–Reference Lindsay23], followed by women in their second trimester [Reference Jain19]. The difference in median age between pregnant women (27·5 years) and women of reproductive age (29·0) years was also consistent with US evidence [Reference Louie5].
Where the Canadian experience differed from other countries was in the proportion of cases that resulted in death, and in the number of cases who reported Aboriginal ethnicity. In Canada 1·5% of all pregnant influenza cases died compared to fatality rates of up to 6·4% reported elsewhere [Reference Louie5]. This difference may be attributable to a decreased severity in cases observed during wave 2 [Reference Helferty9] resulting from an increased awareness among community-based physicians of pregnancy-associated risk [Reference Saleeby24, Reference Brown25]. The outcome of this may have been both an increased administration of early antiviral treatment and immunization as well as a decreased severity threshold for admission of pregnant women relative to the general population. Although there is evidence elsewhere supporting this suggestion [26], in the absence of documented evidence of these interventions, this is merely speculative on our part.
Canada's pandemic experience also displayed higher than expected numbers of hospitalized cases of pregnant women of self-reported Aboriginal ethnicity. Whereas it is estimated that Aboriginal women of reproductive (WRA) age represent about 4·3% of all Canadian WRA [27], during the pandemic it was observed that they represented 16·2% of all hospitalized cases, 18·2% of all ICU cases and 50% of fatalities for which an ethnicity was recorded. Moreover, although the over-representation of Aboriginal cases was higher in wave 1 than in wave 2, wave 2 nonetheless also reported a higher than expected proportion of cases (9·8%). These findings are consistent with some of the early evidence reported in the literature, which suggested that Aboriginal women (especially pregnant ones) were at higher risk of complications than their non-Aboriginal counterparts [Reference Kumar28–Reference Knight31]. Notably, the ANZIC group in New Zealand reported that pregnant Aboriginals in New Zealand and Australia were at higher risk of being admitted to ICU for A(H1N1) [32]. However, unknown Aboriginal status for 15·2% of all pregnant cases means that the findings pertaining to ethnicity reported here need to be interpreted with caution. Furthermore, the small overall number of Aboriginal pregnant cases further contributes to the need for a conservative evaluation of these findings.
The further comparison between the proportion of pregnant cases of Aboriginal ethnicity to the proportion of total cases of Aboriginal ethnicity also exhibited a comparatively high rate in the pregnant subset. However, this finding should also be interpreted with caution as this may have been due to a bias introduced by the active surveillance efforts of the authors exclusively for the pregnant cases.
Comparison of the PHAC data to the CIHI seasonal influenza and P&I numbers estimated that the PHAC admission rates were somewhere between the different CIHI categories – the P&I case definition was less specific as it included cases with a clinical diagnosis of P&I but without systematic virological confirmation whereas the seasonal influenza case definition was more specific as it required a diagnosis of influenza (ICD-10-CA codes J09, J10.- or J11.-). However, the difference between CIHI's seasonal influenza rates and those reported during the pandemic may have been due to more aggressive virological testing during the pandemic relative to inter-pandemic years rather than due to a real difference in the burden of illness.
With respect to the significant difference in trimester distribution between the CIHI seasonal influenza data and the PHAC pregnant case surveillance data, the difference may have been due to the large number of PHAC cases for whom trimester was unreported. However, we posit that a true difference in the proportion of cases by trimester could be attributed to (1) a lack of pre-existing immunity in the population, which rendered women in all trimesters more vulnerable, (2) the particular virulence of the H1N1 2009 virus in some young and healthy adults, or (3) decreased testing and admission thresholds for all pregnant women during pandemic, compared to non-pandemic years.
Our findings suggest that although the volumes of admissions were higher than the seasonal influenza admission volumes in previous inter-pandemic years, overall, the severity of outcomes for pregnant women were less than for all cases admitted for A(H1N1) during the pandemic. However, the data illustrate the need for special consideration for pregnant women of Aboriginal ethnicity who may have been at increased risk. Based on our experience and the findings here, we recommend that during future pandemics, special consideration be taken to closely monitor and document the admissions and clinical outcomes specific to pregnant women so that potential clinical differences such as those described in this study can be confirmed or discredited. In turn, this will lead to better informed clinical decision-making in the management of pregnant women during a pandemic.
Study limitations
Canadian protocols emphasize the testing of inpatients suspected of having the pandemic virus. However, cases may have been missed, either because they were not tested, tested negative, or because subtyping was either not done or was inconclusive. Although every effort was made to collect complete data, this was not always possible. Whenever possible, the incomplete data were highlighted, but may nonetheless have introduced some bias into our findings. Moreover, it is possible that the completeness of the pregnancy cohort may have varied between P/T; however, given that the PHAC must legally rely on the P/T to identify and report cases, this cannot be confirmed. Furthermore, limited surveillance data were available on vaccine and antiviral usage, length of stay and fetal outcomes. In addition, because we collected data only on those with underlying risk conditions described previously by the National Advisory Committee on Immunization (NACI) and Advisory Committee on Immunization Practices (ACIP), we could not systematically assess the impact of potential risk factors for influenza complications, including obesity. Additionally, the lack of differentiation between unknown and absent underlying conditions for cases from two provinces may have underestimated the importance of these factors in relation to case severity [Reference Campbell10]. Conversely, this underestimation may also have been due to superior data quality for severe cases, which may have resulted in an overestimation of the importance of underlying conditions in relation to disease severity overall. Finally, enhanced surveillance for pandemic A(H1N1) 2009 in hospitals resulted in higher than usual testing levels. Comparisons between baseline influenza and P&I should be considered in light of this potential artefactual difference in disease ascertainment.
ACKNOWLEDGEMENTS
The authors thank the provincial and territorial influenza teams for their dedication in collecting and reporting data to the Public Health Agency of Canada throughout the pandemic.
DECLARATION OF INTEREST
None.









