Introduction
Slow release is a fundamental feature of long-acting injectable (LAI) antipsychotics that allows continuous drug exposure between injections.Reference Ceskova and Silhan1 Drugs formulated for a slow rate of dissolution are released into plasma over an extended period and produce sustained plasma drug levels permitting dosing intervals from weeks to months.2 However, the slow dissolution can also result in a significant delay in achieving plasma drug levels associated with symptom improvement when initiating some LAI antipsychotics. This delay has been addressed using one of two strategies: (1) “loading dose” approaches using initial injections delivered more frequently (e.g., weekly) than the dosing interval used for the maintenance dose or (2) prescribing an oral antipsychotic for bridging during the LAI initiation period.Reference Brissos, Veguilla and Taylor3–Reference Gilday and Nasrallah5
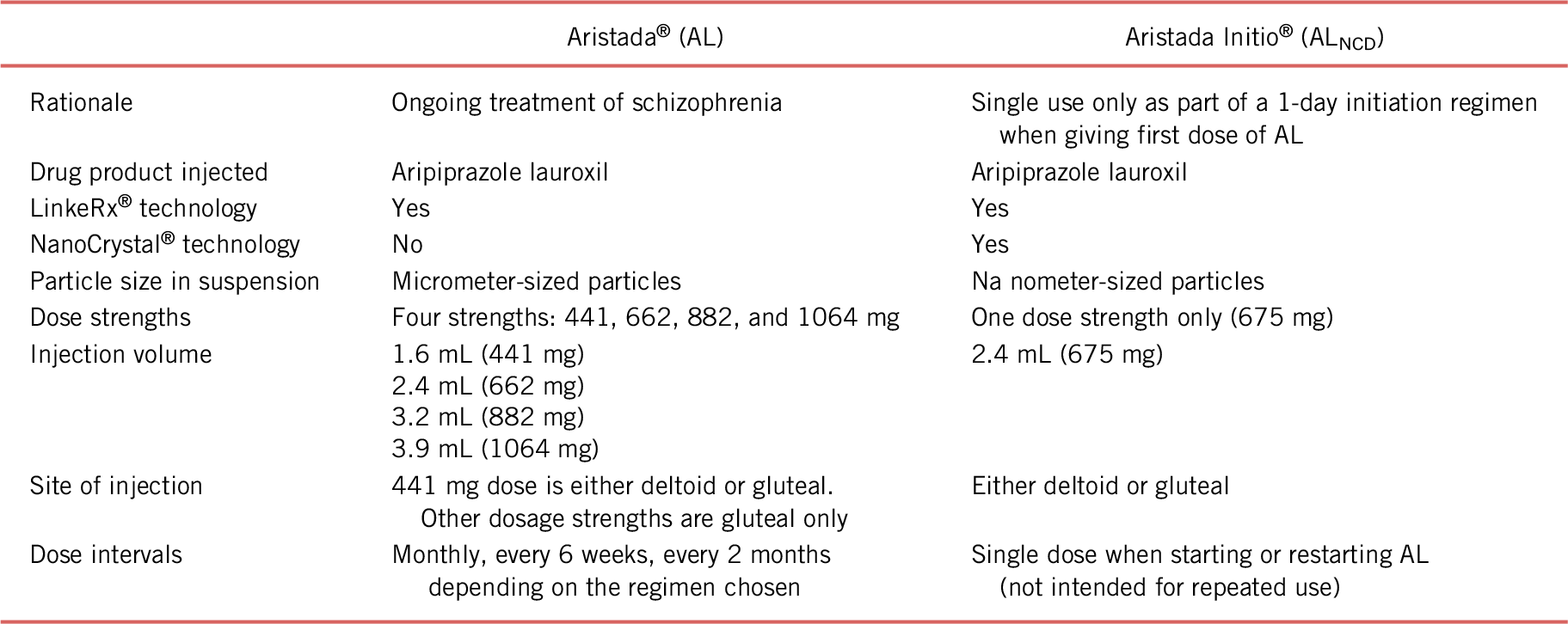
Aripiprazole lauroxil (AL; Aristada®), a prodrug of aripiprazole, is an LAI antipsychotic approved for the treatment of schizophrenia in adults.6 The AL formulation is an injectable suspension of micrometer-sized particles designed for slow dissolution (Figure 1).Reference Ehret, Davis and Luttrell7 The rate of dissolution, or release of the drug from the particles, allows for a dosing interval of up to 2 months.Reference Hard, Mills and Sadler8 But the same slow-release attribute of AL also means that it takes between 6 and 7 weeks for aripiprazole to reach peak plasma levels.Reference Turncliff, Hard and Du9 For other LAIs, this lag time has traditionally been addressed in one of two ways. A loading dose approach has been used for other LAI antipsychotic formulations (e.g., haloperidol decanoate, fluphenazine decanoate, and paliperidone palmitate); however, this approach would not work for AL because this lag time is independent of initial dosing strength and, therefore, would not appreciably change the time delay that occurs before the achievement of relevant aripiprazole levels.Reference Meyer10 Instead, a second option is used to address the lag time between the first AL injection and achievement of relevant plasma drug levels, which involves oral aripiprazole supplementation for the first 21 days with AL initiation.
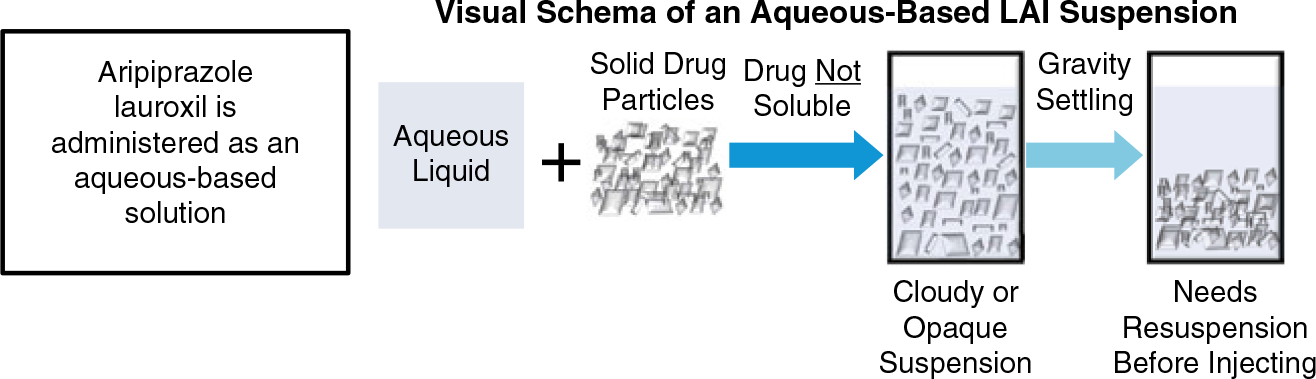
FIGURE 1. Because the aqueous suspension contains drug particles, the size and shape of those particles differ among long-acting injectable antipsychotics and is an important aspect of the specific formulation. Figure not drawn to scale.
Adherence to a 21-day oral-dose regimen may be difficult for some patients starting an LAI medication. Nonadherence rates are notably high for oral antipsychotics,Reference Garcia, Martinez-Cengotitabengoa and Lopez-Zurbano11 with reported rates of partial or complete lack of adherence to antipsychotic medications as high as 25% within the first 10–14 days after hospital discharge.Reference Velligan, Lam and Ereshefsky12 Indeed, it is often the patients who fail to adhere to oral antipsychotic medication who are candidates for LAI treatment.Reference Kane13 Obviously, these individuals may continue to struggle with adherence to even a short, 21-day oral aripiprazole supplementation regimen after their first AL injection. Consequently, these patients may benefit from an AL initiation regimen that provides relevant plasma aripiprazole levels early in the course of therapy, without the need for extended oral supplementation.
This paper describes a new strategy for starting AL therapy as an alternative to the 21-day oral aripiprazole supplementation that was the only option available when AL was initially approved for use by the US Food and Drug Administration. The clinical goal for embarking on this strategy was to reduce the time needed for oral supplementation to a single-day initiation of AL while providing plasma levels of aripiprazole that were comparable to those observed with the 21-day oral aripiprazole initiation regimen.Reference Ehret, Davis and Luttrell7 To that end, a more rapidly dissolving formulation of AL, known as Aripiprazole Lauroxil NanoCrystal® Dispersion (ALNCD), was developed that requires only a 1-day oral aripiprazole initiation regimen.
Particle size and drug kinetics
A key to the development of an AL formulation that has the specific characteristics needed to bridge the plasma aripiprazole level gap at AL initiation is the relationship between particle size and rate of dissolution. In general, larger particle size is associated with a slower rate of dissolution, as the rate of dissolution is related to the surface area-to-volume ratio for a given particle size.Reference Leng, Chen and Li14 A nanometer-sized particle is 1000 times smaller than a micron-sized particle. Because of this, the surface area-to-volume ratio is up to 1000 times larger for a nanometer-sized particle than for its micrometer counterpart.
This net result is that a drug formulated with a smaller particle size has a different plasma concentration profile over time, or kinetics, than one with larger particle size.Reference Leng, Chen and Li14, Reference Hard, Wehr and Sadler15 The observed kinetic profile that results with ALNCD is characterized by a faster rise in plasma drug levels after administration, earlier peak plasma levels, and a shorter half-life than a formulation with a larger particle size (Figure 2). Manipulation of particle size to achieve specific kinetic profiles is a well-known strategy and was employed to develop the 3-month formulation of paliperidone palmitate (PP3M) (Invega Trinza®, Janssen Pharmaceuticals, Inc., Titusville, NJ, USA). In that example, a larger particle size was created from the 1-month formulation (PP1M) (Invega Sustenna®, Janssen Pharmaceuticals, Inc., Titusville, NJ, USA) to facilitate slower release with PP3M.Reference Daghistani and Rey16 Peak plasma drug levels are observed at 30–33 days post-injection for PP3M compared with approximately 7–8 days post-injection for the smaller particle PP1M formulation.Reference Daghistani and Rey16, Reference Coppola, Liu and Gopal17 The aim in developing a new AL initiation regimen, therefore, was to design an AL formulation with a smaller particle size that provided faster kinetics suitable for bridging the AL plasma aripiprazole gap with only a single 30 mg dose of oral aripiprazole, while replicating the kinetics of the original 21-day oral initiation regimen.

FIGURE 2. Kinetics of smaller vs. larger particle size formulations. T last, time last measurable plasma drug concentration; T max, time of peak plasma concentration.
Development of a 1-day initiation regimen
The newly developed formulation is a NanoCrystal Dispersion of AL (Aristada Initio®), which contains the same prodrug that is in AL, but with nanometer-sized particles instead of micrometer-sized particles.Reference Ehret, Davis and Luttrell7 A comparison of the original AL formulation with the new ALNCD formulation is presented in Table 1. The smaller particle size of ALNCD results in faster dissolution of the drug than with AL, and administration of ALNCD was therefore expected to be associated with an earlier rise in plasma aripiprazole levels than with AL. The kinetics of ALNCD were investigated to determine whether this new formulation could provide an earlier rise in plasma aripiprazole levels (than AL) with adequate levels sustained over time such that it could be used in place of the 21-day oral-dose bridging regimen otherwise required for initiating AL therapy.
Plasma levels may vary from patient to patient administered the same dose of a single drug formulation, but population model-based simulations can be used to simulate average plasma levels over time for a population by aggregating numerous plasma concentrations collected after drug administration in multiple studies of drug kinetics. To characterize the kinetics of aripiprazole in plasma after ALNCD administration and to determine whether the new AL formulation could offer an alternative to the oral bridging regimen, observed data and population model-based simulations were used to predict plasma drug levels after administration of ALNCD, AL, or oral aripiprazole (Alkermes, Inc., data on file. Waltham, MA, USA; 2017).Reference Hard, Wehr and Sadler15
Model-based simulations of plasma levels over time are compared for ALNCD and AL in Figure 3(a). As expected based on particle size, plasma aripiprazole levels rise more rapidly after a single injection of ALNCD vs. AL. After initial AL injection, predicted plasma aripiprazole levels rise slowly to peak at approximately 7–8 weeks, which is consistent with observed kinetics study results.Reference Turncliff, Hard and Du9 In contrast, plasma aripiprazole levels are predicted to rise rapidly during the first week after an ALNCD injection and peak at approximately 3–4 weeks (approximately 27 days after the ALNCD injection based on observed follow-up aripiprazole levelsReference Ehret, Davis and Luttrell7). However, although aripiprazole appears more rapidly in plasma after administration of ALNCD than AL, the rise is still slower with ALNCD than with oral aripiprazole.18 A comparison of plasma aripiprazole levels associated with ALNCD vs. a single 30 mg dose of oral aripiprazole shows that, after administration of ALNCD, a small but clinically significant gap in coverage remains (Figure 3(b)). These results indicate that a single ALNCD injection thus could cover most, but not all, of the gap in plasma aripiprazole levels at AL initiation than the 21-day oral aripiprazole supplementation regimen bridges.
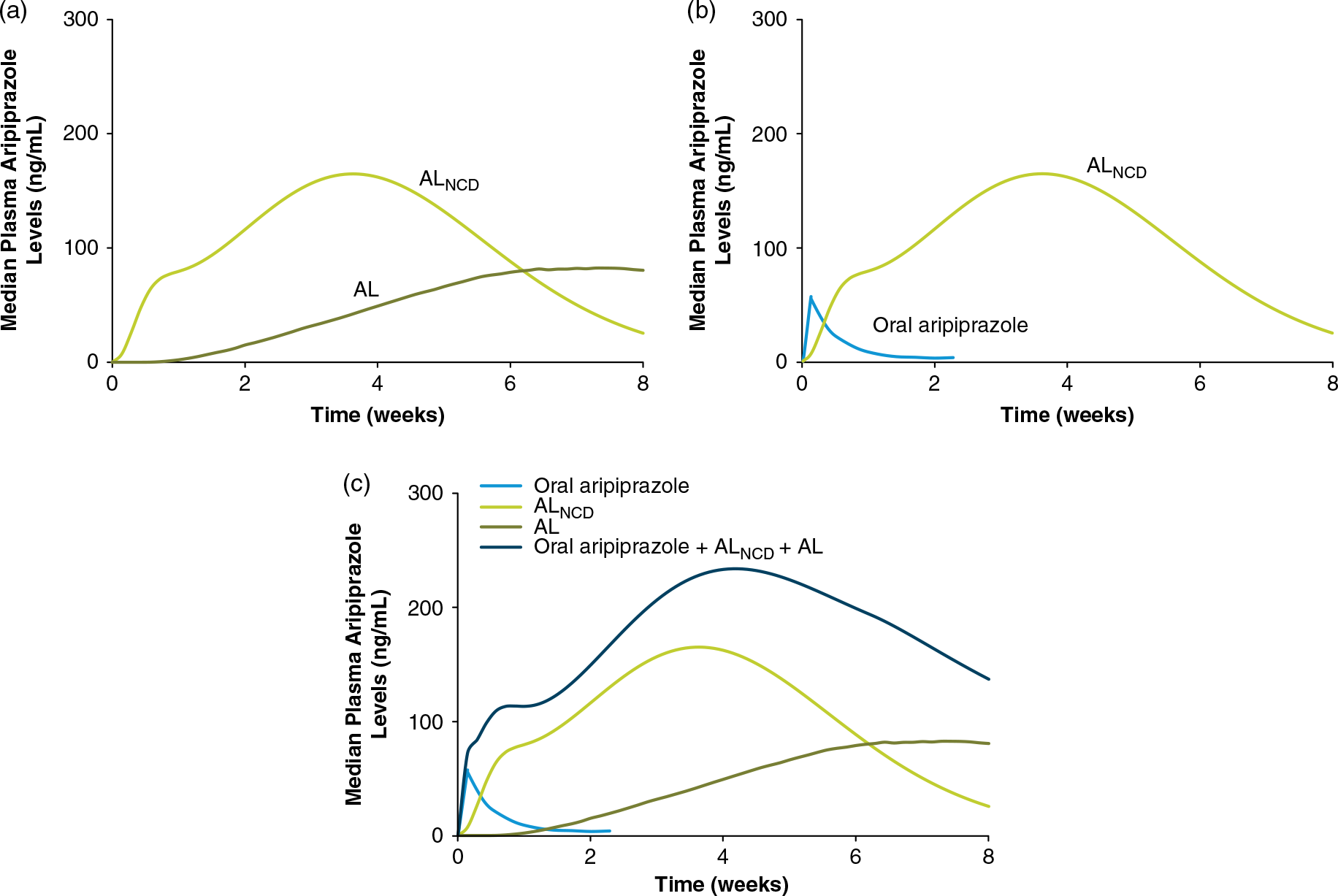
FIGURE 3. (a) ALNCD is associated with a faster rise in plasma aripiprazole levels (plasma levels based on model simulations of aripiprazole levels after administration of ALNCD or AL 1064 mg) at Time 0 than AL and provides consistent levels from week 1 to 4 as levels from AL slowly rise. (b) The rise in plasma aripiprazole levels (ALNCD plasma levels based on model simulations; levels for oral aripiprazole based on observed data) is slower for ALNCD than oral aripiprazole, leaving a gap the first few days after injection. (c) The combination of one injection of ALNCD plus a single 30 mg dose of oral aripiprazole administered at initiation of AL therapy provides consistent target plasma aripiprazole levels (plasma levels based on model simulations of aripiprazole levels after administration of ALNCD, AL 1064 mg, or ALNCD + AL 1064 mg + a single 30 mg dose of oral aripiprazole. Levels for oral aripiprazole based on observed data) in the first few days after administration. AL, aripiprazole lauroxil; NCD, nanocrystalline dispersion.
The solution to this problem of the first week “shortfall” of aripiprazole levels after ALNCD injection came from pharmacokinetic modeling. It turned out that adding a single 30 mg dose of oral aripiprazole on day 1 was what was needed. Thus, giving a single injection of ALNCD along with a single 30 mg dose of oral aripiprazole on the same day would provide an equivalent duration of relevant aripiprazole levels as giving 21 days of oral aripiprazole at 15 mg/day. This concept, illustrated in Figure 3(b), suggests that adding a single 30 mg oral dose of aripiprazole at the time of the ALNCD injection will fill the aripiprazole plasma concentration gap associated with the initiation of AL treatment. Population model-based simulations showed that, when one 30 mg dose of oral aripiprazole was given at the same time as the ALNCD dose, plasma levels rose earlier and relevant levels were attained approximately a week earlier than in simulations without the oral aripiprazole dose (Alkermes, Inc., data on file. Waltham, MA, USA; 2017). These results demonstrate that the ALNCD injection and a single 30 mg dose of oral aripiprazole together provide the profile of plasma aripiprazole levels over time needed for an effective 1-day initiation of AL treatment.
Observed and simulated plasma aripiprazole levels were used to examine how the individual components (i.e., the single injection of ALNCD + a single 30 mg oral dose of aripiprazole, together with the starting dose of AL) contribute to plasma aripiprazole levels through the first 8 weeks of AL therapy (Alkermes, Inc., data on file. Waltham, MA, USA; 2017). As shown in Figure 3(c), the kinetic profile of the combined administration of the two-component initiation regimen (i.e., ALNCD + a single 30 mg dose of oral aripiprazole) with the first dose of AL reflects the additive, time-dependent result of all underlying components. The single dose of oral aripiprazole primarily contributes to plasma aripiprazole levels at the earliest time points, beginning on the day of injection. This is followed by the contribution from the ALNCD injection, which peaks at approximately 27 days. Aripiprazole exposure from the AL starting dose then predominates after the initiation period. The timing of aripiprazole release from the three sources results in sustained plasma exposure, rising rapidly in the first few days and lasting until the next scheduled AL injection. Continuous coverage is provided with no need for further oral antipsychotic supplementation and without producing excessive plasma aripiprazole levels.
The direct test of the 1-day AL initiation regimen vs. 21 days of oral supplementation: exposure, safety, and tolerability
To determine whether the 1-day initiation regimen is comparable to the 21-day regimen, a phase 1, double-blind, placebo-controlled study in adult patients with schizophrenia initiating AL therapy was carried out to assess the kinetics, safety, and tolerability of the two regimens.Reference Hard, Wehr and Du19
The four-arm study compared the 1- and 21-day AL initiation regimens for two individual AL doses (441 or 882 mg administered monthly) as illustrated in Supplemental Figure 1. Patients assigned to the 1-day initiation regimen received a single ALNCD injection given intramuscularly and a single 30 mg dose of oral aripiprazole administered together on day 1 and then received an oral placebo on days 2–21. Patients assigned to the 21-day initiation regimen were administered oral aripiprazole (15 mg/day) on days 1–21, with a placebo injection on day 1. Both 1- and 21-day initiation regimen groups were administered a single injection of AL (441 or 882 mg) on day 1.
Plasma aripiprazole levels were measured through the 21-day AL initiation period and then for 4 months afterward, and safety was monitored throughout the study with collection of adverse events (AEs). A total of 161 patients participated in the study. Their baseline demographic and clinical characteristics are shown in Supplemental Table 1. Reference Hard, Wehr and Du19
Figure 4 shows study patients’ mean plasma aripiprazole levels for 28 days after administration of the 1-day vs. 21-day AL initiation regimens and first AL dose (Figure 4(a), AL 441 mg groups; Figure 4(b), AL 882 mg groups). The comparison between initiation regimens demonstrates that the 1-day initiation regimen of ALNCD + a single 30 mg dose of oral aripiprazole results in rapid achievement of plasma aripiprazole levels comparable to the 21-day 15 mg/day oral aripiprazole initiation regimen and that aripiprazole levels persist through the first month after treatment initiation. Total aripiprazole exposure (the area under the curve) was also similar for the 1- and 21-day initiation regimen groupsReference Hard, Wehr and Du19 (Supplemental Figure 2 and Table 2). Moreover, the two initiation regimens were noted to have comparable peak aripiprazole exposures (Supplemental Figure 3 and Table 2). Importantly, plasma aripiprazole levels observed for the 1-day regimen in this study fell within the range of levels associated with significant improvement in schizophrenia symptoms in the pivotal AL 12-week efficacy study.Reference Meltzer, Risinger and Nasrallah4, Reference Hard, Wehr and Du19
FIGURE 4. Mean (SD) aripiprazole levels over 28 days.Reference Hard, Wehr and Du19 (a) AL 441 mg dose groups. (b) AL 882 mg dose groups. On day 1 (sampling time 0), the pre-dose value is reported. Adapted with permission from Hard et al. Reference Hard, Wehr and Du19 Available at https://journals.lww.com/psychopharmacology/Pages/default.aspx.
The safety profile of the 1-day initiation regimen was comparable to that of the 21-day initiation regimen and consistent with the known safety profile of aripiprazole and AL.Reference Hard, Wehr and Du19 AE rates were similar between patients in the 1- and 21-day initiation regimens (Supplemental Table 3). The incidence of akathisia, an AE associated with oral aripiprazole treatment,Reference Thomas, Caballero and Harrington20 was relatively low (5% for the 1-day initiation regimen and 2.5% for the 21-day initiation regimen). There was no difference in severity of akathisia between the 1- and 21-day regimens. Injection site reactions – most commonly injection site pain – were reported by 17.5% of patients who received ALNCD and 6.2% of patients who received placebo injections.
Using the 1-day initiation regimen in clinical practice
A new 1-day initiation regimen for AL has been developed using ALNCD and a single oral 30 mg aripiprazole tablet. Each component of the 1-day regimen is needed, and these can both be given on the same day as the first dose of AL. There now are two ways to start AL therapy: either with 21 days of oral aripiprazole supplementation (15 mg/day), or, using the 1-day initiation regimen of a single injection of ALNCD and a single 30 mg dose of oral aripiprazole instead. Data demonstrate that the 1-day initiation regimen may be used with any of the approved AL dosing regimens (441, 662, 882 mg q4 weeks; 882 mg q6 weeks; and 1064 mg q8 weeks) and provide relevant aripiprazole levels rapidly that are sustained for up to 2 months.Reference Hard, Wehr and Sadler15 The components of the 1-day regimen have complimentary kinetic profiles that together produce plasma aripiprazole levels that rise rapidly and remain sufficiently elevated until plasma aripiprazole levels from the second AL dose are sufficient (Alkermes, Inc., data on file. Waltham, MA, USA; 2017). The oral aripiprazole supplementation within the 1-day regimen is associated with the early rise in plasma aripiprazole that allows rapid achievement of relevant levels. The relatively rapid delivery of aripiprazole from the nanometer-sized ALNCD particles (vs. micrometer-sized AL particles) allows appropriate aripiprazole levels to be maintained until aripiprazole contributed by AL itself reaches target plasma levels. The double-blind, placebo-controlled, phase 1 study demonstrated that the kinetics of the two components of the new regimen replicate those of the 21-day oral regimen.
Based on these data, ALNCD, in combination with oral aripiprazole, has been approved by the US Food and Drug Administration and is available as a single-dose strength of 675 mg.21 The approved regimen, an ALNCD intramuscular injection administered together with a single 30 mg oral dose of aripiprazole, offers a 1-day AL initiation alternative to the original initiation strategy that required oral aripiprazole supplementation lasting 21 days.21 Although the 1-day initiation regimen was designed to be administered together with the first AL dose, ALNCD maintains target plasma aripiprazole levels long enough to allow the first AL dose to be administered up to 10 days after the 1-day ALNCD + 30 mg oral aripiprazole initiation regimen.Reference Hard, Wehr and Sadler15, 21 ALNCD is approved for use only as a single dose in the AL initiation regimen and not for repeated dosing.21
Prior to the approval of ALNCD, the only option for initiation of AL therapy was to prescribe 21 days of concomitant oral aripiprazole to provide relevant plasma levels during the slow dissolution of the AL dose.Reference Ehret, Davis and Luttrell7 The availability of two options for initiating AL therapy, each offering different advantages, allows the clinician to choose the regimen best suited to the individual patient (Table 2). The 21-day oral supplementation regimen for AL initiation requires one fewer injection than does the 1-day AL initiation regimen; the 1-day initiation regimen, however, can be administered by the clinician in a single office visit. In addition, the 21-day AL initiation regimen should be used in patients who are cytochrome P450 (CYP) 2D6 poor metabolizers, in patients taking strong CYP 2D6 and/or CYP 3A4 inhibitors, and in patients taking strong CYP 3A4 inducers, whereas the 1-day AL initiation regimen using ALNCD should be avoided because no dose adjustment is possible for ALNCD. By eliminating the need for extended oral supplementation, the use of ALNCD + a single 30 mg dose of oral aripiprazole reduces pill burden and removes a potential barrier to adherence during initiation of AL therapy.Reference Correll22 Regardless of the regimens used, the two initiation options are comparable in terms of the plasma aripiprazole levels provided to bridge the gap at AL initiation, and the safety and tolerability of the two regimens are similar and consistent with the known profile of aripiprazole.Reference Hard, Wehr and Du19
TABLE 2. Comparison of the 21- and 1-day initiation regimens for aripiprazole lauroxil
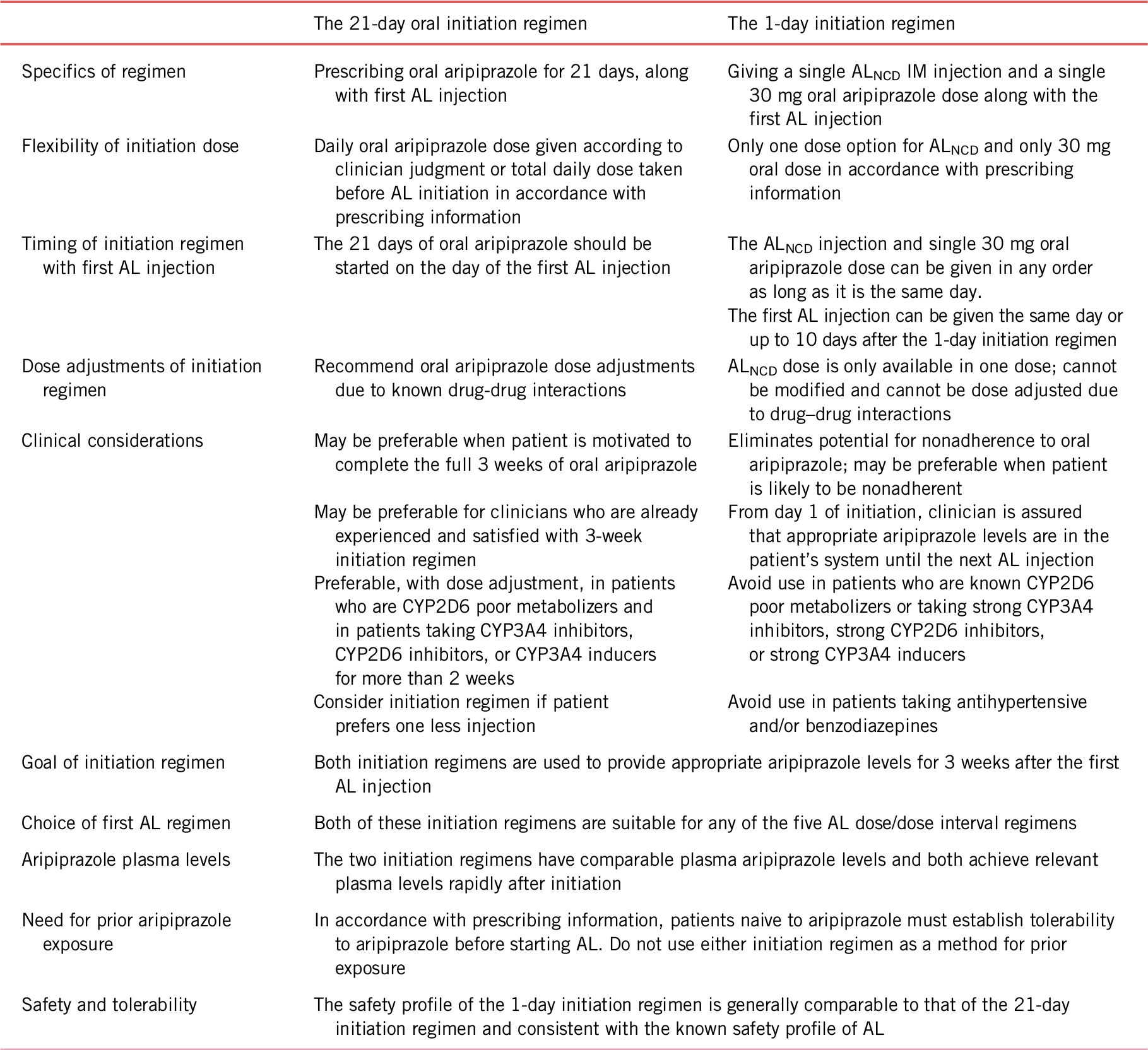
AL, aripiprazole lauroxil; ALNCD, Aripiprazole Lauroxil NanoCrystal® Dispersion; IM, intramuscular.
Conclusion
ALNCD was developed to provide a rapid increase in plasma aripiprazole levels for a new, 1-day AL initiation strategy that is used in conjunction with the first dose of AL. An ALNCD injection administered together with a single 30 mg dose of oral aripiprazole composes a 1-day regimen that results in rapid achievement of aripiprazole levels comparable to the 21-day oral aripiprazole initiation regimen. Treatment with ALNCD, in combination with a single 30 mg dose of oral aripiprazole dose and the starting dose of AL, was well tolerated in adult patients with schizophrenia. An initiation regimen for AL that uses one injection of ALNCD plus a single 30 mg dose of oral aripiprazole offers a 1-day alternative to the 21-day oral aripiprazole bridging strategy for initiating treatment with AL.
Disclosures
Rakesh Jain has served as a consultant to Addrenex, Allergan, Avanir, Janssen, Lilly, Lundbeck, Merck, Neos Therapeutics, Neurocrine Biosciences, Otsuka, Pamlab, Pfizer, Shionogi, Shire, Sunovion, Supernus, Takeda, and Teva; paid speaker for Addrenex, Alkermes, Allergan, Lilly, Lundbeck, Merck, Neos Therapuetics, Otsuka, Pamlab, Pfizer, Rhodes, Shionogi, Shire, Sunovion, Takeda, and Tris Pharmaceuticals; received research support from Allergan, AstraZeneca, Lilly, Lundbeck, Otsuka, Pfizer, Shire, and Takeda; and served on advisory board for Addrenex, Alkermes, Avanir, Forum, Janssen, Lilly, Lundbeck, Merck, Neos Therapeutics, Neurocrine Biosciences, Otsuka, Pamlab, Pfizer, Shionogi, Shire, Sunovion, Supernus, Takeda, and Teva. Jonathan Meyer reports having received speaking or advising fees from Acadia Pharmaceuticals, Alkermes, Allergan, Merck, Neurocrine, Otsuka America, Inc., Sunovion Pharmaceuticals and Teva Pharmaceutical Industries. Angela Wehr, Bhaskar Rege, Lisa von Moltke, and Peter Weiden are employees of Alkermes, Inc.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1092852919000816.