CVD is the most common chronic condition and the highest cause of mortality in the USA(Reference Benjamin, Muntner and Alonso1). In 2016, CVD accounted for more than 840 000 deaths in the USA, one of every three deaths. According to the most recent report from the American Heart Association, up to 48 % of US adults have some type of CVD, a total prevalence higher than the sum for all types of cancer combined(Reference Wilson, D’Agostino and Sullivan2).
During the period 2014–2015, CVD costs accounted for about 14 % of total health expenditure, more than any other diagnostic group, with the total cost exceeding 350 billion USD(Reference Benjamin, Muntner and Alonso1). This scenario will be maintained in the next years, and current forecasts predict that the prevalence of CVD and related costs will increase dramatically in the future(Reference Go, Mozaffarian and Roger3,Reference Heidenreich, Trogdon and Khavjou4) .
Strategies to improve CVD prevention and to reduce the impact of CVD in the USA remain of critical importance. A healthy diet and physical activity appear to be convincing and cost-effective strategies to CVD in baseline healthy subjects(Reference Waxman5). Additionally, robust evidence supports that a healthy diet and physical activity reduce all-cause and CVD mortality(Reference Evenson, Wen and Herring6–Reference Xiao, Murphy and Houston10).
However, for CVD survivors, the scientific evidence regarding the role of diet in association with physical activity is sparse. The current US dietary guidelines for Americans do not specify to what extent a healthy diet and reduced sedentary levels should be considered by individuals living with a chronic CVD condition(Reference DeSalvo, Olson and Casavale11). It seems undeniable that reducing sedentariness and eating a healthy diet would also benefit CVD survivors. Nevertheless, indications regarding what type of diet for CVD survivors should best follow remain unclear. Furthermore, it is unknown to what extent sedentariness may increase and which dietary recommendations would decrease the mortality risk of CVD survivors. Finally, it is unknown whether a healthy diet and reduced sedentariness act independently or whether they interact to improve the survival of individuals living with a chronic CVD condition.
In the present work, we analysed data from the National Health and Nutrition Examination Survey (NHANES) linked to the US mortality registry. First, participants with CVD at baseline were selected, defining the population for analysis. Dietary intakes and sedentary behaviour were related to all-cause and cardiovascular mortality. A non-linear dose–response analysis was undertaken to estimate the duration of sedentariness that results in increased mortality risk and to portray the shape of the dose–response relationship between sedentariness and CVD mortality. An energy partition model and an isoenergetic substitution analysis were undertaken to evaluate how diet and nutrient substitution influence mortality risk in CVD prevalent cases. Finally, an interaction analysis was conducted on both additive and multiplicative scales to identify and typify the interaction between diet and sedentariness and mortality in CVD prevalent cases.
Methods
Ethics statement
The National Centre for Health Statistics Research Ethics Review Board approved the survey protocols. Written informed consent was obtained for all participants.
Study sample and study design
The NHANES is an ongoing series of national surveys aimed to illustrate the general health and the nutritional status of children and adults in the USA. The NHANES study started in 1999, and every year about 5000 US citizens are physically examined and interviewed to obtain data regarding their socio-demographics, health status, smoking, physical activity, body size and dietary intakes. Based on a multistage probability sampling approach, the NHANES study aims to achieve a representative US sample(Reference Casavale12). Approximately every 4 years, the NHANES study is merged with the US mortality registry(Reference Williams13). In the present work, participants to the NHANES surveys, performed during the period 1999–2014, were merged with the US mortality registry updated in 2015, resulting in a prospective study based on 82 091 participants.
After exclusion of participants younger than 18 years of age, those without information on mortality status in 2015 and those without any CVD at the time of the interview, a sample size of 4623 participants was obtained. After further exclusion of participants without information regarding sedentary time, nutrient intakes and covariates used in the models, a final sample size of 2473 individuals were included. The flow chart of participant selection is reported in Fig. 1.

Fig. 1. Flow chart of 2473 CVD survivors from the National Health and Nutrition Examination Survey (NHANES) 1999–2014. * BMI, systolic blood pressure, diastolic blood pressure, alcohol use, smoking status, ethnicity and education.
Dietary and sedentariness assessment
The dietary interview was undertaken in three sessions. First, a 24-h dietary recall interview was performed. Afterwards, an evaluation of nutritional supplement use was conducted. Finally, a dietary post-recall by telephonic interview 3 to 10 d after the first dietary evaluation was performed(Reference Nocon, Hiemann and Müller-Riemenschneider14). Two days of dietary intake data were collected for all surveys performed after 2002. Data regarding sedentariness were collected using the Global Physical Activity Questionnaire reporting daily activities at work, leisure-time and sedentary activities at home. Total time spent on sedentary activities (television watching, computer use, etc.) was computed by summing time spent on all sedentary activities.
Statistical methods
Data description was performed considering the overall sample, participants who survived up to the end of the observational time and participants who died (all-cause and CVD mortality). Continuous data are reported using age-adjusted means and 95 % confidence limits, while categories are reported as counts and age-adjusted prevalence (Table 1). Nutrient intakes are reported as age- and energy-adjusted means and 95 % confidence limits (Table 2).
Table 1. Baseline characteristics of 2473 CVD survivors from the National Health and Nutrition Examination Survey 1999–2014*
(Numbers and percentages; medians and interquartile ranges (IQR))

SBP, systolic blood pressure, DBP, diastolic blood pressure.
* Results were standardised considering 10 years age classes. Categories were reported as number of subjects and standardised age prevalence. Continuous variables were reported by standardised age means and 95 % confidence limits.
† Alcohol use among users.
Table 2. Tertiles of daily dietary intakes of dietary fibres and micronutrients in relation to mortality risk (first tertile as reference category)*
(Hazard ratios (HR) and 95 % confidence intervals)
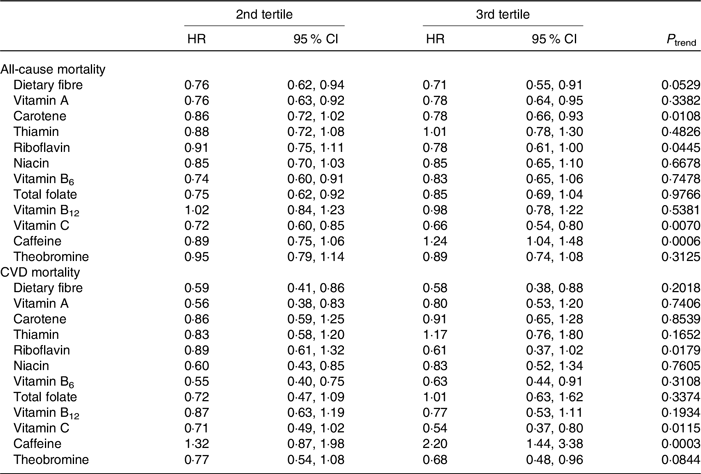
* All models were adjusted for ethnicity, education, BMI (continuous), alcohol use (continuous), smoking status, cancer type, any cardiovascular disease, time spent in sedentary time (continuous), mean of systolic and diastolic blood pressure (continuous) and energy intake (continuous).
Multivariate adjusted Cox regression was used to derive mortality hazards. All models were based on age to event as the underlying time variable and were stratified by sex, 10-year age class and survey. A sequential modelling approach was used to define adjusting variables to be retained in the final multivariate models. Unless otherwise specified, all reported analyses were adjusted for ethnicity (White, Black, Hispanic, others and mixed), education (more than high school), smoking status (smoked at least 100 cigarettes in life), alcohol use (continuous), type of CVD (congestive heart failure, CHD, heart attack or stroke as self-reported), cancer of any type (self-reported), the arithmetic mean of systolic and diastolic blood pressure (continuous) and BMI (continuous). Analyses regarding micronutrients were further adjusted for energy intake (continuous).
The non-linear dose–response analysis evaluating the relation between sedentariness and all-cause mortality was performed by means of restricted cubic splines with three knots at the 1st, 50th and 90th percentiles(Reference Croxford15,Reference Harrell16) .
The association between macronutrient intakes and all-cause and CVD mortality was evaluated using energy partition and isoenergetic substitution analyses(Reference Willett, Howe and Kushi17). In the present study, the first partition model aimed to estimate all-cause and cause-specific mortality hazard in relation to an increase of 1046 kJ from protein, carbohydrate and fat.
Our second partition model estimated the hazard for all-cause and cause-specific mortality in relation to an increase of 418·4 kJ from SFA, MUFA and PUFA.
Isoenergetic substitution analyses were undertaken to estimate the mortality hazard in relation to a substitution of 5 % of total energy from total fat, carbohydrate and protein. A final isoenergetic substitution analysis was undertaken for fat intake only. That analysis estimated the mortality hazard due to the mutual substitution of 10 % of fat-related energy from SFA, MUFA and PUFA(Reference Ricci, Baumgartner and Zec18). Isoenergetic substitution models were adjusted also for energy intake. Interaction analyses considering diet and sedentariness were conducted on an additive and a multiplicative scale. Sedentariness was dichotomised according to the risk threshold arising from the above-mentioned dose–response analysis. Micronutrient intakes were dichotomised at their medians. The test for statistical significance of the additive interaction was performed on the proportion of deaths attributable to the interaction(Reference Li and Chambless19). An ordinary statistical test was performed for the multiplicative interaction term. The significance of interaction terms was reported considering both ordinary P values for significance (α = 0·05) and Bonferroni adjusted type I error significance. Schoenfeld’s residuals visual inspection was used to evaluate the assumption of hazards proportionality of the Cox models. Statistical analyses were conducted taking the complex multi-stage probability sampling design adopted by the NHANES study into account, using specific survey procedures. In brief, survey weights, sample strata and sample cluster were considered to avoid differential probabilities of selection among sub-groups and to compensate for exclusion of sampling areas from the sampling frame.
Finally, sensitivity analyses were undertaken to confirm the results (data not showed but available by request to the corresponding author). First, models were additionally adjusted for medication use coded for the treatment of hypertension, dyslipidaemia, diabetes and anticoagulants. To investigate possible reverse causation, participants who died during the first year of follow-up were excluded in a further supplementary sensitivity analysis.
All statistical tests were two-tailed, and type I error rate was set to 5 % (α = 0·05). Statistical analyses were performed using SAS version 9.4 and STATA version 15.
Results
Characteristics of the study sample
The final sample of 2473 participants was followed for a median period of 5·6 (interquartile range = 2·8, 8·3) years, resulting in a cumulative observation of 14 356 person-years. During this period, 761 deaths occurred, of which, 199 deaths were due to CVD. Baseline characteristics of the study sample are reported in Table 1. The majority of the study sample were men (age standardised prevalence = 56 %). The median age of the study sample was 69 (interquartile range 60, 78) years. Survivors spent less time on sedentary activities, tended to be better educated, smoked less, were less frequently alcohol non-drinkers, had a lower systolic blood pressure, but a higher diastolic blood pressure, compared with those who died (Table 1). Daily nutrient intakes are reported in online Supplementary Table S2. Survivors tended to have marginally higher intakes of a number of nutrients including dietary protein, dietary fibre, vitamin C and carotene compared with those who died.
All-cause and CVD mortality
A linear monotonic positive relationship between time spent on sedentary activities and all-cause mortality risk was observed (online Supplementary Fig. S2). According to our dose–response analysis, when time spent on sedentary activities exceeded a threshold of 360 min/d, a statistically significant increased all-cause mortality risk was observed (hazard ratio (HR) = 1·39, 95 % CI 1·16, 1·67).
Furthermore, one standard deviation increase of time spent in sedentary behaviour (188 min) resulted in 20 % higher mortality (HR = 1·20, 95 % CI 1·09, 1·31 and HR = 1·19, 95 % CI 1·00, 1·42 for all-cause and CVD mortality, respectively). When looking at dietary fibre and micronutrient intakes, we found that higher intakes (2nd and 3rd tertile v. 1st tertile) of dietary fibre (between 18·9 and 52·39 g v. below 11·93 g), vitamin A (between 512·3 and 1999 retinol equivalents (RE) v. below 302·5 RE), carotene (between 2389 and 18 000 RE v. below 730·2 RE), riboflavin (between 2·52 and 6·85 mg v. below 1·67 mg) and vitamin C (between 106·3 and 385·9 mg v. below 51·58 mg) were statistically significantly inversely associated with an all-cause mortality in the range of 22–34 %. On the other hand, higher intakes of caffeine (3rd v. 1st tertile, between 172 and 1088 mg v. below 43 mg) were positively associated with all-cause mortality. Inverse associations with CVD mortality ranging between 32 and 46 % were observed for higher intakes (2nd and 3rd tertile v. 1st tertile) of dietary fibre, vitamin B6 (between 2·30 and 6·38 mg v. below 1·45 mg), vitamin C and theobromine (between 37·25 and 306·6 mg v. below 4·27 mg). Higher caffeine intakes (3rd v. 1st tertile) were, however, positively related to CVD mortality. Dietary fibre and micronutrient intakes in relation to all-cause and CVD mortality are reported in Table 2.
When looking at the energy partition analyses, we noted a statistically significant inverse association between fat intake and all-cause mortality. Furthermore, a positive trend existed between protein intake and all-cause and CVD mortality, while an inverse trend existed between PUFA intake and all-cause and CVD mortality. These suggestive results were confirmed by the isoenergetic substitution analyses where the isoenergetic substitution of protein and carbohydrate with fat resulted in a significant reduced all-cause mortality in a range of 63–83 %, respectively. On the other hand, the isoenergetic substitution of SFA and MUFA with PUFA appeared as beneficial with respect to all-cause and CVD mortality risk, in spite of lack of statistical significance. Partition model and isoenergetic substitution analyses are reported in Table 3.
Table 3. Energy partition and isoenergetic substitution analyses for macronutrients*
(Hazard ratios (HR) and 95 % confidence intervals)

Ref., reference.
* All models were adjusted for ethnicity, education, BMI (continuous), alcohol use (continuous), smoking status, cancer type, any cardiovascular disease, cancer, time spent in sedentary time (continuous) and mean of systolic and diastolic blood pressure (continuous). Isoenergetic substitution model1 based on 5 % substitution of energy from single macronutrients and isoenergetic substitution model2 based on 10 % substitution of energy from single types of fat. Isoenergetic substitution models were adjusted for energy intake.
† To convert kcal to kJ, multiply by 4·184.
A significant additive interaction between sedentariness and dietary fibre intake and all micronutrients was observed for all-cause and CVD mortality risk. Notably, the interaction between sedentariness and dietary fibre intake and all micronutrients was NS when considering the Bonferroni significance threshold taking into account the multiple comparisons. According to this evaluation, the benefit afforded by sedentariness below 360 min/d and dietary fibre and vitamin intakes above the median act on an additive scale. Notably, when low sedentariness and high dietary fibre and vitamin intakes were mutually present, we observed an inverse all-cause mortality risk in the range of 35–41 % and a reduced CVD mortality risk in the range of 32–61 %. No significant association was observed for an interaction on a multiplicative scale for sedentariness below 360 min/d and dietary fibre and vitamin intakes above the median daily intake. All-cause and cause-specific mortality risks in relation to micronutrients and sedentariness are reported in Table 4.
Table 4. Interaction between dietary fibres and micronutrients (nutr) with sedentariness (sed)*
(Hazard ratios (HR) and 95 % confidence intervals)

HR1, mortality risk for subjects with low sedentariness and low nutrient intake; HR2, mortality risk for subjects with high sedentariness and high nutrient intake; HR3, mortality risk for subjects with low sedentariness and high nutrient intake; P 1, additive scale interaction, test on the attributable proportion; P 2, multiplicative scale interaction, test on the multiplicative interaction term.
* All models were adjusted for ethnicity, education, BMI (continuous), alcohol use (continuous), smoking status, cancer type, any cardiovascular disease, mean of systolic and diastolic blood pressure (continuous) and energy intake (continuous). The reference category is given by subjects with high sedentariness and low nutrient intake.
† Bonferroni adjusted significant P values.
Finally, when looking at other generic determinants of all-cause and CVD mortality, we report that a previous event of congestive heart failure and stroke increased all-cause mortality by 72 and 32 %, respectively. When more than one CVD morbidity co-existed, the addition of each further CVD morbidity increased all-cause mortality risk by 34 %. Obesity (BMI > 30 kg/m2), high sedentary time (3rd tertile v. 1st tertile, between 480 and 840 min v. below 300 min), alcohol use (3rd tertile v. 1st tertile between 16·9 and 104·9 g v. below 7·6 g) and smoking increased all-cause mortality risk by 29, 31, 47 and 61 %, respectively. An increased CVD mortality risk was confirmed for congestive heart failure (HR = 2·17, 95 % CI 1·58, 2·98) and stroke (HR = 1·44, 95 % CI 1·04, 1·99) with respect to subjects without these conditions. Furthermore, when CHD was present, a 50 % increased CVD disease mortality risk was observed. When more than one CVD morbidity co-existed, a further CVD morbidity increased CVD mortality risk by 50 % (HR = 1·50, 95 % CI 1·28, 1·77). Moreover, when present, cancer was associated with a reduced CVD mortality risk of 32 %.
Sedentary time (3rd tertile v. 1st tertile) and smoking increased CVD mortality risk by 61 and 80 %, respectively. Hazards for all-cause and CVD mortality by CVD morbidity, adiposity, sedentary time, alcohol use, smoking and baseline presence of cancer are reported in online Supplementary Table S2.
All present results were confirmed when excluding participants who died during the first year of follow-up and when using models additionally adjusted for medication use. In particular, the exclusion of participant died during the first year of follow-up resulted in a non-significant shift of the second decimal of relative risk estimates. Models further adjusted for medication use had a slightly non-significant fitting improve according to a likelihood ratio test.
Discussion
In this prospective analysis among US adults aged 20–85 years, we found that reduced sedentariness and a varied diet rich in dietary fibre and vitamins were inversely associated with all-cause and CVD mortality among CVD survivors. Although these relationships have been reported for the general population(Reference Williams13,Reference Nocon, Hiemann and Müller-Riemenschneider14,Reference Li and Siegrist20–Reference Threapleton, Greenwood and Evans22) , our findings emphasise the potential prolongation of life in those who have already suffered a CVD event and who are known to have a reduced life expectancy. A novel finding was that low levels sedentariness in combination with a healthy diet were more strongly inversely associated with all-cause and CVD mortality as compared with each of these two lifestyle factors alone.
Our finding regarding the beneficial contribution of not being sedentary with respect to all-cause and CVD mortality is supported by evidence from previous studies conducted on CVD survivors from other populations(Reference Hamer, Ingle and Carroll23,Reference Hamer and Stamatakis24) . In addition, numerous biological mechanisms have been proposed to support the role of physical activity to counteract CVD risk and related mortality(Reference Mora, Cook and Buring25). First, physical activity improves energy balance promoting the maintenance of a healthy weight, which in turn has been demonstrated to reduce CVD and all-cause mortality(Reference Flegal, Kit and Orpana26). Second, it is hypothesised that being physically active reduces inflammation and oxidative stress, two major determinants of endothelial dysfunction, which in turn is associated with increased all-cause and CVD mortality(Reference de Jager, Dekker and Kooy27).
We observed an inverse association between a diet rich in dietary fibre and vitamins with all-cause and CVD mortality in CVD survivors. More specifically, we showed a beneficial association between dietary fibre, micronutrients with anti-oxidant properties and B vitamins (specifically thiamine, riboflavin, niacin, vitamin B6, folate and vitamin B12) with all-cause and CVD mortality. Three different biological mechanisms have been proposed to explain the role of these nutrients in reducing all-cause and CVD mortality risk. First, high intakes of dietary fibre reduce fat absorption, thus acting on metabolic balance reducing energy intake and promoting a healthy body weight(Reference Pereira and Ludwig28). This is confirmed by robust epidemiological evidence reporting that higher dietary fibre intakes are associated with reduced mortality risk in healthy US adults(Reference Park, Subar and Hollenbeck29). Second, antioxidants such as vitamin A, vitamin C and carotene act by reducing endothelial dysfunction and thus reducing CVD risk(Reference Frei30). As confirmation, low intakes of these vitamins are associated with increased CVD mortality in Europeans(Reference Gey, Stähelin and Eichholzer31). Third, the beneficial role of B vitamins on all-cause and CVD risk is well reported(Reference Albert, Cook and Gaziano32–Reference Huang, Chen and Yang34), and several biological mechanisms have been revealed for B vitamins(Reference Robinson35). For example, they act as coenzymes in the metabolism of fat, protein, and carbohydrates, thus acting on energy balance(Reference Gropper and Smith36). Also, a remarkable benefit of B vitamins is their ability to reduce circulating homocysteine levels(Reference Clarke, Halsey and Lewington37). This is probably the most important mechanism through which B vitamins may act since high homocysteine levels have been widely associated with increased risk of CVD(Reference Albert, Cook and Gaziano32–Reference Huang, Chen and Yang34,Reference Chambers, Ueland and Obeid38,Reference Ganguly and Alam39) .
In agreement with previous studies based on healthy participants(Reference Estruch, Ros and Salas-Salvadó40,Reference Ford and Caspersen41) , we found that decreased sedentary behaviour combined with a healthy diet reduce all-cause and CVD mortality risk in subjects with a cardiovascular condition.
Notably, as reported above, physical activity and diet share some common mechanisms of action, such as adiposity control, antioxidant activity and inflammation reduction. According to this, the observed additive interaction could be interpreted as the result of joint action given by the sum of these two separate factors.
In the present work, we observed a beneficial, nevertheless controversial, association between fat intake and all-cause and CVD mortality in CVD survivors. This evidence is currently under discussion because there is still partly conflicting evidence regarding the relationship of fat intake with CVD morbidity and mortality(Reference Hooper, Summerbell and Higgins42–Reference Siri-Tarino, Sun and Hu44).
In the present study, we also confirmed previous evidence regarding all-cause and CVD mortality in subjects with CVD. First, we found that the co-existence of multiple cardiovascular morbidities increases all-cause and CVD mortality risk. Moreover, according to our results, congestive heart failure represents the cardiovascular condition with the highest all-cause and CVD mortality risk for CVD survivors, more so than myocardial infarction, CHD or stroke. We confirmed that obesity, alcohol use and smoking increased all-cause and CVD mortality risk in subjects with CVD and that, when present, cancer significantly reduces CVD mortality risk. This is probably because CVD survivors who also have cancer are at higher risk of dying of cancer. We investigated this result in a supplementary analysis considering cancer mortality as outcome and observed a 2-fold higher cancer mortality risk in CVD survivors who also have cancer (HR = 2·82, 95 % CI 1·76, 4·51). This result highlights the complexity of CVD survivors and how CVD mortality represents only a limited part of possible outcomes for subjects with CVD.
Finally, in the present work, we reported that higher caffeine intake would result in increased all-cause and CVD mortality risk. This is opposite to recently reported evidence(Reference Gunter, Murphy and Cross45,Reference Poole, Kennedy and Roderick46) . However, first, we observed a particularly high caffeine intake up to 1000 mg which could have influenced mortality risk. Second, it should be considered that we considered a sample of US subjects with an already existing CVD condition.
The present work has several strengths. First, it is based on a robust sampling methodology and study design, thus reporting results that are generalisable to the US population of CVD survivors. Second, the present work provides novel scientific evidence regarding modifiable mortality risk factors in CVD survivors, a population of high epidemiological interest. Notably, the evidence provided herein is of quantitative nature and may provide useful guidance in addressing the current cardiovascular threat in the USA. Finally, the present results are robust with respect to the sensitivity analyses performed; thus, the risk of bias and reverse causation appear minor.
The work also has some limitations. First, the specific nature of the population under study limited our sample size, and we cannot exclude the possibility that this may have reduced statistical power leading to false-negative results. Furthermore, the limited sample size prevented us from investigating other possible mortality causes, such as cancer or type 2 diabetes. Also, it would have been of interest to investigate the nature and origin of macronutrient intakes and type of dietary fibre (soluble, insoluble), but this information was not reported in the final version of the NHANES database. Moreover, the observational nature of the study does not allow the interpretation of the present results in terms of causation and biases including reverse causation and residual confounding cannot be ruled out. Another possible pitfall is represented by use of a dietary post-recall by telephonic interview and by use of self-reported data on physical activity, which could have determined misreporting and bias of dietary intakes and physical activity, respectively. Moreover, after exclusion of individuals without information on mortality status, without prevalent cardiovascular disease and with missing information regarding covariates used in the model our sample reduced from 82 091 to 2473 individuals. It is possible that this could have biased our analysis resulting in an underestimation of the mortality risks due to non-response from categories such as smokers, drinkers and subjects with sedentary behaviour.
Finally, we acknowledge that the major limitation of the present study is the lack of information on time since CVD diagnosis and the lack of information on disease severity and CVD therapy.
Future studies with an experimental design should be planned and executed to better disentangle the causal relationship between dietary intakes and sedentariness in relation to all-cause and CVD mortality risk in CVD survivors.
Conclusions
In summary, reduced sedentariness, along with a varied diet rich in dietary fibre and vitamins, appears to be related to reduced all-cause and CVD mortality in US CVD survivors. These two factors appear to interact on an additive scale so that the most appropriate recommendation for CVD survivors is to have a healthy diet rich in dietary fibre and vitamins combined with reduced sedentariness. Finally, the fat component of diet looks as crucial to improve the survivorship of CVD survivors. Further studies are needed to this regard to investigate for sub-types such as individual fatty acids or protein and carbohydrates coming from different food sources.
Acknowledgements
The present work can be considered spontaneous research and none of the authors received a grant.
C. R., M. F. L., H. F. and M. P. contributed to the conception and the design of the work. C. R. contributed to data acquisition and statistical analysis. C. R. and M. P. drafted the first version of the manuscript. M. F. L., H. F., A. E. S., R. S., S. H. K. and C. M. S. critically revised the manuscript. All authors participated to the interpretation of results, gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
The authors declare that there are no conflicts of interest.
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/WHO, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/WHO.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520002391








