The prevalence of obesity throughout the USA has increased significantly over the past 25 years(Reference Flegal, Carroll and Ogden1). National Health and Nutrition Examination Survey results indicate that over one-third of the adult population in the USA is obese, encompassing 35·5 % of women and 32·2 % of men(Reference Flegal, Carroll and Ogden1). This upward trend is not without consequences. A review of the health consequences of obesity shows that as BMI increases so does the risk of many health problems, including some forms of cancer, CVD, type 2 diabetes and other life-threatening disorders(Reference Li, Bowerman and Heber2).
One of the key health problems associated with obesity is insulin resistance, a common metabolic condition that can lead to a host of serious chronic diseases(Reference Bogardus, Lillioja and Mott3). Because insulin resistance is a precursor of several diseases, it has received considerable attention. Some of the diseases closely connected to insulin resistance are hypertension, type 2 diabetes and CVD(Reference Facchini, Hua and Abbasi4–Reference Jeppesen, Hansen and Rasmussen6). Facchini et al. (Reference Facchini, Hua and Abbasi4) examined prospectively over 4–11 years the extent to which insulin resistance predicts age-related diseases, including hypertension, CHD, stroke, cancer and type 2 diabetes. Results showed that approximately one out of three of the initially healthy subjects in the upper tertile of insulin resistance developed an age-related disease(Reference Facchini, Hua and Abbasi4). However, among the individuals who were more insulin sensitive, no age-related events were observed(Reference Facchini, Hua and Abbasi4). Clearly, insulin resistance is a serious health risk. Consequently, additional research is warranted to determine strategies that will reduce insulin resistance in adults and curb the risk of many life-threatening diseases.
Fortunately, insulin resistance can be improved through lifestyle changes, particularly weight loss and regular physical activity (PA)(Reference Anderson, Baird and Davis7). Moreover, the literature shows that diets high in carbohydrates and fibre and low in fat are associated with increases in insulin sensitivity(Reference Anderson, Baird and Davis7–Reference McClenaghan9). Consuming a healthy diet may reduce the pathogenesis of insulin resistance and thus decrease the risk for type 2 diabetes and CVD.
Fibre intake has been one of the main focuses of studies examining dietary approaches to reducing the risk of insulin resistance. To date, many investigations have examined the link between fibre intake and insulin sensitivity(Reference Lau, Faerch and Glumer10–Reference Ludwig, Pereira and Kroenke18). Comparison of these studies is difficult, given the many different methods used for detecting insulin resistance and the limitations of some dietary assessment methods. No doubt, research methods in this area can be improved.
Studies focusing on dietary fibre consumption and insulin resistance have displayed several consistent weaknesses. First, most studies have used BMI when controlling for obesity(Reference Lau, Faerch and Glumer10–Reference Ylonen, Saloranta and Kronberg-Kippila13, Reference Marshall, Bessesen and Hamman17). BMI is not a high-quality index of percentage body fat(Reference Rothman19). Very few studies have used percentage body fat to index obesity. Additionally, PA has a strong influence on insulin sensitivity, and the predominant assessment form in epidemiological studies has been questionnaires(Reference Laporte, Montoye and Caspersen20). This measurement method relies on self-report and thus contains significant error, as memory and a desire to appear favourable are key factors associated with such self-reported data(Reference Mahabir, Baer and Giffen21). Lastly, most studies that have examined the relationship between fibre intake and insulin resistance have focused on total fibre consumption(Reference Lau, Faerch and Glumer10–Reference McKeown, Meigs and Liu12, Reference Lovejoy and DiGirolamo14, Reference Rave, Roggen and Dellweg15, Reference Marshall, Bessesen and Hamman17, Reference Ludwig, Pereira and Kroenke18). However, since dietary fibre consists of two main categories, namely, soluble and insoluble, the independent influence of each should be considered, given these fibres have vastly different effects on food absorption and digestion.
The purpose of the present study was to examine the relationship between total, soluble and insoluble fibre and insulin resistance, as estimated by the homeostasis model assessment of insulin resistance (HOMA-IR), in pre-menopausal, non-diabetic women. Additionally, the influence of age, body fat percentage, body weight, total PA, intensity of PA, dietary fat intake and total energy consumption were measured and controlled while examining the fibre and insulin resistance relationship.
Experimental methods
Design
A cross-sectional design was employed in the present study to examine the relationship between fibre intake, including total, soluble and insoluble fibres, and insulin resistance (HOMA-IR). A total of 264 women were included in the analysis and were recruited through the use of newspaper advertisements, flyers and emails. Distribution included two metropolitan areas in the Mountain West. Telephone interviews were used to screen applicants according to the study requirements. All of the qualified subjects were healthy, non-smoking, pre-menopausal women. The mean age of the subjects was 40·1 (sd 3·0) years. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Institutional Review Board. Prior to the collection of data, written informed consent was obtained from all subjects/patients.
Procedures
At the first appointment, measurements of dietary intake, total PA, intensity of PA, body weight, percentage body fat, HOMA-IR and age were obtained. The Human Performance Research Center at the university served as the location where all laboratory measurements were made. Subjects were informed at the start of their first appointment of any potential risks, as well as the benefits, from participating in the study.
During the first appointment, height, weight and body fat percentage were measured while wearing a one-piece swimsuit in bare feet. Subjects also received a digital food scale (Ohaus 2000, Ohaus Corporation), 7 d dietary records and an ActiGraph accelerometer (formerly called CSA; Health One Technology), all of which were explained so that each subject had knowledge of proper weighing and logging methods and appropriate use of the activity monitor. Recording of dietary intake and continuous wear of the accelerometer occurred simultaneously during the seven consecutive days.
Insulin resistance
Qualified hospital personnel obtained blood samples from subjects who had fasted for at least 12 h before their appointment. The antecubetal vein served as the location where blood samples were taken and the sample was then centrifuged at 2000 g for 15 min at a temperature of 4°C. Final storage of samples was in aliquots at temperatures of − 20°C. The hospital laboratory determined fasting insulin levels (μU/ml) and glucose levels (mg/dl) utilising two separate methods, namely, Access® Ultrasensitive Insulin assay (Beckman Coulter, Inc.) and Dimension Vista System® and the Flex reagent cartridge (Siemens), respectively. Insulin resistance was indexed using HOMA-IR, which was estimated using fasting glucose and insulin concentrations in the following equation(Reference Matthews, Hosker and Rudenski22):
HOMA-IR provides comparable assessment of insulin resistance to other validated methods. Matthews et al. (Reference Matthews, Hosker and Rudenski22) demonstrated that HOMA-IR produced estimates of insulin resistance similar to measurements obtained by the hyperinsulinaemic euglycaemic clamp (R s= 0·88, P <0·0001), which is considered one of the ‘gold standard’ tests. A review article revealed that when the HOMA-IR model is used in epidemiological investigations, valuable data can be obtained(Reference Wallace, Levy and Matthews23).
Dietary intake
Total energy, fat and fibre intakes were measured using 7 d diet records in which subjects weighed and recorded all food and drink consumed within a consecutive 7 d time frame. A digital food scale was issued to each subject along with an explanation of how to properly weigh and record all food and drink consumed, including water. Food description and food weight were recorded daily on the records provided. During the 7 d, research personnel contacted each woman at least twice to provide support and to ensure that accurate records were being kept. Following completion of the 7 d, assessment of dietary intake was accomplished using ESHA Research software, version 7.6 (ESHA Research, Inc.) to provide objective dietary results. If energy intake was not at least 130 % of RMR estimated through the Ravussin metabolism formula(Reference Ravussin, Lillioja and Anderson24), the women were required to redo their weighed food records for an additional 7 d.
Using 7 d dietary records, where all food and drink are weighed prior to eating, provides many benefits. Subjects' ability to recall foods eaten and also portion sizes are not a problem with food records. Also, with the occurrence of day-to-day variations in eating habits, recording foods for seven consecutive days documents habitual dietary intake when compared with other dietary assessments(Reference Hartman, Brown and Palmgren25).
Total physical activity
ActiGraph accelerometers (Health One Technology) provided a means to objectively assess PA during the same seven consecutive days that dietary intake was measured. Instructions on how to appropriately use this device were provided during the initial appointment. The accelerometer was worn constantly throughout the day and night, with the exception of water activities, during which subjects were required to remove the activity monitor. The accelerometer was attached to a nylon belt that was worn comfortably around the subjects' waist and positioned over the left hip.
Objective and reliable measurements can be obtained through the use of Actigraph accelerometers (Health One Technology) to evaluate levels of PA(Reference Liu, Li and Song26, Reference Bassett, Ainsworth and Swartz27). Validation of this accelerometer among adults has been conducted and shown to provide a close representation of PA levels in free-living subjects in comparison to doubly labelled water and portable metabolic system(Reference Liu, Li and Song26, Reference Bassett, Ainsworth and Swartz27).
In the present study, total PA was indexed using the sum of all the activity counts acquired over the 7 d of assessment. Concurrent validity for this measure has been shown by several investigations(Reference Tucker and Tucker28–Reference Davidson, Tucker and Peterson32).
Intensity of physical activity
Intensity of PA was measured using Actigraph accelerometers (Health One Technology) in which participant movement was recorded in 10 min segments for a total of 144 bouts (epochs) each day, 1008/week. The reason for choosing 10 min as the length for assessing intensity of PA was based on the American College of Sports Medicine guidelines, indicating that multiple 10 min bouts are sufficient for accumulating PA(33).
The following categories for PA intensity were utilised based on previous research(Reference LeCheminant, Tucker and Bailey34). Each category included the activity counts associated with the three levels of intensity (low, moderate and vigorous) and corresponding speeds (miles per h; mph) on a treadmill: low intensity, 0–29 999 counts in one 10 min bout ( < 3 mph; 4.8km/h); moderate intensity, 30 000–49 999 counts in one 10 min epoch (3–4 mph; 4.8-6.4km/h); vigorous intensity, 50 000 counts or greater in one 10 min bout (>4 mph; >6.4km/h)(Reference LeCheminant, Tucker and Bailey34).
Each participant had a total of 1008 10 min bouts of monitored activity distributed over the three intensity categories over the course of the week. The amount of time subjects engaged in PA within each intensity category was used to differentiate among participants. For example, one subject might have 0 bouts of vigorous activity across the 7 d of recording, whereas another subject might have 20 min and another might have 120 min of vigorous activity over the week. Many investigations have employed these guidelines when using the Actigraph accelerometer (Health One Technology) to assess the intensity of PA(Reference Tucker and Tucker28, Reference Nokes and Tucker30, Reference LeCheminant, Tucker and Bailey34).
Body fat percentage
Body fat percentage was measured through the use of air displacement plethysmography, the BOD POD (COSMED, USA, Inc.). Thoracic lung volume was also evaluated directly via the BOD POD. Before performing any measurements, the BOD POD was calibrated in order to minimise measurement error. Subjects were asked to fast for 3 h prior to their appointment. A university-issued, one-piece swimsuit was worn by each woman as well as a swim cap. Subjects were instructed to use the restroom immediately prior to the measurements. Two measurements were obtained for each subject to ensure accuracy. A maximum difference of 1 percentage point was allowed between the two results. If a difference of more than 1 percentage point resulted, a third measurement was obtained. An average of the two measurements within 1 percentage point of each other was then used.
The BOD POD provides a valid and reliable measurement of body fat percentage, as concluded by several studies(Reference LeCheminant, Tucker and Peterson35, Reference Ballard, Fafara and Vukovich36). For the present study, reliability was established by performing a test–retest on 100 women from the study sample, which resulted in an intra-class correlation of 0·999 (P <0·0001)(Reference Bailey, Tucker and Peterson37). On the same 100 women, validity of the BOD POD was also examined through comparison of the results with findings obtained from dual-energy X-ray absorptiometry (Hologic, Inc.) (intra-class correlation of 0·97 (P <0·001))(Reference LeCheminant, Tucker and Peterson35). Ballard et al. (Reference Ballard, Fafara and Vukovich36) concluded after comparing the BOD POD with the dual-energy X-ray absorptiometry that the BOD POD was a valid and reliable method of evaluating percentage body fat in female athletes and non-athletes.
Body weight
Each subject was weighed on an electrical scale (Tanita), which measured body weight to the nearest 0·005 kg. Calibration of the scale occurred daily before any measurements were obtained. Subjects refrained from eating anything for 3 h before their appointment. The same one-piece swimsuit used for the BOD POD was also worn during the weigh in. The data for weight were the average of two measurements taken a week apart.
Statistical methods
Dietary fibre intake (total, soluble and insoluble fibres) was expressed as g of fibre per 4184 kJ (1000 kcal). HOMA-IR values were log transformed because the values were not normally distributed, but to facilitate interpretation of the findings, HOMA-IR data in the Results section and tables were reported in common clinical units. Regression analysis using the general linear model procedure was employed to determine the bivariate relationships between each of the three key fibre variables, total, soluble and insoluble fibres and insulin resistance, specifically HOMA-IR. Partial correlation, using the general linear model framework, was used to determine the extent to which each of the potential confounding variables, i.e. age, body weight, body fat percentage, dietary fat intake, total energy consumption, total PA and intensity of PA, influenced the fibre and HOMA-IR associations, considered individually and collectively. The value of α was set at the 0·05 level. Additionally, to assist with interpretation of the data, fibre intake and HOMA-IR scores were each divided into two categories using the median, i.e. low and high. Specifically, the median value for HOMA-IR was 1·3, and for total, soluble and insoluble fibres, the intake (g) per 4184 kJ (1000 kcal) was 8·9, 1·6 and 3·5, respectively. OR were calculated to determine the relationships between the two dichotomous variables. To determine the statistical significance of the OR, 95 % CI were used. Logistic regression was employed to determine the effect of each of the potential confounding variables on the OR, considered individually and in combination. The SAS (SAS Institute, Inc.) software program (version 9.3) was utilised for all of the statistical analysis.
Results
The present cross-sectional investigation had 264 participants. The majority of the women were Caucasian (approximately 90 %), married (approximately 80 %) and were employed either part- or full-time (approximately 60 %). Approximately half had received some college education (approximately 50 %). Additional characteristics for the key variables of the study are displayed in Table 1, including age, weight, body fat percentage, PA, fasting insulin, fasting glucose, HOMA-IR, total energy intake, total fibre weight, total fibre intake per 4184 kJ (1000 kcal), soluble and insoluble fibre weight and soluble and insoluble fibre intake per 4184 kJ (1000 kcal). Average HOMA-IR for these women was 1·5 (sd 1·0) and average total, soluble and insoluble fibre intake (g) per 4184 kJ (1000 kcal) was 9·3 (sd 2·9), 1·7 (sd 0·9) and 3·8 (sd 1·9), respectively. The average BMI of this sample was 23·8 (sd 3·3) kg/m2, and based on official BMI cut-points, this falls within the normal category. The mean body fat percentage was 31·7 (sd 6·9) %. Approximately 51 % of the sample was obese using a cut-point of 32 % body fat(Reference Cotton and Green38).
Table 1 Descriptive statistics (n 264) (Mean values and standard deviations; medians, 25th and 75th percentiles)

HOMA-IR, homeostasis model assessment of insulin resistance.
* Minimum and maximum represent the lowest and highest values within the entire sample.
† Actual counts were measured objectively through accelerometers and are averages of weekly activity counts divided by 1000.
Soluble fibre and homeostasis model assessment of insulin resistance
When both soluble fibre intake and HOMA-IR were treated as continuous variables, there was a 0·112 decrease in HOMA-IR for every 1 g increase in soluble fibre intake when no variables were controlled statistically (F= 7·97, P =0·0051) (Table 2). Table 2 shows that, after controlling for the individual confounding variables, the relationship remained statistically significant. Further analysis showed that the relationship was weakened slightly, but remained statistically significant, after controlling the following variables individually: body weight (F= 7·62, P =0·0062), percentage body fat (F= 6·86, P =0·0094), total energy intake (F= 6·82, P =0·0095), dietary fat intake (F= 6·78, P =0·0098), total PA (F= 6·86, P =0·0093), time in sedentary activity (F= 5·91. P =0·0157), time in moderate activity (F= 5·98, P =0·0151) and lastly, time in vigorous activity (F= 6·69, P =0·0102). Controlling for age was the only confounding variable that strengthened the relationship (F= 8·44, P =0·0040). With all the potential confounders controlled simultaneously, the association between soluble fibre intake and HOMA-IR changed minimally and remained statistically significant (F= 7·82, P =0·0055).
Table 2 Differences in insulin resistance (homeostasis model assessment of insulin resistance (HOMA-IR)) corresponding to a 1 g difference in soluble fibre intake, independent of key potential confounding variables
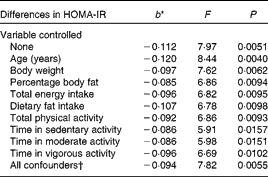
* b = regression coefficient.
† In the full model, the following variables were controlled statistically: age, percentage body fat, body weight, total energy intake, dietary fat intake, total physical activity and physical activity intensity.
When the relationship between soluble fibre intake and HOMA-IR was analysed with both variables treated as categorical, OR were calculated. Both soluble fibre intake and HOMA-IR were divided into two categories using the median. The OR was 0·58 and statistically significant (95 % CI 0·36, 0·94), with no variables controlled statistically (Table 3). The relationship remained significant, even after controlling for several potential confounding variables individually, including age, percentage body fat, body weight, total energy intake, dietary fat intake and total PA. After controlling for each of the intensity of PA measures individually, the relationship between soluble fibre intake and HOMA-IR no longer remained significant. As shown in Table 3, after adjusting for all the potential confounding variables simultaneously, the OR of having insulin resistance among those with high soluble fibre intake was about one-half that of the women with low soluble fibre intake (OR 0·52; 95 % CI 0·29, 0·93).
Table 3 Insulin resistance in women with low soluble fibre intake compared with high soluble fibre intake in women (Odds ratios and 95 % confidence intervals)
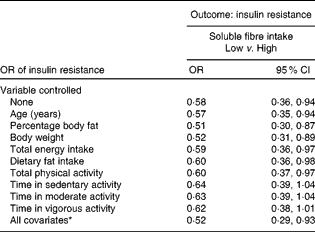
* In the full model, the following variables were controlled statistically: age, percentage body fat, body weight, total energy intake, dietary fat intake, total physical activity and physical activity intensity.
Total fibre and homeostasis model assessment of insulin resistance
Table 4 displays the relationship between total fibre intake and HOMA-IR, both treated as continuous variables, without and with control of the potential confounding variables. With no variables controlled statistically, the association was statistically significant (F= 4·58, P =0·0332). For every 1 g increase in total fibre consumption, there was a 0·026 decrease in HOMA-IR. The relationship was weakened slightly, but remained statistically significant, after controlling for body weight (F= 3·91, P =0·0490), total PA (F= 3·97, P =0·0473) and time in vigorous activity (F= 3·90, P =0·0493). The following confounding variables, however, weakened the relationship to the point that it was no longer significant: percentage body fat (F= 1·96, P =0·1631), total energy intake (F= 3·53, P =0·0613), dietary fat intake (F= 3·41, P =0·0659), time in sedentary activity (F= 3·37, P =0·0676) and time in moderate activity (F= 3·36, P =0·0680), with the last four potential confounders resulting in borderline significance. Age strengthened the relationship after being controlled (F= 4·81, P =0·0291). However, this relationship between total fibre intake and HOMA-IR was completely nullified when differences in soluble fibre intake were controlled statistically (F= 0·01, P =0·9409).
Table 4 Differences in insulin resistance (homeostasis model assessment of insulin resistance (HOMA-IR)) corresponding to a 1 g difference in total fibre intake, independent of key potential confounding variables
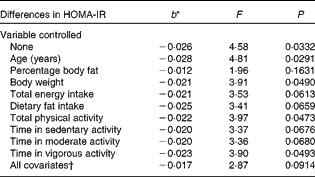
* b = regression coefficient.
† In the full model, the following variables were controlled statistically: age, percentage body fat, body weight, total energy intake, dietary fat intake, total physical activity and physical activity intensity.
Treating total fibre and HOMA-IR as categorical variables resulted in the relationship failing to reach statistical significance (Table 5). The relationship remained insignificant even after controlling for the various confounding variables. However, borderline significance was seen after controlling for total energy intake (OR 0·75; 95 % CI 0·46, 1·00).
Table 5 Insulin resistance in women with low total fibre intake compared with high total fibre intake (Odds ratios and 95 % confidence intervals)
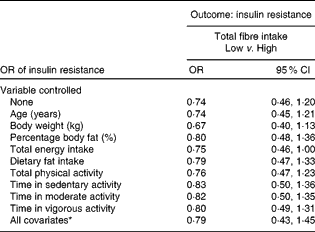
* In the full model, the following variables were controlled statistically: age, percentage body fat, body weight, total energy intake, dietary fat intake, total physical activity and physical activity intensity.
Insoluble fibre and homeostasis model assessment of insulin resistance
None of the relationships between insoluble fibre and HOMA-IR was statistically significant when treated as continuous variables. After controlling for each of the potential confounding variables, the relationships remained insignificant. Similarly, with insoluble fibre and HOMA-IR treated as categorical variables, none of the OR was statistically significant, without and with control of the potential confounders.
Covariates, fibre intake and homeostasis model assessment of insulin resistance
To better understand how PA intensity influenced the relationship between fibre intake and insulin resistance, additional analyses were conducted. Results showed that time spent in sedentary pursuits was related directly to HOMA-IR (r 0·153, P =0·0128). Moderate-intensity PA was inversely associated with insulin resistance (r − 0·144, P =0·0191) and time spent in vigorous PA was also inversely related to HOMA-IR (r − 0.155, P =0·0114).
Further analyses showed that PA intensity was also predictive of fibre intake. Specifically, time spent in sedentary behaviours was inversely related to total fibre (r − 0·214, P =0·0005), soluble fibre (r − 0·208, P =0·0007) and insoluble fibre consumption (r − 0·178, P =0·0037). Time spent in moderate-intensity activities was a significant predictor of each of the fibre variables: total fibre (r 0·223, P =0·0003), soluble fibre (r 0·221, P =0·0003) and insoluble fibre (r 0·188, P =0·0022). Lastly, time spent in vigorous PA was predictive of total fibre (r 0·145, P =0·0185) and soluble fibre intake (r 0·149, P =0·0157), and the insoluble fibre relationship was borderline significant (r 0·104, P =0·0913).
The relationship between fibre intake and insulin resistance was influenced by several mediating factors other than body fat and PA. Controlling for dietary fat intake weakened the fibre–HOMA-IR relationship, which can be explained by the inverse association between dietary fat consumption and total fibre (r − 0·42, P <0·0001), soluble fibre (r − 0·29, P <0·0001) and insoluble fibre (r − 0·35, P <0·0001). Total energy (kJ) intake was not related significantly to any of the fibre variables, because each fibre variable was corrected for differences in energy intake (i.e. g/4184 kJ (1000 kcal)). However, kJ intake was predictive of fibre intake, not expressed per 4184 kJ (1000 kcal), as shown by the following: g of total fibre (r 0·37, P <0·0001), soluble fibre (r 0·23, P =0·0002) and insoluble fibre consumed (r 0·22, P =0·0004).
Additional data analyses were conducted using food groups that showed that servings of non-starchy vegetable intake was the best predictor of soluble fibre intake per 4184 kJ (1000 kcal) (r 0·42, P< 0·0001). In other words, the more non-starchy vegetables in the diet, the more soluble fibre the participants consumed. Servings of fruit were also directly related to soluble fibre intake (r 0·40, P< 0·0001), as were servings of starch (r 0·24, P< 0·0001), whereas servings of simple carbohydrate (r − 0·38, P< 0·0001), dietary fat (r − 0·28, P< 0·0001) and meat (r − 0·17, P= 0·0061) were significantly and inversely related to soluble fibre intake.
Discussion
The present investigation uncovered a significant inverse association between soluble fibre intake and insulin resistance in non-diabetic, middle-aged women. However, insoluble fibre consumption was not a significant predictor of insulin resistance. Total fibre intake was also inversely associated with insulin resistance, but the relationship was much weaker than the link between soluble fibre and HOMA-IR, and actually became nullified after controlling for differences in soluble fibre intake.
Important to the fibre intake and insulin resistance relationship is the fact that obesity and insulin resistance are strongly related. As obesity increases, risk of insulin resistance and type 2 diabetes increases dramatically(Reference Ludvik, Nolan and Baloga39). Moreover, fibre intake is inversely related to weight gain and obesity(Reference Tucker and Thomas40). Consequently, to isolate the relationship between dietary fibre and insulin resistance, obesity must be controlled. To date, almost all studies have achieved this adjustment by controlling for differences in BMI, yet BMI is not a good index of body composition. Hence, in the present study, body fat percentage was controlled statistically instead of BMI.
Controlling for differences in body fat percentage weakened the relationship between soluble fibre and HOMA-IR by 32 %, but the association remained significant (P =0·0094). The weaker link between total fibre intake and HOMA-IR was also attenuated substantially by adjusting for differences in body fat percentage ( − 66 %), causing this relationship to become non-significant (P =0·1631).
From these findings, it can be argued that part of the association between fibre intake and insulin resistance is a function of differences in body fat percentage. Although a meaningful relationship remains between soluble fibre and insulin resistance after removing the influence of body fat, the significant relationship between total fibre intake and HOMA-IR is nullified when differences in body fat are taken into account. In short, if all women had the same body fat percentage, the relationship between soluble fibre and insulin resistance would be weaker and the total fibre–HOMA-IR relationship would not exist.
PA also has a strong effect on insulin sensitivity(Reference Borghouts and Keizer41). Those who exercise or engage in PA regularly have a much lower risk of insulin resistance and type 2 diabetes(Reference Helmrich, Ragland and Leung42, Reference Ivy43). However, few investigations that have studied the relationship between fibre intake and insulin resistance have controlled for differences in PA, and those which have(Reference Lau, Faerch and Glumer10, Reference McKeown, Meigs and Liu12, Reference Ylonen, Saloranta and Kronberg-Kippila13, Reference Marshall, Bessesen and Hamman17, Reference Ludwig, Pereira and Kroenke18) have relied on activity questionnaires, which harbour significant measurement error. To overcome this problem, the present study assessed PA objectively using accelerometry over a 7 d period. Moreover, not only was total PA evaluated, but the mediating roles of PA intensity at the sedentary, moderate and vigorous levels were also ascertained.
The soluble fibre and HOMA-IR relationship was weakened by controlling for PA intensity, as shown in Table 2, but remained statistically significant. However, when the relationships between soluble fibre intake and HOMA-IR were expressed using OR, and PA intensity was controlled, the results were weakened to the point of non-significance (Table 3). Further, most of the associations between total fibre intake and insulin resistance were weakened to the point of non-significance when the various levels of PA intensity were controlled. Apparently, a significant portion of the relationship between fibre intake and insulin resistance is a function of differences in PA, particularly PA intensity. To date, this has not been shown in the literature.
The relationship between fibre intake and HOMA-IR was also evaluated using OR. In women who had high soluble fibre intake (upper 50 %), the OR of having an elevated HOMA-IR level was 0·58 (95 % CI 0·36, 0·94) times that of women with low soluble fibre intake (lower 50 %). After controlling for all the potential confounding factors simultaneously, the OR was 0·52 (95 % CI 0·29, 0·93). In other words, women with high fibre intake had only one-half the likelihood of having insulin resistance compared with those with low fibre consumption, a substantially lower probability.
The relationship between fibre intake and insulin resistance has been researched before. However, there are many differences among previous studies, especially regarding the confounding variables that were measured and accounted for when examining this relationship. Regardless of variations, other cross-sectional studies similarly found an inverse association between dietary fibre intake and HOMA-IR(Reference Lau, Faerch and Glumer10, Reference McKeown, Meigs and Liu12, Reference Ylonen, Saloranta and Kronberg-Kippila13). Ylonen et al. (Reference Ylonen, Saloranta and Kronberg-Kippila13) further expanded upon this relationship by also breaking total fibre into soluble and insoluble fibres. Results demonstrated a difference in comparison with the present study, in that both soluble and insoluble fibres were inversely associated with HOMA-IR. Prospective studies also support this relationship, even though different measurements of insulin resistance were utilised(Reference Marshall, Bessesen and Hamman17, Reference Ludwig, Pereira and Kroenke18).
Collectively, these studies not only support the present findings, but also demonstrate the large differences among the confounding variables adjusted for in the examination of this relationship. Of the five studies listed, all controlled for BMI and measured PA using a questionnaire, one accounted for percentage of saturated and polyunsaturated fat, four adjusted for total energy intake and only one accounted for all four mediating variables. The present study also accounted for these variables, but used higher-quality measurement methods. Specifically, PA was measured objectively, and instead of BMI, percentage body fat was controlled statistically. These improvements make the present study unique when compared with previous research examining this relationship.
Comparison of total and soluble fibre intakes of this cohort with other studies reveals similar ingestion. Very few studies have actually made a distinction between soluble and insoluble fibres in the analysis of this relationship. However, one comparable sample of non-diabetic women consumed a median of 17·1 g of total fibre and 4·0 g of soluble fibre(Reference Ylonen, Saloranta and Kronberg-Kippila13). The median for total and soluble fibres of this sample was 18·0 and 3·2 g, respectively(Reference Ylonen, Saloranta and Kronberg-Kippila13).
Strengths of the present study include its large sample size (n 264), measurement of soluble and insoluble fibres in addition to total fibre intake, statistical control of percentage body fat instead of BMI, objective assessment and statistical control of total PA as well as PA intensity and statistical adjustment for differences in total energy intake and dietary fat intake.
The present study was not without weaknesses, however. The cross-sectional design prevents cause-and-effect conclusions to be drawn because of the issue of temporality. Also, because the investigation focused on non-diabetic, middle-aged, non-smokers, and the sample included mostly White, non-Hispanic women, in the strictest sense, generalisation should be limited to women with similar characteristics. Additionally, the subjects' behaviour may have been affected as a result of the weighing and recording of foods eaten as well as wearing of an accelerometer; thus, potentially influencing the conclusions drawn from the data obtained from these time-consuming processes.
The observed results for soluble fibre support the proposed mechanisms by which soluble fibre influences the digestion of carbohydrates. Soluble fibre, especially that with high viscosity, becomes a gelatinous substance in the stomach after consumption, which slows gastric emptying time, food digestion and, therefore, absorption(Reference Jenkins, Axelsen and Kendall44). This delay is achieved through the use of the small intestine as a storage location, which gradually releases glucose into the circulation, and thus, corresponds to a lower insulin response(Reference Jenkins, Axelsen and Kendall44).
Several other mechanisms have been proposed as well. Because fibre is found only in plant foods, there is also the possibility that other plant constituents affect the process by which fibre influences insulin resistance. Mg is one example which has been researched and is suspected to influence insulin resistance(Reference Anderson, Baird and Davis7). As observed in the present study, another factor, body fat, attenuated the relationship between soluble fibre and insulin resistance. In short, a portion of the fibre and insulin resistance relationship can be explained by differences in body fat percentage. Moreover, fibre intake is inversely related to weight gain and obesity(Reference Tucker and Thomas40). Fibre-rich foods promote satiation and satiety and may reduce energy consumption, which over time leads to weight loss or prevention of further weight gain(Reference Burton-Freeman45). Additional research is needed regarding the mechanisms by which fibre, particularly soluble fibre, reduces the risk of insulin resistance.
Experimental studies employing the euglycaemic clamp have been able to find a link between insoluble fibre and insulin sensitivity. The use of different measures of insulin sensitivity between the clamp studies and the present study may account for this difference. In a 3 d intervention among overweight and obese women, insoluble fibre, mainly from cereal fibre, was observed to improve whole-body insulin sensitivity(Reference Weickert, Mohlig and Schofl46). Moreover, Pereira et al. (Reference Pereira, Jacobs and Pins47) compared two diets: whole-grain v. refined-grain, by employing a randomised crossover controlled trial. Significant improvements to insulin sensitivity were observed after consuming the whole-grain diet in comparison with the alternative refined-grain diet. Another study employing similar methodology with the addition of a glucose tracer found a significant increase in peripheral insulin sensitivity after consuming a high-carbohydrate, high-fibre diet(Reference Fukagawa, Anderson and Hageman48). Unfortunately, no distinction between soluble and insoluble fibres was made.
In summary, the relationship between fibre intake, particularly soluble fibre, and insulin resistance appears meaningful. However, the insoluble fibre and HOMA-IR association is weak. Total fibre also appears to be a good predictor of insulin sensitivity, but the association appears to be mostly a function of soluble fibre intake. A moderate portion of the soluble fibre and insulin resistance relationship appears to be a result of differences in body fat and PA intensity, as well as energy intake and dietary fat consumption. However, independent from these factors, soluble fibre remains a good predictor of lower levels of insulin resistance.
In conclusion, the literature contains dozens of investigations showing that dietary fibre tends to reduce insulin resistance. The vast majority of these studies, however, have not used high-quality measurement methods when accounting for potential mediating factors. Future researchers will need to be careful to isolate the effects of fibre intake on insulin sensitivity, since body fat, intensity of PA and other dietary factors can influence the relationship between fibre intake and insulin resistance
Acknowledgements
The present study has been funded internally by the Brigham Young University. C. B. B. and L. T. have no conflicts of interest. C. B. B. and L. T. designed the research; L. T. organised and supervised data collection; L. T. analysed data; C. B. B. and L. T. wrote the paper. C. B. B. and L. T. had primary responsibility for final content. All authors read and approved the final manuscript.







