Introduction
Obesity is considered to be a major risk factor for chronic diseases such as CHD and hypertension, type 2 diabetes, and some types of cancer(1). Its prevalence is increasing, with 400 million obese and 1·6 billion overweight adults around the world(1). Although genetics plays a role in the regulation of body weight, body size and body composition and the metabolic response to feeding in humans(Reference Ichihara and Yamada2–Reference Martínez-Hernandez, Enríquez and Moreno-Moreno6) and in animals(Reference Reuter7, Reference Speakman, Hambly and Mitchell8), the increase in worldwide obesity in a short period of time cannot be explained by genetics; there are individual differences in genetic susceptibility to environmental factors such as diet(Reference Ichihara and Yamada2, Reference Martínez-Hernandez, Enríquez and Moreno-Moreno6, Reference James9–Reference Rosengren, Lissner, Rosengren and Lissner11).
Dietary fat intake often has been claimed as responsible for the increase in adiposity. Human studies have shown that high-fat diets ( ≥ 30 % of energy from fat) can easily induce obesity(Reference Jequier10, Reference Hill, Melanson and Wyatt12–Reference Schrauwen and Westerterp15). Epidemiological studies conducted in countries such as China, Canada and the USA have shown that, when the average amount of fat in the diet increases, the incidence of obesity also increases(Reference George, Tremblay and Despres16–Reference Tucker and Kano19). This has led to a worldwide effort to decrease the amount of fat in the human diet.
Diets rich in fat not only induce obesity in humans but also make animals obese(Reference Rothwell and Stock20–Reference Warwick and Schiffman22). In both rats(Reference Boozer, Schoenbach and Atkinson23, Reference Ghibaudi, Cook and Farley24) and mice(Reference Bourgeois, Alexiu and Lemonnier25, Reference Takahashi, Ikemoto and Ezaki26) a positive relationship has been found between the level of fat in the diet and body weight or fat gain. In the scientific literature it was first shown that rats consuming diets containing high proportions of fat gained weight faster than those on diets containing minimal amounts of fat(Reference Deuel, Movitt and Hallman27, Reference Deuel, Meserve and Straub28). In 1949, obesity was induced for the first time in rats by ad libitum feeding of a semi-liquid palatable diet(Reference Ingle29). Then in 1953, Fenton & Dowling used high-fat diets with fat at 50 % of total energy in weanling mice to induce obesity; they called it nutritional obesity(Reference Fenton and Dowling30) but the model was later renamed as dietary obesity(Reference Sclafani and Springer31).
Since under-reporting is an important bias in epidemiological studies on diet and obesity in human subjects(Reference Hebert, Patterson and Gorfine32–Reference Voss, Kroke and Klipstein-Grobusch34), animal models have been widely utilised for experiments on dietary obesity(Reference Reuter7, Reference Speakman, Hambly and Mitchell8, Reference Thibault, Woods and Westerterp-Plantenga35, Reference Young and Kirkland36). Usually high-fat diets within the range of 30–78 % of total energy intake are used(Reference Buettner, Scholmerich and Bollheimer21) – either by adding a particular fat to the animal's diet or using an assortment of fat- and sugar-rich supermarket foods (cafeteria diet) – for studying obesity in rats(Reference Ghibaudi, Cook and Farley24, Reference Mickelsen, Takahashi and Craig37–Reference Levin and Dunn-Meynell46) and mice(Reference Bourgeois, Alexiu and Lemonnier25, Reference Bell, Spencer and Sherriff47–Reference Ikemoto, Takahashi and Tsunoda52). The use of high-carbohydrate–low-fat diets has not been found as efficient as high-fat–low-carbohydrate diets in inducing obesity(Reference Ghibaudi, Cook and Farley24, Reference Harrold, Williams and Widdowson41, Reference Ellis, Lake and Hoover-Plow53).
It has been reported that despite the growing problem of obesity, Canadians and Americans are eating less fat than a generation ago(Reference Lissner, Heitmann and Bengtsson54, Reference Gray-Donald, Jacobs-Starkey and Johnson-Down55). This shows that the increasing rate of obesity cannot be totally explained by high intakes of fat in the diet, suggesting that the type of fat may also play a role, although the results of the studies in human subjects and animals have not been conclusive(Reference Moussavi, Gavino and Receveur56). Some studies have reported that not all fats are obesogenic and the dietary fatty acid profile rather than the amount of energy from fat is an important variable in developing dietary obesity(Reference Bourgeois, Alexiu and Lemonnier25, Reference Bell, Spencer and Sherriff47, Reference DeLany, Windhauser and Champagne57–Reference Wang, Storlien and Huang60), but there is some controversy on this matter since there are reports showing non-significant differences in final body weight and/or body-weight gain of the animals consuming various fatty acids(Reference Ellis, Lake and Hoover-Plow53, Reference Okere, Chandler and McElfresh61–Reference Su and Jones66).
Other factors that may contribute to obesity induced by a diet rich in fat include failure to adjust oxidation of fat to the extra fat in the diet(Reference Schrauwen and Westerterp15), increase in adipose tissue lipoprotein lipase activity(Reference Preiss-Landl, Zimmermann and Hämmerle67), increased meal size and decreased meal frequency(Reference Westerterp-Plantenga68), as well as overconsumption of energy attributed to high energy density of the diet(Reference Blundell and Macdiarmid69–Reference Poppitt and Prentice72), orosensory characteristics of fats and poorly satiating properties of the high-fat diets(Reference Warwick and Schiffman22, Reference Blundell and Macdiarmid69, Reference Golay and Bobbioni70). Reviews of dietary obesity describe potential mechanisms of body weight and food intake regulation involving the central nervous system – mainly the hypothalamus – neuropeptides such as ghrelin and neuropeptide Y, and hormones such as insulin and leptin(Reference Skelton, DeMattia and Miller73, Reference Kiess, Petzold and Topfer74). Adipose tissue per se is considered to be an endocrine organ that secretes cytokines such as IL-6 and TNFα; thus obesity could possibly be regarded as a chronic inflammatory disease(Reference Skelton, DeMattia and Miller73–Reference Sorisky76).
Obesity occurs when energy uptake surpasses energy expenditure in the individual animal and so the stores of energy in body fat are enlarged, particularly in adipose tissues. Obesity involves both or either an increase in the number of adipocytes (hyperplasia) and their size (hypertrophy)(Reference Jequier10, Reference de Ferranti and Mozaffarian77, Reference Avram, Avram and James78). Initially it was hypothesised that adipocyte number was determined in early childhood and that the obesity developed during adulthood was a result of an increase in adipocyte size(Reference Hirsch and Han79, Reference Greenwood and Hirsch80). However, it is now known that hyperplasia is an ongoing event not limited to childhood. At any stage of life when adipocytes enlarge to the point of hypertrophy, they release factors such as TNFα and insulin-like growth factor that stimulate hyperplasia of the adipocytes(Reference Sorisky76, Reference de Ferranti and Mozaffarian77, Reference Prins and Orahilly81). Conversely, recent studies on reversal of obesity in human subjects have found decreases not only in the size of the fat cells but also in their number: the loss of weight is followed by apopotosis of adipocytes(Reference Avram, Avram and James78, Reference Prins and Orahilly81).
This paper summarises the present literature on factors that can play a role in the development of obesity and explores mechanisms that have been proposed for obesity induced by a diet rich in fat. The adequacy of the paradigm of high-fat diets in animal models of human obesity will be discussed. The possibility of reversing dietary obesity in animal models will be explored. Physical activity is another important factor in obesity; however, the present paper focuses on dietary factors only. Some reviews have been published about diverse areas of dietary obesity that have been cited in this introduction but the aim of the present review is to summarise the range of relevant results and to provide a conclusive coverage of the different aspects of obesity from high-fat diets in non-human species.
Assessment of dietary obesity
In animal models, as in humans, obesity can be assessed by criteria based on (1) gain of body weight or the Lee obesity index and/or (2) increase of body fat content. However, standard thresholds for obesity have not been developed like BMI in human beings. In most studies, the degree of obesity has been evaluated by comparing body weight (or fat) of the experimental group fed a high-fat or energy-dense diet with control animals that show normal growth while fed chow or low-fat diets(Reference Rothwell and Stock20, Reference Ghibaudi, Cook and Farley24, Reference Harrold, Williams and Widdowson41, Reference Woods, Seeley and Rushing42, Reference Schemmel, Mickelsen and Tolgay45, Reference Levin and Dunn-Meynell82). Researchers that have attempted to do so differed in the values that are 10–25 % greater body weight than age-matched control rats fed chow (normal pattern of body-weight gain) as moderate obesity(Reference Harrold, Williams and Widdowson41, Reference Woods, Seeley and Rushing42) and greater than 40 % as severe obesity(Reference Levin and Dunn-Meynell82).
The Lee index for assessing obesity in rats is similar to BMI in humans. It was defined by Lee in 1929(Reference Lee83) as the cube root of body weight (g) divided by the naso–anal length (cm) and multiplied by 1000. Lee considered values greater than 310 as an indicator of obesity. Since then some researchers have used the Lee index to assess the levels of obesity in rats(Reference Sclafani and Gorman44, Reference Li, Wu and Xie84–Reference Stephens88). Reliable correlations were found in some studies between the Lee index and fat content of the body(Reference Kanarek and Markskaufman86, Reference Bernardis89–Reference Rogers and Webb91).
In human subjects body composition assessment with methods such as air displacement plethysmography or dual-energy X-ray absorptiometry gives a more precise idea of the degree of obesity than do anthropometric measurements alone(Reference Lindsay, Hanson and Roumain92, Reference Tzotzas, Krassas and Doumas93). For example, children and adolescent males have smaller fat mass than females of a similar BMI, and this difference is more pronounced in the older age group; and so the relationship between BMI and the direct measures of adiposity is influenced by factors such as sex and age(Reference Lindsay, Hanson and Roumain92). Dual-energy X-ray absorptiometry is also used in rats for assessing body composition(Reference Ghibaudi, Cook and Farley24, Reference Holemans, Caluwaerts and Poston94). In rats fed diets high in fat, a linear increase in body fat with increasing body weight has been shown(Reference Bourgeois, Alexiu and Lemonnier25, Reference Schemmel, Mickelsen and Tolgay45). However, results of the study of Woods et al. (Reference Woods, Seeley and Rushing42) showed that measuring body fat is a more sensitive criterion for assessing obesity in animals, since rats fed a high-fat diet (40 % of energy) for 10 weeks displayed a 10 % increase in total body weight but a 35–40 % increase in total body fat compared with the animals fed a low-fat diet.
In models of dietary obesity, animals are classified as prone and resistant based on their body weight, body-weight gain, body fat, or noradrenaline concentrations in urine. Tulipano et al. (Reference Tulipano, Vergoni and Soldi95) categorised Sprague–Dawley rats fed a high-fat diet based on their final body weight, with rats in the highest quartile designated as obesity prone and those in the lowest quartile assigned as obesity resistant. In some studies upper (prone) and lower (resistant) tertiles of body-weight gain(Reference Levin and Dunn-Meynell46, Reference Huang, Xin and McLennan51, Reference Dourmashkin, Chang and Hill96) or body fat(Reference Dourmashkin, Chang and Gayles97) of the animals fed high-fat diets have been used for this classification. Before developing obesity while fed with chow, prone and resistant animals have also been identified based on high and low levels of urinary noradrenaline, respectively(Reference Hassanain and Levin98, Reference Michel, Levin and Dunn-Meynell99).
High-fat diets
Energy density
In humans, a significant positive relationship has been found between the amount of dietary energy from fat and the proportion of the population who are overweight (in epidemiological studies), and in clinical studies between the level of dietary fat and body-weight gain as well as between the reduction in the dietary fat and weight loss(Reference George, Tremblay and Despres16, Reference Popkin, Keyou and Fengying17, Reference Tucker and Kano19, Reference Lissner, Levitsky and Strupp100). These associations have also been shown in animal studies(Reference Boozer, Schoenbach and Atkinson23–Reference Takahashi, Ikemoto and Ezaki26, Reference Bartness, Polk and McGriff101). This relationship in humans or in animal models of more dietary fat leading to greater obesity shows that the fat content of the diet is an important factor in energy balance. In general, diets containing more than 30 % of total energy as fat lead to the development of obesity.
Researchers have induced obesity by diets having different percentages and sources of fats in rats(Reference Ghibaudi, Cook and Farley24, Reference Sclafani and Springer31, Reference Mickelsen, Takahashi and Craig37–Reference Schemmel, Mickelsen and Tolgay45, Reference Ellis, Lake and Hoover-Plow53, Reference Levin and Dunn-Meynell82, Reference Jen, Buison and Pellizzon102–Reference Rolland, Roseau and Fromentin105), mice(Reference Bourgeois, Alexiu and Lemonnier25, Reference Bell, Spencer and Sherriff47–Reference Ikemoto, Takahashi and Tsunoda52, Reference Wang, Storlien and Huang60) and hamsters(Reference Wade106). Furthermore, the characteristics of the diets used have differed within and between laboratories in macronutrient composition, energy density and orosensory properties. In many animal studies the composition of the control diet was not shown or a non-purified chow control diet was used. This could have confounding effects arising from comparisons made with the high-fat diets.
Since the original observations of dietary obesity, obesity has been induced in animals by diets containing fat as low as 13 % of total energy in a high-energy diet(Reference Harrold, Williams and Widdowson41) (Table 1; line 26) (which is more than the rat's requirement for fat: 5 %) to as high as 85 % of energy(Reference Mickelsen, Takahashi and Craig37) (Table 1; line 1). Several researchers have reviewed the amount of fat required to induce obesity in animals. The most recent review was by Buettner et al. (Reference Buettner, Scholmerich and Bollheimer21) who summarised studies conducted between 1997 and 2007, and concluded that the best method to induce obesity in animals was to use semi-purified high-fat diets containing animal fats at 40 % of energy, with a low amount of n-3 fatty acids and a low amount of plant oils rich in n-6 and n-9 fatty acids.
Table 1 Studies of high-fat diet-induced obesity (DIO) in animal models
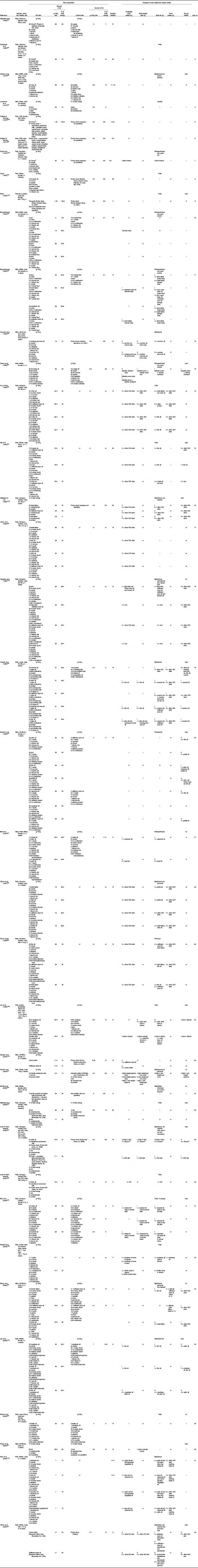
nr, Not reported; i, increase v. control diet or as specified; 0, no change or difference v. control diet or as specified; –, not measured; d, decrease v. control diet or as specified; DR, dietary obesity resistant.
Interestingly, some recent studies have indicated that the development of obesity is prevented in humans and rats when the increase in dietary fat is accompanied by an increase in protein (high protein:carbohydrate and low carbohydrate:fat ratios)(Reference Pichon, Huneau and Fromentin107–Reference Weigle, Breen and Matthys109). This has been related to greater satiety with high-protein diets, lower insulin levels with low-carbohydrate diets and the energy required to convert amino acids in glucose compounds for gluconeogenesis(Reference Pichon, Huneau and Fromentin107). High-protein diets were also found to increase cholecystokinin and decrease plasma levels of the orexigenic hormone ghrelin(Reference Potier, Darcel and Tome110, Reference Blom, Lluch and Stafleu111), reduce gastric emptying(Reference Blom, Lluch and Stafleu111) and increase central nervous system leptin sensitivity(Reference Weigle, Breen and Matthys109, Reference Saris112). Moreover, high-protein diets resulted in a decrease in fatty acid synthase enzyme activity in the liver that reduces hepatic lipogenesis(Reference Pichon, Huneau and Fromentin107). The increase in circulating amino acids per se is a satiety signal and inhibits food intake through suppressing the gene expression of agouti-related protein (a neuropeptide in the brain that increases appetite)(Reference Potier, Darcel and Tome110, Reference Morrison, Xi and White113). However, Huang et al. (Reference Huang, Liu and Rahardjo114) showed that increasing the dietary protein:carbohydrate ratio could not reduce the degree of obesity when obesity had already been induced in high-fat diet-fed mice (at 40 % of energy). Therefore they suggested that these diets might be efficient in preventing obesity but may not reverse obesity once established.
In the human diet, an increase in dietary fat is usually accompanied by a decrease in carbohydrate while the protein is relatively constant (for example, fat 35–45 %, carbohydrate 45–55 %, protein 15–20 %). This is why a presumably positive relationship between the level of fat of the diet and degree of obesity is usually found in epidemiological studies without controlling for dietary protein level.
Dietary profile of fatty acids
Fatty acid composition of the diet may play an important role in body-weight regulation and cellularity of adipose tissue (fat cell volume and number)(Reference Moussavi, Gavino and Receveur56, Reference Storlien, Huang and Lin59, Reference Ailhaud, Massiera and Weill115, Reference Clarke116). Studies in human subjects have shown that SFA are more obesogenic than PUFA(Reference DeLany, Windhauser and Champagne57, Reference Kien, Bunn and Ugrasbul58, Reference Lawton, Delargy and Brockman117–Reference Piers, Walker and Stoney119). This idea has been supported by animal studies by showing either greater accumulation of body fat(Reference Yaqoob, Sherrington and Jeffery43, Reference Bell, Spencer and Sherriff47, Reference Shillabeer and Lau120–Reference Takeuchi, Matsuo and Tokuyama122) (Table 1; lines 19, 21, 16, 18 and 37, respectively) or higher body weight(Reference Bourgeois, Alexiu and Lemonnier25, Reference Bell, Spencer and Sherriff47, Reference Wang, Storlien and Huang60, Reference Takeuchi, Matsuo and Tokuyama122) (Table 1; lines 11, 21, 33 and 18, respectively) on feeding with diets moderate or rich in SFA. A study conducted by Ellis et al. (Reference Ellis, Lake and Hoover-Plow53) in 3-week-old female Sprague–Dawley rats comparing diets rich in low-SFA maize oil or high-SFA coconut oil (40 % of total energy) for 8 weeks found higher fat cell number in animals fed coconut oil and greater fat cell size in the rats fed maize oil. Since hypertrophy of adipocytes is a prerequisite for hyperplasia, those results show that more severe form of obesity developed from feeding a diet high in SFA.
The obesogenic effect of SFA can be explained by the fact that SFA are poorly used for energy, and so remain to be acylated into TAG and stored in adipose tissue, whereas PUFA and MUFA are readily used for energy and so stored less(Reference Storlien, Huang and Lin59). In other words, the effective energy content of a diet is greater when the fats in it are high in SFA. In addition, the rate of oxidation of SFA decreases with increase of carbon chain length(Reference DeLany, Windhauser and Champagne57). Furthermore, unlike MUFA and PUFA, SFA decrease RMR and diet-induced thermogenesis(Reference Clarke116, Reference Lichtenbelt, Mensink and Westerterp118, Reference Casas-Agustench, Lopez-Uriarte and Bullo123–Reference Soares, Cummings and Mamo125). Moussavi et al. (Reference Moussavi, Gavino and Receveur56) suggested that PUFA suppress the expression of lipogenic transcription genes while MUFA and SFA do not.
Another possible mechanism is that saturation of fatty acids decreases their suppressive effect on dietary intake: thus fats and oils containing high proportions of linoleic acid are more satiating than fats and oils rich in oleic or stearic acid(Reference French and Robinson13, Reference Lawton, Delargy and Brockman117, Reference Beardshall, Morarji and Bloom126). PUFA inhibit appetite more strongly than MUFA or SFA through an increase in the release of cholecystokinin which augments other signals of satiety(Reference Lawton, Delargy and Brockman117, Reference Beardshall, Morarji and Bloom126). Another study, however, failed to confirm that SFA induced less satiety than MUFA(Reference Alfenas and Mattes127).
A study in adult male Wistar rats showed that feeding high-fat diets (60 % of energy) for 8 weeks resulted in greater intrathoracic fat mass in animals fed a SFA-rich diet (cocoa butter) and greater intra-abdominal and epididymal fat mass in those fed PUFA (safflower-seed oil)(Reference Okere, Chandler and McElfresh61) (Table 1; line 38). There are also reports of studies that did not show any specific effect of SFA and PUFA on body weight or fat mass(Reference Jones, Toy and Cha64, Reference George, Mulkins and Casey128) (Table 1; lines 17 and 25, respectively).
Short-chain (C2 : 0–C4 : 0) and medium-chain (C6 : 0–C12 : 0) fatty acids are directly transported to the liver via the portal system, are not dependent upon carnitine for entering the mitochondria and therefore are oxidised more and deposited less in adipose tissue than long-chain fatty acids (C14 : 0–C24 : 0)(Reference Moussavi, Gavino and Receveur56, Reference Nagao and Yanagita129, Reference Takeuchi, Sekine and Kojima130). Short-chain and medium-chain fatty acids also increase diet-induced thermogenesis and energy expenditure(Reference Moussavi, Gavino and Receveur56, Reference Takeuchi, Sekine and Kojima130). The lower obesogenic effect of medium-chain TAG, which are composed of medium-chain fatty acids, was shown in many studies. Isoenergetic diets (with fat at 12 % of total energy) containing olive oil or medium-chain fatty acid (octanoic acid) offered for 23 d to overweight adult female Wistar rats led to a lower final weight and fat mass in medium-chain fatty acid-fed animals(Reference Simón, Fernández-Quintela and Del Puy Portillo131). Similarly, lower body weight and fat gain were found in adult male Sprague–Dawley rats fed medium-chain TAG-rich high-fat diets (at 50 % of energy) for 8 weeks than in rats fed high-fat diets based on long-chain fatty acids(Reference Takeuchi, Noguchi and Sekine132). Other studies in animals(Reference Bray, Lee and Bray133–Reference Noguchi, Takeuchi and Kubota135) and in human subjects(Reference Stubbs and Harbron136, Reference Tsuji, Kasai and Takeuchi137) reported similar findings.
The location of the terminal double bond of PUFA may affect their action. Diets rich in n-3 fatty acids have been shown to prevent obesity better than other subclasses of PUFA(Reference Moussavi, Gavino and Receveur56, Reference Clarke116). This effect has been reported in studies in human subjects(Reference DeLany, Windhauser and Champagne57, Reference Garaulet, Perez-Llamas and Perez-Ayala138, Reference Nogi, Yang and Li139), mice(Reference Cunnane, McAdoo and Horrobin48, Reference Wang, Storlien and Huang60) and rats(Reference Cha and Jones62, Reference Hill, Peters and Lin63, Reference Okuno, Kajiwara and Imai65, Reference Su and Jones66, Reference Jen, Buison and Pellizzon102). In most of the animal studies lower fat deposition in subjects fed n-3 fatty acids was shown despite comparable food and energy intake among the groups(Reference Cunnane, McAdoo and Horrobin48, Reference Wang, Storlien and Huang60, Reference Cha and Jones62, Reference Okuno, Kajiwara and Imai65, Reference Su and Jones66) (Table 1; lines 12, 33, 23, 22 and 14, respectively); therefore this effect can be related to the metabolic effects of n-3 fats. Suggested mechanisms involved in this effect of n-3 PUFA are: (1) low expression of lipogenic transcription genes with diets high in n-3 PUFA(Reference Storlien, Huang and Lin59, Reference Wang, Storlien and Huang60, Reference Clarke116, Reference Lichtenbelt, Mensink and Westerterp118); (2) increased concentrations of thromboxane A2, leukotriene B4 and some cytokines that are elevated by an increase in n-6 PUFA intake and decrease in n-3 PUFA, and a low dietary n-6:n-3 ratio is beneficial for preventing them(Reference Simopoulos140); (3) inhibition of PG synthesis by n-3 PUFA leading to suppression of terminal differentiation of adipocytes(Reference Okuno, Kajiwara and Imai65).
The configuration of the double bonds of PUFA may also affect the development of obesity. Conjugated fatty acids are PUFA that have at least one double bond separated by one single bond. Conjugated linoleic acid was shown to prevent obesity, and this effect has been attributed to: lower energy intake by decreasing the expression of neuropeptide Y and agouti-related protein, increased diet-induced thermogenesis, decreased pre-adipocyte differentiation via decreasing the expression of PPARγ which is a key factor for adipogenesis, and decreased lipogenesis through decreasing lipoprotein lipase activity and fatty acid synthase expression(Reference Kennedy, Martinez and Schmidt141, Reference Park142). The antiobesity effect of conjugated linoleic acid was reported in studies conducted in rodents(Reference Moon, Lee and Seo143, Reference Parra, Palou and Serra144) and human subjects(Reference Norris, Collene and Asp145, Reference Sahin, Uyanik and Inanc146). However, animal(Reference Halade, Rahman and Fernandes147) and human(Reference Ingelsson and Risérus148) studies have found that feeding conjugated linoleic acid-rich diets might also lead to insulin resistance.
The studies mentioned varied in species, strain, age and/or sex of the animals used, which may explain some divergences among the results. Using different fats or fatty acids at various percentages of animal or plant origin and in a wide range of durations might have affected the results as well. For example, in a diet containing 40 %(Reference Bell, Spencer and Sherriff47) or 58 %(Reference Wang, Storlien and Huang60) energy as beef tallow (48 % SFA and 52 % PUFA), the percentages of SFA and PUFA would be 19 and 21 % (in the 40 % diet) and 28 and 30 % (in the 58 % diet), respectively. These percentages might have been high enough to reveal the obesogenic effect of SFA when used for a 7–8-week period. The source of fat (plant origin v. animal origin) also might have affected the results, for example, because SFA of plant origin might not be as effective as SFA of animal origin in developing obesity. Indeed, SFA of plant origin mainly contain medium-chain fatty acids (lauric acid in coconut oil and palm kernel oil) rather than long-chain fatty acids which are abundant in fats of animal origin(Reference Van Nieuwenhuyzen and Adelmann149).
Taken together, the findings indicate that rats and mice are appropriate models for studying the effects of various fatty acids in developing obesity.
Physiological mechanisms of high-fat diet-induced obesity
Food efficiency and diet-induced thermogenesis
Some reports have attributed obesity induced by high-fat diets to their high food efficiency (g body-weight gain per kJ food consumed). Energy from fat has a larger effect on body-weight gain than has energy from non-fat sources(Reference Hill, Melanson and Wyatt12, Reference Bray and Popkin14, Reference Warwick and Schiffman22, Reference Herberg, Doppen and Major49, Reference Wade106, Reference Prpic, Watson and Frampton150, Reference Roberts, Berger and Barnard151). Diet-induced thermogenesis is the energy for digesting, absorbing and storing nutrients and produces a loss of energy for the body which is 2–3 % for fats, 25–30 % for proteins and 6–8 % for carbohydrates. Therefore, the efficiency of nutrient utilisation differs among macronutrients and fats have an efficiency of 97–98 %, whereas efficiency is 70–75 % for proteins and 92–94 % for carbohydrates(Reference Jequier10, Reference Hill, Melanson and Wyatt12, Reference Warwick and Schiffman22, Reference Saris112). In addition, it costs energy to build long-chain fatty acids from glucose or amino acids, whereas dietary fat contains long-chain fatty acid pre-formed.
Energy density
Some studies have shown that a fat-rich diet induces obesity by increasing energy intake(Reference Ghibaudi, Cook and Farley24, Reference Oscai38, Reference Ainslie, Proietto and Fam39, Reference Harrold, Williams and Widdowson41, Reference Woods, Seeley and Rushing42). If weight of intake is not increased at least in proportion, this implicates the high energy density of high-fat diets.
Individuals with ad libitum access to diets with different energy densities ate the same amount of food by weight (in a meal or over a few days)(Reference Poppitt and Prentice72, Reference Drewnowski152–Reference Rolls and Bell155). On the other hand, after 2 weeks of exposure, subjects learned to compensate for the higher energy density of the diet, and ate less weight of food(Reference Stubbs and Whybrow156). Rats and mice have been labelled as hyperphagic when fed a fat-rich diet, which was based on animals ingesting more energy and not necessarily weight of food(Reference Ghibaudi, Cook and Farley24, Reference Ainslie, Proietto and Fam39, Reference Woods, Seeley and Rushing42, Reference Huang, Xin and McLennan51). Although in the mentioned studies, the wieght of food ingested was not always reported, rats might have attempted to adjust their intake according to the energy density of the fat-rich diet. While some of the high-fat diets were less dense in other macronutrients and micronutrients, rats could not fully adjust for the extra dietary energy while ingesting a minimal amount of the high-fat diet to meet their requirements (for example, 5 % protein for maintenance and 15 % for growth)(157) with carrying extra energy. Therefore, high-fat diets used to induce obesity in animal models should meet macronutrient and micronutrient requirements of the animals, so that hyperphagia can be better interpreted.
Satiating effects of fat
Weaker satiety signals from fat than from carbohydrates and proteins have been suggested to play a role in overconsumption of energy from fat-rich diets(Reference Blundell and Macdiarmid69, Reference Golay and Bobbioni70, Reference Blundell, Lawton and Cotton158, Reference Lucas, Ackroff and Sclafani159). To clarify if the hyperphagia from fat-rich diets is due to their post-ingestive effect, rats were administered by gastric self-infusion for 16 d isoenergetic high-fat (59·9 % of energy) and high-carbohydrate (fat: 16·7 % of energy) liquid diets(Reference Warwick and Weingarten160). Rats self-infused more energy per d of the high-fat diet than of the high-carbohydrate diet; thus, when orosensory effects are minimised, hyperphagia on high-fat diets remains. Poorly satiating post-ingestive effects of fat produced more frequent meals and resulted in large meals(Reference Warwick and Weingarten160).
Post-ingestive effect of nutrients also may increase food intake by conditioning sensory preference(Reference Lucas, Ackroff and Sclafani159). In a 9 d study, adult female Sprague–Dawley rats were infused intragastrically with isoenergetic high-fat (59·6 % of energy) and high-carbohydrate (14·6 % of energy) diets paired with different flavours (cherry, grape or strawberry). The rats drank substantially more (38 %) of the solution paired with the infusion of the high-fat diet than the solution paired with the infusion of the high-carbohydrate diet, hence the post-ingestive effect of the diets high in fat enhances preference for the sensory features of high-fat diets(Reference Lucas, Ackroff and Sclafani159).
Various mechanisms have been suggested for a reduction in satiety signals with high-fat feeding and attenuation of suppression of energy intake by high-fat diets. These include: (1) attenuated enterogastric inhibition of gastric emtpying and secretion of satiety hormones (cholecystokinin, peptide YY (PYY) and glucagon-like peptide-1) which are normally stimulated by the presence of fat in the small intestine, and thus decrease late satiety(Reference Covasa and Ritter161, Reference Little, Horowitz and Feinle-Bisset162); (2) inhibition of fatty acid oxidation(Reference Scharrer163, Reference Kahler, Zimmermann and Langhans164), so that high-fat diets lower the rate of oxidation of fatty acids, hence they may increase intake; (3) insensitivity to the food intake reducing the effect of apoA-IV, which is a peptide that decreases meal size(Reference Tso and Liu165, Reference Woods, D'Alessio and Tso166). Low-energy-dense diets have greater volume and so induce more stomach distension than diets with higher energy density(Reference French and Robinson13).
Hormones
Signals from adipose tissue (leptin, adiponectin and resistin), stomach (ghrelin and obestatin), pancreas (insulin) and intestine (cholecystokinin, PYY and incretins including glucagon-like peptide-1 and gastric-inhibitory peptide) are sent to the brain to regulate energy balance(Reference Coll, Farooqi and O'Rahilly167–Reference Gale, Castracane and Mantzoros169). The present review reports the most extensively studied hormonal effects on energy balance (by reducing energy expenditure or increasing energy intake) associated with high-fat feeding.
Leptin
Leptin, first identified in 1994 by Rockefeller University scientists, is an important hormone in the control of food intake and body weight(Reference Zhang, Proenca and Maffei170). It is as an obese gene product produced by adipose tissue, generally in proportion to fat mass, with rises in plasma levels resulting in a decrease in food intake and increase in energy expenditure(Reference Zhang, Proenca and Maffei170–Reference Halaas, Gajiwala and Maffei174). Plasma leptin levels display a circadian rhythm. In humans, leptin is increased during the night and peak values are reached at about 24.00 hours, while minimum values are found at midday(Reference Randeva, Karteris and Lewandowski175, Reference Saad, Riad-Gabriel and Khan176). Studies in human subjects have shown that obesity is associated with higher concentrations of plasma leptin(Reference Saad, Riad-Gabriel and Khan176). Moreover, in healthy men, leptin levels increased in response to a high-fat meal; however, no differential effects among fatty acid chain length or saturation were reported(Reference Poppitt, Leahy and Keogh177).
Laboratory rats have similar circadian variations of plasma leptin, although maximum levels are reached in the middle of their active phase (at night) and minimum levels in the middle of their resting phase (daytime)(Reference Bodosi, Gardi and Hajdu178, Reference Chacon, Esquifino and Perello179). In a study in weanling male and female normal FVB mice, 12 weeks of feeding a high-fat diet (Western diet, Teklad Adjusted Calories Western-Type Diet, no. 88 187, fat at 40 % of total energy; Harlan-Teklad, Madison, WI, USA) produced 2·6- to 4·6-fold elevation in plasma leptin levels (measured between 09.00 and 11.00 hours) relative to control mice fed chow, but intake of energy was not less than that of the chow-fed controls(Reference Frederich, Hamann and Anderson180). Higher leptin levels were also found after a 2 h high-fat meal at dark onset compared with pre-meal levels in adult male obesity-prone Sprague–Dawley rats(Reference Leibowitz, Chang and Dourmashkin181).
In adult male Osborne–Mendel rats, adapted to a high-fat diet (56 % of energy) for 2 weeks, no reduction in food intake at 2, 4, 6 and 24 h following intraperitoneal injection of leptin (0·5 mg/kg body weight) after an overnight fast was found(Reference Lin, Martin and Schaffhauser182). In contrast, when the rats had been adapted to a low-fat diet, the injection suppressed the food intake at all time points. Thus, the intake response to peripheral leptin was impaired by chronically high levels of fat intake(Reference Lin, Martin and Schaffhauser182). Harrold et al. (Reference Harrold, Williams and Widdowson41) found hyperleptinaemia after 1 week of feeding adult male Wistar rats a raised level of energy as fat (13 % of energy). Levin & Dunn-Meynell(Reference Levin and Dunn-Meynell46) showed that when adult male Sprague–Dawley rats were fed a high-fat diet (31 % of energy) for 1 week and were then switched to 3 weeks of chow feeding, leptin levels (time of sampling not mentioned) were higher in rats that were prone to developing obesity on the high-fat diet than in rats that were resistant to dietary obesity despite having comparable body weights. Both obesity-prone and -resistant Sprague–Dawley rats fed high-fat diets (at 20 % of total energy) showed resistance to the anorectic effect of centrally administered leptin (10 μg; intracerebroventricular; ICV), while control animals fed a low-fat diet (3 % of total energy) decreased their energy intake following leptin administration(Reference Tulipano, Vergoni and Soldi95). However, in another study resistant animals did not show compromised responsiveness to the food-lowering effect of leptin when fed high-fat diets(Reference Surwit and Collins183). Overall, these results indicate that high-fat feeding induces hyperleptinaemia and leptin resistance and that this effect is independent of obesity-induced leptin resistance.
The mechanism thought to be involved in hyperleptinaemia and leptin resistance on the high-fat diet involves hypothalamic leptin receptors and their signalling pathways(Reference Frederich, Hamann and Anderson180). Animals susceptible to dietary obesity have reduced hypothalamic leptin receptor gene expression and show an early leptin response to an increase in dietary fat(Reference Levin, Dunn-Meynell and Ricci184).
In contrast to this, Ainslie et al. (Reference Ainslie, Proietto and Fam39) showed that female hooded Wistar rats aged between 20 and 22 weeks fed a high-fat diet (36 % of energy) for 4 weeks had significantly lower plasma leptin levels (measured after an overnight fast) than control rats fed low-fat diets (6·5 % of energy)(Reference Ainslie, Proietto and Fam39). A more recent study showed that adult male Sprague–Dawley rats fed a high-fat diet (60 % of energy) for 2 weeks were hypersensitive to the food intake-lowering effect of ICV administration of leptin (3 μg); however, after 5 weeks on the high-fat diet, rats became insensitive to this effect of injected leptin(Reference Fam, Morris and Hansen185). Another study in weanling C57BL/6J mice led to similar conclusions(Reference Lin, Thomas and Storlien186). The researchers suggested that early in high-fat feeding, animals are sensitive to the food-lowering effect of leptin but despite the reduction in food intake animals become fat as a result of the increase in food efficiency, leading to an increase in plasma leptin levels that is followed by insensitivity to its action(Reference Lin, Thomas and Storlien186). This implies that leptin resistance after long-term feeding on a high-fat diet is an effect of the obese state rather than the cause of obesity development.
Animal studies found that the fatty acid composition of a high-fat diet may influence leptin levels in the circulation. Lower serum leptin levels (measured 3–6 h after initiation of the dark phase) were found in 8-week-old lean male Wistar rats fed a diet rich in long-chain SFA (cocoa butter at 60 % of energy) than in animals fed a diet rich in long-chain PUFA (safflower-seed oil at the same percentage) or chow for 8 weeks(Reference Okere, Chandler and McElfresh61). Although total body fat was similar across dietary groups, SFA-fed rats had less abdominal and epididymal fat, and more intrathoracic fat compared with the other groups. Another study found that adult male Sprague–Dawley rats fed a beef tallow-based diet for 10 weeks had lower leptin levels than animals fed safflower-seed or fish oil, while fish oil-fed animals had the lowest amount of perirenal fat(Reference Cha and Jones62). These studies suggest that the site of fat accumulation depends on the fatty acid profile of the diet, and various adipose tissue depots can differently contribute to circulating leptin. However, no differences were found between moderate-SFA and -MUFA beef tallow, high-PUFA safflower-seed oil and high-n-3 PUFA fish oil in the increased fasting leptin levels in adult male Sprague–Dawley rats fed these diets for 10 weeks(Reference Hynes, Heshka and Chadee187). Greater leptin levels were found in weanling C57BL/J6 male mice fed high-fat diets (at 58 % of energy) based on beef tallow for 7 weeks than mice fed high-fat diets based on fish oil, safflower-seed oil or animals fed low-fat diets (at 10 % of energy); leptin levels were correlated with body fat as well(Reference Wang, Storlien and Huang60). Similar results were found in other studies(Reference Jen, Buison and Pellizzon102, Reference Jang, Hwang and Chae188).
Ghrelin
Ghrelin is a peptide released by cells in the fundus of the stomach that stimulates the release of growth hormone from the pituitary and was identified by Kojima et al. in 1999(Reference Kojima, Hosoda and Date189). Ghrelin rises before and falls after each ad libitum meal and increases food intake(Reference Cummings, Purnell and Frayo190, Reference Tschop, Smiley and Heiman191). In humans ghrelin levels peak in the morning (08.00 hours), at noon (12.00 to 13.00 hours) and in the evening (17.00 to 19.00 hours) and fall after each peak(Reference Natalucci, Riedl and Gleiss192). Obese individuals have lower fasting ghrelin levels than lean individuals and reduced suppression of ghrelin secretion after a meal(Reference English, Ghatei and Malik193–Reference le Roux, Patterson and Vincent196). A fat-rich meal has a smaller suppressive effect on plasma ghrelin concentration than a carbohydrate-rich meal regardless of obesity status(Reference Tentolouris, Kokkinos and Tsigos197). So far, no effect of dietary fatty acid profile on total ghrelin levels has been reported(Reference Poppitt, Leahy and Keogh177, Reference Lithander, Keogh and Wang198).
In rats there is a peak of plasma levels of ghrelin 5 h after light onset (resting phase) which remains relatively high for 9 h(Reference Bodosi, Gardi and Hajdu178). There is also a second rise just before the beginning of the dark phase, followed by a sharp drop and then a gradual rise during the remainder of the dark phase(Reference Sanchez, Oliver and Pico199). Ghrelin gene expression and plasma ghrelin concentrations have been found to be lower in mice with dietary obesity than in their lean counterparts, coupled with a decrease in sensitivity to the orexigenic effects of ghrelin as well as impairment in suppression of ghrelin in response to a meal(Reference Moesgaard, Ahren and Carr200, Reference Perreault, Istrate and Wang201). A study was conducted by Liu et al. (Reference Liu, York and Bray202) in two strains of rats with different susceptibilities to develop obesity (Osborne–Mendel prone and S5B/P1 resistant) fed a diet high in fat (56 % of energy) for 2 weeks. Ghrelin gene expression was increased in the stomach of fasted susceptible rats but plasma ghrelin concentrations remained unchanged, while in resistant rats both expression and plasma levels of ghrelin remained unchanged. This indicated that ghrelin may play a role in susceptibility to dietary obesity. In adult Long–Evans rats, 2 weeks of high-fat feeding (70 % of energy) was associated with lower levels of ghrelin than was feeding on a high-carbohydrate diet(Reference Beck, Musse and Stricker-Krongrad203). In an attempt to distinguish between the effects of a high-fat diet and of dietary obesity on ghrelin concentrations, Greeley et al. (Reference Greeley, Reed and Qi204) fed adult male Sprague–Dawley rats high- (45 % of energy) or low (12 % of energy)-fat diets for 3 weeks. Both groups were tested with triiodothyronine (T3) to prevent accumulation of fat. Decreased ghrelin levels in high-fat-fed animals were not restored by T3 treatment, despite the fact that the groups had comparable weights. Moreover, duodenal and jejunal infusion of fat suppressed plasma ghrelin less than glucose and amino acids in adult male Sprague–Dawley rats(Reference Overduin, Frayo and Grill205).
The mechanisms suggested for ghrelin's actions are twofold. It stimulates hypothalamic secretion of neuropeptide Y that increases food intake, decreases fat oxidation and utilisation of fat and plays a role in meal initiation(Reference Cummings, Purnell and Frayo190, Reference Beck, Musse and Stricker-Krongrad203). Ghrelin also decreases the utilisation of fat(Reference Tschop, Smiley and Heiman191). High-fat diets are known to down-regulate ghrelin secretion(Reference Moesgaard, Ahren and Carr200, Reference Beck, Musse and Stricker-Krongrad203) and an inverse relationship between leptin and ghrelin has been reported(Reference Beck, Musse and Stricker-Krongrad203). On the other hand, hypothalamic expression of ghrelin receptors was enhanced and ghrelin levels were greater in adult male Wistar rats fed a fat-rich meal(Reference Sánchez, Cladera and Llopis206). Thus, regulation of ghrelin concentration through fat intake remains inconclusive.
Since suppression of ghrelin levels after a meal is associated with postprandial satiety, the lower suppression of ghrelin secretion following high-fat diets might be an explanation for hyperphagia on high-fat diets. Thus, in an environment with abundant high-fat foods, impairment of ghrelin suppression after a meal leads to overconsumption of energy and induces obesity. Furthermore, the obesity itself impairs the suppression of ghrelin secretion after a meal which further exacerbates the development of obesity.
Insulin
Obesity is associated with elevated basal plasma insulin levels and resistance to the metabolic effects of insulin(Reference de Ferranti and Mozaffarian77, Reference Lichtenstein and Schwab207). Independent of obesity, high-fat feeding itself contributes to impaired glucose tolerance and insensitivity to the blood glucose-lowering effect of insulin(Reference Lichtenstein and Schwab207, Reference Riccardi, Giacco and Rivellese208). The fatty acid profile of the diet plays a crucial role in insulin resistance dependent on a high-fat diet(Reference Lichtenstein and Schwab207–Reference Bray, Lovejoy and Smith209). In a human study, intake of SFA and MUFA was positively correlated with plasma levels of glucose and insulin(Reference Lovejoy, Champagne and Smith210). Replacing SFA with MUFA had no beneficial effect on blood glucose and insulin levels during 4 weeks of high-fat feeding in adult overweight and obese men(Reference Piers, Walker and Stoney119). On the other hand, some studies have shown beneficial effects of MUFA intake on glucose homeostasis and insulin sensitivity(Reference Vessby, Uusitupa and Hermansen211).
Animal studies have also shown that hyperinsulinaemia and insulin resistance are induced by high-fat feeding(Reference Woods, Seeley and Rushing42, Reference Woods, D'Alessio and Tso166, Reference Barnard, Roberts and Varon212). In female C57BL/6J mice fed high-fat diets (at 10, 20, 30, 40, 50 and 60 % of total energy) for 15 weeks, a linear relationship between the percentage of dietary fat and glucose intolerance was found(Reference Takahashi, Ikemoto and Ezaki26). This dose-dependent effect was also seen in weanling male Sprague–Dawley rats fed diets with different percentages of energy as fat (10, 32, 45 %)(Reference Ghibaudi, Cook and Farley24).
Mechanisms of the hyperinsulinaemia and insulin resistance with high-fat diets and obesity are discussed in reviews by Lichtenstein & Schwab(Reference Lichtenstein and Schwab207), and Riccardi et al. (Reference Riccardi, Giacco and Rivellese208). These authors suggest that decreases in insulin receptors, glucose transport and metabolism are involved, plus reduction in liver and muscle glycogen synthase activity and storage of glucose as glycogen(Reference Lichtenstein and Schwab207, Reference Riccardi, Giacco and Rivellese208). These abnormalities thus develop when the intake of fat is more that 40 % of total energy. Excessive amounts of adipose tissue (hypertrophy and hyperplasia) stress the endoplasmic reticulum, resulting in secretion of cytokines and decrease in the responsiveness of the cells to insulin(Reference de Ferranti and Mozaffarian77).
Differences among dietary fatty acids affect the composition of the cell membranes and this in turn influences the affinity of receptors for insulin and so its action on the cell(Reference Lichtenstein and Schwab207, Reference Riccardi, Giacco and Rivellese208, Reference Storlien, Higgins and Thomas213). Some studies have found that insulin secretion and sensitivity are enhanced as the degree of unsaturation of fatty acids increases, especially with n-3 feeding, and thus feeding diets rich in SFA results in more insulin resistance than MUFA and PUFA(Reference Clarke116, Reference Lichtenstein and Schwab207, Reference Riccardi, Giacco and Rivellese208, Reference Storlien, Higgins and Thomas213). In a study in 7-week-old female C57BL/6J mice fed high-fat diets (60 % of energy) composed of palm oil, lard, fish oil, perilla oil or rapeseed oil for 18 weeks, blood glucose levels were higher in all the high-fat-fed animals 30, 60 and 120 min after an oral glucose challenge than in the group fed a high-carbohydrate–low-fat diet (fat: 11 % of energy), but the increase in fasting blood insulin levels was only reliable in the group fed palm oil(Reference Ikemoto, Takahashi and Tsunoda52). In weanling female Wistar rats, no difference in insulin levels was found between soyabean oil and palm oil groups(Reference Jen, Buison and Pellizzon102), whereas lower plasma insulin levels were found in adult male Wistar rats fed a high-fat diet (60 % of energy) rich in SFA (cocoa butter) than in the control animals (10 % of energy)(Reference Okere, Chandler and McElfresh61). These disparities might be related to different fats used in these studies: palm oil and cocoa butter differ in SFA content and so diets will vary in SFA at the different percentages of total energy used in the studies. The same can be said for lard, soyabean oil and safflower-seed oil. Beneficial effects of n-3 PUFA on action of insulin are reported in many studies(Reference Ikemoto, Takahashi and Tsunoda52, Reference Cha and Jones62, Reference Tsitouras, Gucciardo and Salbe214, Reference Lombardo, Hein and Chicco215).
Since human and animal studies have shown comparable relationships of hormones to obesity, these models can be used to clarify the uncertain areas such as effects of fatty acid profile of the diet on these hormones. However, relating hormone action to obesity itself requires demonstration of its effect on energy intake and/or expenditure.
Behavioural mechanisms of high-fat diet-induced obesity
As discussed in the previous sections, one explanation why high-fat diets induce obesity is hyperphagia(Reference Ghibaudi, Cook and Farley24, Reference Oscai38, Reference Ainslie, Proietto and Fam39, Reference Harrold, Williams and Widdowson41, Reference Woods, Seeley and Rushing42), i.e. increased weight or volume of daily dietary intake. Effects of energy density were reviewed earlier. A possible lack of inhibitory effects of fats on intake (‘satiety’) was discussed above. Here the intake-facilitatory effects of sensory characteristics (or palatability) of high-fat diets will be considered(Reference French and Robinson13, Reference Warwick and Schiffman22, Reference Blundell and Macdiarmid69, Reference Golay and Bobbioni70). Feeding rhythmicity(Reference Toschke, Kuchenhoff and Koletzko216, Reference Ma, Bertone and Stanek217), social environment(Reference De Castro5, Reference De Castro218–Reference Sclafani and Springer228) and stress(Reference Dallman, La Fleur and Pecoraro229–Reference Torres and Nowson231) may also promote obesity. Each of these will be reviewed below. Because social environment is not documented in relation to high fat intake, only feeding rhythmicity and stress will be reviewed below.
Sensory facilitation of intake
Facilitation of intake by the sensory characteristics of high-fat foods is an important influence on ingestion. Sensory stimulation from food consumption can influence energy intake directly(Reference Yeomans, Blundell and Leshem232), by promoting selection, consumption, digestion and absorption of a food(Reference Yamaguchi and Ninomiya233). It also increases diet-induced thermogenesis(Reference Swinburn and Ravussin234, Reference LeBlanc and Labrie235). Foods high in fat are usually preferred by rats to those that are low in fat and are consumed in greater amounts as a result(Reference French and Robinson13, Reference Golay and Bobbioni70, Reference Stubbs and Whybrow156, Reference Holt, Delargy and Lawton236). A variety of sensory properties contribute to this high palatability of fat-rich diets, mainly texture and odour(Reference Blundell and Macdiarmid69, Reference Warwick and Weingarten160, Reference Sclafani237, Reference Warwick, Schiffman and Anderson238).
In a study on adult male Long–Evans rats, Warwick & Weingarten(Reference Warwick and Weingarten160) compared the sensory effects of a high-fat (59·9 % of energy) and a high-carbohydrate diet (fat at 16·7 % of energy). In order to minimise the post-ingestive effect of diets on intake, they used a preparation in which most of the ingested liquid food drained out of the stomach via a fistula. When both diets were offered simultaneously, rats consumed more of the high-fat diet than the high-carbohydrate diet, demonstrating a sensory preference. Warwick et al. (Reference Warwick, Schiffman and Anderson238) concluded from a study in weanling female Sprague–Dawley rats that consuming high-fat diets early in life can lead to a sensory preference for this fat product which is relatively stable.
Evidence for sensory preferences for fats in rat and mouse animal models is likely to be based on NEFA released from the TAG in food(Reference Fushiki and Kawai239, Reference Mizushige, Inoue and Fushiki240). Lingual lipase has such activity in rodents; taste receptor cells in the oral cavity of rats can easily detect these NEFA; these gustatory signals are transmitted to the brain where they cause release of neurotransmitters such as dopamine and endorphin(Reference Fushiki and Kawai239–Reference Gilbertson, Fontenot and Liu241). Long-chain PUFA stimulate T-cell receptors more efficiently and thus are more strongly preferred than other types of fatty acid(Reference Gilbertson, Fontenot and Liu241). Preference for fat is also found in humans, with textural, olfactory and gustatory cues being involved(Reference Mattes242).
Rhythmicity of feeding
Rhythmicity in feeding (variation over time in total amount ingested, size and frequency of meals) may play a role in the development of obesity. In human subjects, a lower risk of obesity was reported in both adults and children with a high frequency of eating episodes(Reference Toschke, Kuchenhoff and Koletzko216, Reference Ma, Bertone and Stanek217, Reference Fabry, Hejda and Cerny243). A greater number of meals each day was consumed by obese women than healthy-weight women in Sweden in a cross-sectional survey(Reference Bertéus Forslund, Lindroos and Sjöström244). However, similar meal patterns were found in obese and healthy-weight Swedish men in a dietary survey(Reference Andersson and Rössner245).
Time of eating also may play a role in the development of obesity. In humans, meals eaten late in the evening have been suggested to be one of the risk factors of obesity(Reference Ma, Bertone and Stanek217, Reference Qin, Li and Wang246). In free-living individuals food intake in the morning was more satiating and associated with less overall intake throughout the day than evening food(Reference De Castro5). However, in another study, percentage energy from evening food intake and weight changes were unrelated(Reference Kant, Schatzkin and Ballard-Barbash247). Taylor et al. (Reference Taylor, Missik and Hurley248) and Bellisle(Reference Bellisle249) suggested that the effects of meal patterns on human obesity have yet to be clarified.
Unlike humans, rats are nocturnal animals that ingest 70–80 % of their food during the dark phase(Reference LeMagnen and Devos250). There are two peaks in meal frequency and rate of intakes: at the beginning of the night and towards the end, i.e. dusk and dawn feeding(Reference Clifton251, Reference Prins, Dejongnagelsmit and Keijser252). In adult male Wistar rats fed a stock diet containing 10 % energy as fat, the greater intake during the dark phase resulted in positive energy balance and fat deposition, with negative energy balance along with the oxidation of fat in the light phase over fourteen 24 h cycles(Reference LeMagnen and Devos250, Reference LeMagnen253). Altered circadian rhythmicity of intake characterised by larger meal size and decreased meal frequency has been found in genetically obese animals fed non-purified diets(Reference Prins, Dejongnagelsmit and Keijser252, Reference Becker and Grinker254–Reference Strohmayer and Smith257).
Some animal studies have found a relationship between sizes of meals and susceptibility to obesity. Adult male Sprague–Dawley rats that ingested chow in larger meals had a higher rate of weight gain when fed high-fat diets than rats that were fed on chow in smaller meals(Reference Drewnowski, Cohen and Faust258). When weanling male obesity-prone Sprague–Dawley rats were fed high-fat diets (45 % of energy) for 19 weeks, they ate larger meals than resistant animals(Reference Farley, Cook and Spar259). In adult inbred obesity-prone and -resistant rats fed chow, on the other hand, the obesity-prone rats ingested smaller meals more frequently(Reference Cottone, Sabino and Nagy260). These results suggest that an irregular meal pattern is not a cause of developing obesity in obesity-prone animals. A 6 h meal pattern analysis during the dark phase in adult male Sprague–Dawley rats exposed to isoenergetic high- and low-fat diets (soya oil at 38 and 10 % of total energy) for 2 weeks revealed comparable amounts of food ingested in the first meal, but less food ingested in the second and third meal of high-fat-fed rats, as well as greater meal frequency, shorter inter-meal interval (IMI) and lower rate of weight gain than animals fed the low-fat diet(Reference Paulino, Darcel and Tome261). However, when feeding period was prolonged to 8 weeks, the size of the second meal and IMI increased. Increased meal size and decreased meal frequency have also been found in rats acclimatised to a mixture of high-fat and high-carbohydrate diets (providing 38·5 % energy as fat) for 14 d and then fed a fat-rich diets (at 60 % of total energy) for an additional 8 d(Reference Synowski, Smart and Warwick262).
There is a shift of food intake from the dark phase to the light phase in genetically obese rats and mice(Reference Becker and Grinker254–Reference Ho and Chin256). Mistlberger et al. (Reference Mistlberger, Lukman and Nadeau263) reported higher weight gain in genetically obese Zucker rats when fed ad libitum than in those fed only during the 14 h dark phase, while both groups had similar food intakes. In addition, rats differ in their macronutrient selection during the light–dark cycle. It has been reported that when rats are offered a two- or three-way selection between macronutrients, they eat more carbohydrate at the beginning of the dark phase, and more protein and fat at the end of the dark phase and during the light period(Reference Thibault and Booth264). Thus it is probable that, with high-fat feeding, more food will be ingested in the light period that may further facilitate the development of obesity.
Obesity-prone rats respond more than resistant animals with an increase in meal size. This might account for the hyperphagia with high-fat feeding in dietary obesity. Further research is needed to find out the cause–effect relationship between eating patterns and obesity.
Stress
Many studies have shown that long-term stress increases food intake and promotes weight and fat gain in human subjects(Reference Branth, Ronquist and Stridsberg265, Reference Laitinen, Ek and Sovio266). In addition, obesity was found to be associated with depression(Reference Rivenes, Harvey and Mykletun267). Higher levels of obesity in depressed individuals as well as higher prevalence of depression in overweight and obese women and extremely obese men (BMI ≥ 40 kg/m2) were found(Reference Zhao, Ford and Dhingra268, Reference Allison, Newcomer and Dunn269). Depressed individuals with eating disorders often describe themselves as chronically stressed and usually are obese, suggesting that they eat more when stressed in an attempt to cope with the situation and feel better(Reference Dallman, Pecoraro and Akana230). Energy-dense foods with high fat and sugar are known as ‘comfort food’ and are more often eaten during stress(Reference Dallman, La Fleur and Pecoraro229, Reference Laitinen, Ek and Sovio266, Reference Pecoraro, Reyes and Gomez270). On the other hand, some individuals show loss of appetite during stress(Reference Oliver and Wardle271). It has been suggested that this difference is based on the dieting history of the individual: usually dieters increase and non-dieters decrease their intake while in a stressful situation(Reference Oliver and Wardle271).
A different pattern of responsiveness to stress has been shown in a variety of rodent models(Reference Michel, Levin and Dunn-Meynell99, Reference Levin, Richard and Michel272–Reference Rowland and Antelman274). Rowland & Antelman(Reference Rowland and Antelman274) discovered that in adult female Sprague–Dawley rats mild stress induced by six daily sessions (10–15 min) of pinching of the tail for 5 d at equal intervals while they had free access to sweetened milk and tap water resulted in greater food intake and body-weight gain than in the control animals. However, chronic exposure of adult male Sprague–Dawley rats to an immobilisation stressor led to a decrease in food intake, independent of the duration of the stress, while handling stress did not result in change in food intake(Reference Marti, Marti and Armario273).
Obesity-prone and -resistant animals are also different in their responsiveness to stress. A study was conducted by Levin et al. (Reference Levin, Richard and Michel272) in 2·5-month-old selectively bred male obesity-prone and -resistant Sprague–Dawley rats fed a high-fat diet (31 % of energy) for 1 week. They were then randomly assigned to a stress group or control group while fed the high-fat diet for 3 weeks and then the high-fat diet plus Ensure® (Ross Products Division, Medical Supplies Depot, AL, USA) for another 2 weeks. Rats in the stress group had daily exposure to different stressors for 5 weeks, which were restraint for 15 min, moving the animal to the cage of another, exposure to another male rat for 10 min, 2 min swimming or saline injection. Results showed that stressed obesity-resistant rats gained less weight without any decrease in energy intake with little effect of the stressors on body-weight gain and energy intake of obesity-prone animals. Adding Ensure® to the high-fat diet increased energy intake and rate of weight gain in resistant animals, but cumulative weight gain over 5 weeks was still lower in stressed rats than in control animals. Weight gain and intake of the obesity-prone rats were unaffected by the addition of Ensure®. It was suggested that resistant rats had a lowered sympathetic activity compared with their unstressed controls, which was shown by lower noradrenaline levels in their urine.
The effect of a high-fat diet on weight gain after stress was investigated in a study in 3- to 4-month-old male obesity-prone and -resistant Sprague–Dawley rats that were restrained once for 20 min, and after release were presented either a high-fat diet (at 31 % of energy) or chow for 9 d(Reference Michel, Levin and Dunn-Meynell99). Stressed prone rats fed the high-fat diet gained more weight than unstressed prone rats fed the same diet while having similar food intakes. However, when stressed prone rats were fed chow, they gained less weight than unstressed prone rats fed the same diet. These results showed that prone rats were less responsive to the weight-reducing effect of immobilisation stress when fed a high-fat diet; at the same time they were more responsive to this effect when fed chow. Immobilisation stress had no effect on body-weight gain in resistant rats fed either diet(Reference Michel, Levin and Dunn-Meynell99). In another study, adult male Sprague–Dawley rats fed high-fat (at 40 % of total energy) or low-fat diets (at 12 % of total energy) for 4 d were divided into two groups of stressed (restraint tubes with no food and water access followed by tail blood sampling, 3 h daily for three consecutive days) and mildly stressed rats (moved to new cages, food and water deprived for the same period and blood sampled)(Reference Legendre and Harris275). On the days of restraint, stressed rats lost weight regardless of the diet. High-fat-fed mildly stressed animals stopped gaining weight; however, low-fat-fed mildly stressed rats gained weight throughout the experiment(Reference Legendre and Harris275). Results showed that low- and high-fat diets resulted in similar body-weight changes under a severe stress, whereas with a mild stress high-fat-fed animals were more responsive to the weight-lowering effect of stress. In adult male Long–Evans rats, the weight loss resulting from chronic stress was regained after recovery from stress and body-weight and fat gain were greater in high-fat-fed rats than in chow-fed control animals(Reference Tamashiro, Hegeman and Sakai276). Higher preference for high-fat feeding during chronic stress was reported in mice(Reference Teegarden and Bale277).
Mechanisms that influence food intake during acute and chronic stress are different. Physiologically, the initial response of the body to an acute stress is secretion of corticotrophin-releasing factor from the paraventricular nucleus of the hypothalamus that stimulates the secretion of adrenocorticotropin hormone from the anterior pituitary which in turn leads to the release of cortisol from the adrenal cortex to provide energy for the brain and/or muscles. Then cortisol itself makes a negative feedback for its further secretion. However, with a chronic exposure to stressor, the negative feedback does not work efficiently and thus induces an increase in food intake and body-weight gain through increased secretion of glucocorticoids which elevate appetite, food intake and fat storage especially in the abdomen(Reference Dallman, Pecoraro and Akana230, Reference Torres and Nowson231, Reference Adam and Epel278). In adult male Wistar rats, a chronic stress of keeping rats in cages filled with water to a height of 2 cm for 5 d led to delayed gastric emptying during the first 24 h of exposure, but after that it was accelerated and exceeded that of the control group by day 5. In addition, catecholamines were increased during the first 24 h and then decreased while active ghrelin levels were high on day 3 and remained elevated until day 5(Reference Ochi, Tominaga and Tanaka279). It was suggested that the increased sympathetic activity after 24 h stimulated ghrelin secretion, and therefore the increased food intake found during chronic stress might be a result of enhanced plasma ghrelin. Plasma ghrelin levels were also found to be increased with acute stress(Reference Kristenssson, Sundqvist and Astin280).
Susceptibility to obesity
There is a genetic background for susceptibility to obesity with interacting environmental factors; the environment alone has an impact on the inherent risk of obesity in individuals(Reference De Castro5, Reference Martínez-Hernandez, Enríquez and Moreno-Moreno6, Reference Karnehed, Tynelius and Heitmann281–Reference Speechly and Buffenstein283). This has been shown in many studies in human subjects(Reference Ichihara and Yamada2).
An underlying genetic predisposition to be obesity prone or resistant is also shown in animal models(Reference Levin and Dunn-Meynell46, Reference Levin, Dunn-Meynell and Ricci184). Rats and mice known as the standard models for studying dietary obesity are different in their susceptibility to obesity: outbred Sprague–Dawley rats, Wistar rats and C57BL/6C mice can be easily categorised to prone and resistant phenotypes with ad libitum access to high-fat diets(Reference Reuter7, Reference Speakman, Hambly and Mitchell8, Reference Buettner, Scholmerich and Bollheimer21). There are also strains known as genetically obese, such as Zucker fa/fa rats and ob/ob mice(Reference Speakman, Hambly and Mitchell8, Reference Thibault, Woods and Westerterp-Plantenga35).
When exposed to high-fat diets, some animals are sensitive to high-fat diet-induced obesity and become obese (obesity-prone animals), while others resist to this obesogenic effect and grow normally (resistant animals)(Reference Levin and Dunn-Meynell82, Reference Levin, Dunn-Meynell and McMinn284). Some researchers have attributed this difference to higher energy intakes in obesity-prone animals(Reference Huang, Xin and McLennan51, Reference Wang, Storlien and Huang60, Reference Dourmashkin, Chang and Gayles97, Reference Farley, Cook and Spar259, Reference Commerford, Pagliassotti and Melby285, Reference Commerford, Pagliassotti and Melby286), while others have found similar intakes in prone and resistant animals, and suggested that susceptible animals were capable of storing energy with greater efficiency(Reference Tulipano, Vergoni and Soldi95, Reference Abdoulaye, Wetzler and Goubern287, Reference Levin and Dunn-Meynell288).
Suggested mechanisms for the difference between prone and resistant animals in responding to high-fat diets are that prone animals have lower fat oxidation(Reference Chang, Graham and Yakubu40, Reference Hassanain and Levin98, Reference Levin and Dunn-Meynell288, Reference Jackman, Kramer and MacLean289), increased lipoprotein lipase activity in their adipose tissue and no change in lipoprotein lipase activity of their muscles which favours fat storage in these animals(Reference Dourmashkin, Chang and Gayles97, Reference Jackman, Kramer and MacLean289, Reference Pagliassotti, Knobel and Shahrokhi290). However, Commerford et al. (Reference Commerford, Pagliassotti and Melby286) fed 7-week-old male Wistar rats high-fat diets (45 % of energy) for 1 or 5 weeks and found comparable fat accumulation and lipogenesis in prone and resistant rats after provided with an isoenergetic 14C-labelled high-fat meal, suggesting that the increased energy intake is the main reason for accelerated weight gain in prone animals.
Dietary obese-prone animals also had increased arcuate neuropeptide Y mRNA expression (an orexigenic neuropeptide)(Reference Levin and Dunn-Meynell291), decreased noradrenaline turnover and α2-adrenoceptor binding in some parts of the hypothalamus (ventromedial, dorsomedial and lateral) compared with resistant animals, as well as in the pancreas and heart, which shows a reduced sympathetic activity in these organs(Reference Levin292). The reduction in noradrenaline turnover in the pancreas leads to an increase in insulin release and development of obesity.
Sex differences
In humans, there are differences between the two sexes in energy expenditure and requirements as well as in fat metabolism and fat distribution(Reference Blaak293–Reference Sweeting295). Greater storage of fat in the lower body in females (gynoid) due to lower basal fat oxidation and greater number of α2-adrenoceptors, as well as decreased α2-adrenergic sensitivity in the abdominal region, all lead to more fat storage in the thigh region and less in the abdomen compared with men who have greater storage of fat in the upper body (android)(Reference Blaak293–Reference Sweeting295). Moreover women have more subcutaneous fat than men(Reference Geary296). Despite all these differences, in a recent review of the genetic studies of obesity in different countries it was shown that overall obesity rates of males and females as determined by BMI were small and inconsistently different, with no indication of obesity in either sex being more prevalent(Reference Sweeting295).
These differences can also been found in animal models of obesity(Reference Thibault, Woods and Westerterp-Plantenga35). In laboratory rats, males gain weight steadily throughout their lives while the body weight of female rats becomes stable in early adulthood(297). As a result, female rats are better models for studying obesity during adulthood since they are more like humans in their growth patterns. Besides, more subcutaneous fat is found in females due to higher concentrations of oestrogen and progesterone receptors in these depots while males have more visceral fat related to high concentrations of androgen receptors in this area(Reference Mayes and Watson298).
The sex of the animals may also affect the cellular response of the adipose tissue to high-fat feeding. This was shown in adult rats fed cafeteria diets for 9 weeks which led to a more rapid development of obesity in female rats, and their difference in weight compared with control animals became obvious after 5 d while in males this became significant after 40 d(Reference Sclafani and Gorman44). The same report showed that weight gain of male and female rats fed a supermarket diet is more similar to each other than that of rats fed chow. Therefore, the sex difference in weight gain normally seen in rats is reduced when animals are developing dietary obesity. Female golden hamsters, aged 10 weeks, fed a fat-rich diet (52 % of energy) ate significantly more energy and gained more weight than males(Reference Wade106). Likewise, 10 d old female Wistar rats fed a cafeteria diet for 14 weeks gained more weight than their male counterparts fed the same diet, suggesting less thermogenic capacity in females when fed the cafeteria diet(Reference Rodríguez, Quevedo-Coli and Roca299). A study in 3-month-old Sprague–Dawley rats showed that female rats fed chow had higher food intake and greater increase in ghrelin and decrease in leptin levels than males following a 12 h fast(Reference Gayle, Desai and Casillas300). Moreover, an interaction between sex and site of fat accumulation was found in 6-week-old NMRI mice given different amounts of fat (17, 27, 43·5, 60 % of energy) for 14 weeks, with more fat accumulation in retroperitoneal and parametrial sites in females, and in subcutaneous depot in males(Reference Bourgeois, Alexiu and Lemonnier25).
All together, similar to humans, male and female rats have different body fat distribution which makes them appropriate models for studying adipose tissue. Besides, female animal models are better responders to high-fat feeding, mimicking susceptibility to obesity in humans. However, in a recent review, male mice and rats are introduced as ‘gold standards’ for studying dietary obesity(Reference Reuter7). This might be because of the oestrous cycle of the female animals which is repeated every 4–6 d and can affect the food intake of the animal during this period(Reference Andersen, Clewell and Gearhart301).
Reversibility
Animal studies have shown that low-fat diets can induce weight loss in dietary obese rats. A reduction in energy intake and obesity reversal was found when adult male Wistar rats fed a high-fat diet at 60 % of total energy for 17 weeks were switched to chow for 13 weeks(Reference Hill, Dorton and Sykes302). Decreased energy intake and obesity reversal with significant weight and fat loss were found in weanling male C57BL/6 mice switched from 17 weeks of high-fat feeding (at 58 % of energy) to low-fat feeding (at 11 % of energy) for 17 weeks(Reference Parekh, Petro and Tiller303) or after 13 weeks of high-fat feeding (at 59 % of energy) to low-fat feeding (10 % of energy) for 6 weeks(Reference Huang, Xin and McLennan51). Likewise, a reduction in energy intake and a complete reversal of diet-induced obesity were found when 13-week-old female Wistar rats originally fed high-fat diets (at 30 or 60 % of energy provided by Crisco®; Proctor & Gamble, Cincinnati, OH, USA) for 8 or 14 weeks were switched to chow(Reference Bartness, Polk and McGriff101).
In other studies, however, ad libitum low-fat feeding was not an efficient method to completely reverse dietary obesity. For example, an initial decline in body weight followed by a plateau have been found in adult male obesity-prone and -resistant Sprague–Dawley rats after switching from 10 weeks of a high-fat diet (at 31 % of energy) to chow feeding for 2 weeks(Reference Levin and Dunn-Meynell288); however, when animals were restricted on chow to 50 % of their energy intake for 3 weeks, their body weights reached the level of chow-fed control animals. Decline in body weight and a plateau were also found in adult male obesity-prone Sprague–Dawley rats switched from 10 weeks of being fed a high-fat diet (at 31 % of energy) to 7 weeks of chow feeding, although their energy intake was decreased while on chow(Reference Levin and Dunn-Meynell82). Wade(Reference Wade304) reported that young male and female golden hamsters fed a high-fat diet (at 52 % of energy) for 4 weeks and then switched to chow for 4 weeks (fat at 4·5 % energy) decreased their energy intake and lost 80 % of their weight gain from feeding on the fat-rich diet. Adult male obesity-prone Sprague–Dawley rats fed a high-fat diet (at 31 % of energy) for 12 weeks and then switched to chow for 1 week decreased their energy intake (by 10–20 %) but failed to lose weight(Reference Levin and Keesey305).
Both hyperplasia and hypertrophy of fat cells are involved in developing obesity(Reference de Ferranti and Mozaffarian77, Reference Avram, Avram and James78, Reference Prins and Orahilly81). A total of 8 weeks of energy restriction in normal-weight adult male Sprague–Dawley rats did not result in hypoplasia despite the significant decrease in body weight and body fat(Reference Miller, Faust and Goldberger306). When weanling male Sprague–Dawley rats made obese by feeding a diet containing chow, Crisco®, sweetened condensed milk and sucrose solution for 19 weeks were then food deprived for 8, 15 or 25 d, body weight and fat cell size decreased, but fat cell number was comparable with that of the chow-fed control animals(Reference Yang, Presta and Bjorntorp307). Another study in adult male C57BL/6J mice fed a high-fat diet (45 % of energy, Research Diets, New Brunswick, NJ, USA) for 10 weeks and then switched to chow for 2 weeks had similar results(Reference Shi, Akunuru and Bierman308). Centrally administered leptin (10 μg, ICV) for 4 d in normal-weight adult male Sprague–Dawley rats caused a decrease in the number of inguinal fat cells(Reference Gullicksen, Hausman and Dean309). Leptin injections (5 μg; ICV) over 5 d decreased the number of fat cells in young (aged 3 months) male Sprague–Dawley rats, but not in mature (aged 8 months) animals(Reference Qian, Azain and Hartzell310). This was also found in a study in mice(Reference Shi, Akunuru and Bierman308). However, a decrease in fat cell number was not found following dietary reversal of obesity in rats and mice(Reference Miller, Faust and Goldberger306–Reference Shi, Akunuru and Bierman308). These findings contrast with human studies that showed hypoplasia of adipocytes following reversal of obesity(Reference Avram, Avram and James78, Reference Prins and Orahilly81).
Conclusions
The physiological mechanisms involved in high-fat diet-induced obesity are overconsumption of high-fat diets due to their low satiating effects, the high efficiency of dietary fat in being stored in the body as well as the alterations in the hormones involved in energy balance, such as high-fat diet-induced hyperleptinaemia and hyperinsulinaemia accompanied by leptin and insulin resistance, and lowered suppression of ghrelin secretion following high-fat diets. Among the behavioural mechanisms, the sensory facilitation of intake with high-fat diets is well understood. Meal pattern analysis of high-fat-fed animals in a pre-obese v. obese state could be useful to understand the development of obesity. An area for future research is to investigate whether different patterns of eating in animal models before obesity development can be a predictor of prone and resistant phenotypes, and to assess their feeding circadian rhythms. There has been extensive research on the obesogenic effects of fatty acids with different degrees of saturation but no constant pattern of outcome under different conditions has been found. More work is needed to prove that body weight can be regulated by the fatty acid profile in high-fat diets. An important key point in designing animal studies is that high-fat diets meet animals' minimal nutrient requirements, especially for protein, vitamins and minerals, to eliminate the possibility of overconsumption of the diet to fulfill these nutrient needs. The ineffectiveness of low-fat diets fed ad libitum to reverse dietary obesity induced by long-term high-fat feeding stresses the use of restricted regimens. This could help to investigate whether a significant and sustainable weight loss accompanied by decrease in fat cell number can be achieved.
Acknowledgements
The present review was supported by a grant to Professor Louise Thibault by the Natural Sciences and Engineering Research Council of Canada (NSERC RGPIN 39636-01). The authors thank Professor David A. Booth for his insightful comments and suggestions.
Both authors contributed equally to the preparation of this paper.
The authors had no conflicts of interest.



