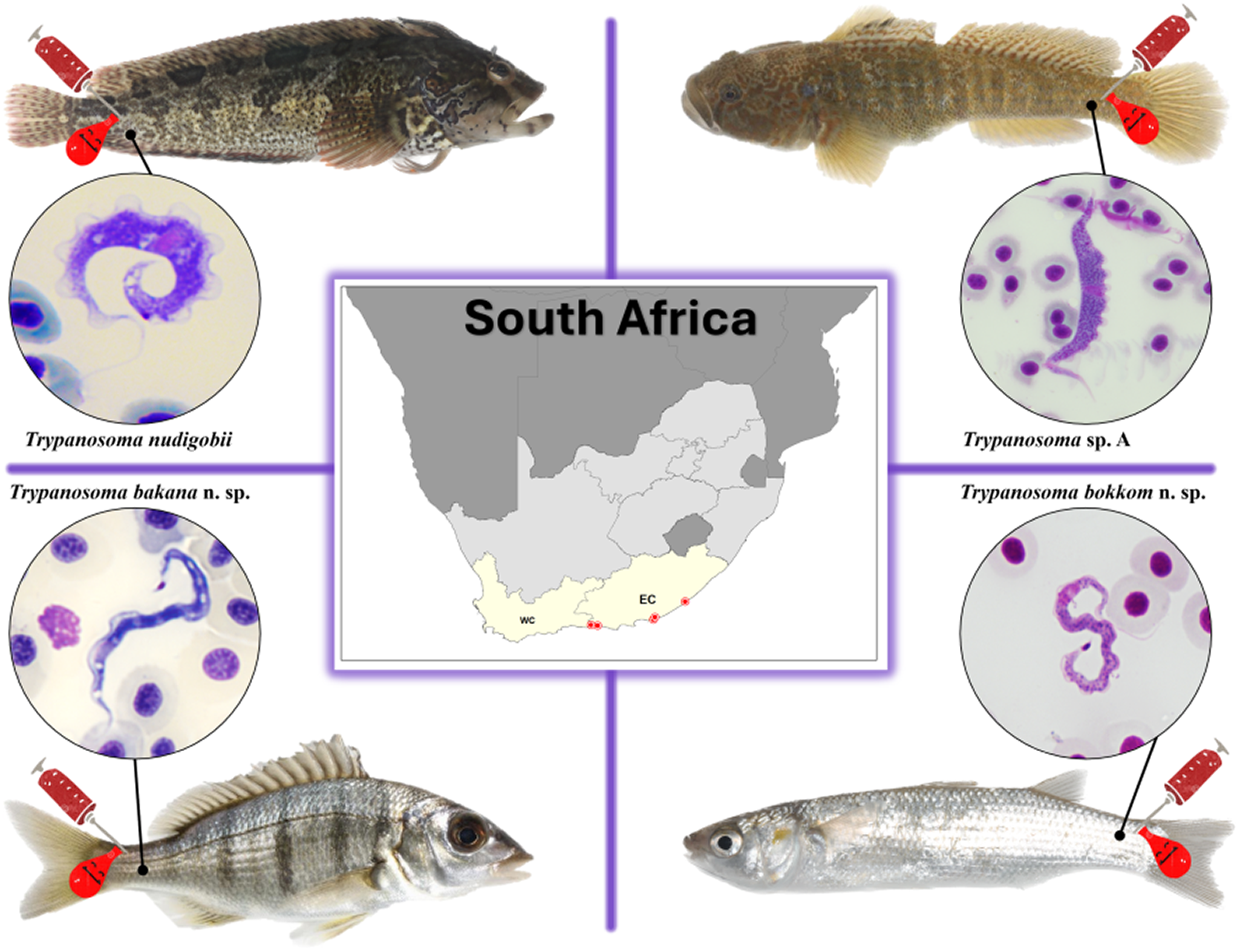
Introduction
Marine trypanosomes are single-celled eukaryotic organisms belonging to the phylum Euglenozoa (Kinetoplastida). Often overshadowed by their terrestrial counterparts, such as the well-known Trypanosoma brucei (Plimmer and Bradford, 1899), which causes human sleeping sickness, marine trypanosomes have recently emerged as an intriguing topic of scientific inquiry (Hoare, Reference Hoare1972; Karlsbakk et al., Reference Karlsbakk, Haugen and Nylund2005; Hayes et al., Reference Hayes, Smit, Seddon, Wertheim and Davies2006, Reference Hayes, Lawton, Smit, Gibson and Davies2014; Yeld and Smit, Reference Yeld and Smit2006; Su et al., Reference Su, Feng, Jiang, Guo, Liu and Xu2014; Pretorius et al., Reference Pretorius, Smit, Schaeffner and Cook2021). These parasites have been documented in various marine environments worldwide, ranging from coastal regions to the open ocean (Burreson, Reference Burreson2007; Hayes et al., Reference Hayes, Lawton, Smit, Gibson and Davies2014). Understanding their ecological roles and potential impact on marine ecosystems is essential to advance our knowledge of marine parasitology and ecosystem health. Although most trypanosome life cycles are unknown, leeches have been identified as the invertebrate hosts or vectors for some marine and freshwater fish trypanosomes (Hayes et al., Reference Hayes, Lawton, Smit, Gibson and Davies2014; Smit et al., Reference Smit, Joubert, Lawton, Hayes and Cook2020). Leal et al. (Reference Leal, O’dwyer, Ribeiro, Silva, Ferreira and Rodrigues2009) proposed that the dissonance in the characterization of species of Trypanosoma Gruby, 1843 is due to the extreme polymorphism that may be found in a single species, as well as individual species being able to infect numerous vertebrate and invertebrate host species. Additionally, by restudying trypanosomes using fresh material from type hosts and utilizing modern methods such as molecular data, additional inferences on species identification can be obtained. This approach may help infer likely synonymies of species described in different hosts or geographical regions (Leal et al., Reference Leal, O’dwyer, Ribeiro, Silva, Ferreira and Rodrigues2009; Hayes et al., Reference Hayes, Lawton, Smit, Gibson and Davies2014). The inclusion of non-human trypanosome molecular data in phylogentic analyses is important for better understanding of evolutionary and ecological relationships of all the host species infected with trypanosomes. The most significant division in the phylogenetic relationships of non-human trypanosomes is between the species infecting terrestrial and aquaticvertebrates. Based on phylogenetic evidence of 18S rRNA, amphibian and fish trypanosomes are the origin from which all other trypanosomes are derived (Hamilton et al., Reference Hamilton, Gibson and Stevens2007; Fermino et al., Reference Fermino, Paiva, Viola, Rodrigues, Garcia, Campaner, Takata, Sheferaw, Kisakye, Kato and Jared2019). Despite the value of the division of the clades, the utilization of subgenera (see Kostygov et al., Reference Kostygov, Karnkowska, Votýpka, Tashyreva, Maciszewski, Yurchenko and Lukeš2021) has been largely disregarded, possibly due to the expectation of conflicts between morphology- and phylogeny-based systems that did not complement each other. The subgenus Haematomonas (Mitrophanow, Reference Mitrophanow1883 emend. Votýpka and Kostygov, 2021) comprises leech-transmitted parasites that infect aquatic vertebrates, and its classification is based on phylogenetic analyses using the 18S rRNA gene region. Mitrophanow (Reference Mitrophanow1883) was the first to describe 2 monoflagellates from freshwater fish, placing them in the genus Haemotomonas (Haematomonas cobitis Mitrofanov, and Haematomonas carassii Mitrofanov, 1972), but Doflein (Reference Doflein1901) later reassigned them to Trypanosoma. Following Mitrophanow (Reference Mitrophanow1883)’s description of the first fish trypanosomes, more than 190 trypanosome species have been described from both marine and freshwater aquatic hosts including amphibians, teleost, elasmobranchs, crocodilians, turtles and the platypus (Woo, Reference Woo2006). Of these, 29 are known from marine teleost fish and 12 from elasmobranchs, of which only 2 have been reported from the coast of South Africa. The first record of marine trypanosomes from South Africa was by Fantham (Reference Fantham1919), who described 2 new species: Trypanosoma capigobii (Fantham, Reference Fantham1919) and Trypanosoma nudigobii (Fantham, Reference Fantham1919), from the heart blood of a barehead goby, Caffrogobius nudiceps (Valenciennes) at Kalk Bay on the southern coast. Later, Trypanosoma blenniclini (Fantham, Reference Fantham1930) was recorded from the blenniid, Parablennius cornutus (Linnaeus) and the clinid Blennophis anguillaris (Valenciennes). Fantham (Reference Fantham1930) also reported larger forms of T. capigobii from Ca. nudiceps collected close to Kalk Bay, at St James. However, following a redescription and molecular analysis of the trypanosomes of the Blenniidae and Clinidae, Hayes et al. (Reference Hayes, Lawton, Smit, Gibson and Davies2014) proposed that the 3 species described by Fantham (Reference Fantham1919, Reference Fantham1930) are a single pleomorphic species, retaining the name T. nudigobii and considering T. capigobii and T. blenniclini as junior synonyms. The second species of marine trypanosome from South Africa is Trypanosoma haploblephari (Yeld and Smit, Reference Yeld and Smit2006), originally described from the dark shyshark, Haploblepharus pictus (Müller and Henle) and the puffadder shyshark, Haploblepharus edwardsii (Schinz) from False Bay and Granger Bay, and more recently molecularly characterized from H. pictus and the leopard catshark, Poroderma pantherinum (Smith), also from Granger Bay (Yeld and Smit, Reference Yeld and Smit2006; Pretorius et al., Reference Pretorius, Smit, Schaeffner and Cook2021). South Africa is known for hosting more than 1900 marine fishes, including many endemic species (Smit and Hadfield, Reference Smit and Hadfield2015). Surprisingly, as mentioned above, only 2 trypanosomes have been described across this diverse range of fish hosts. Thus, the current study aimed to increase the known diversity of marine fish trypanosomes by collecting and screening blood from different species of teleost hosts from selected locations along the south coast of South Africa. All trypanosomes found were described using morphological and morphometrical characterization and molecular analyses.
Materials and methods
Collection of research specimens
Nearshore fish species, mainly of the family Sparidae, were collected using rod and line fishing; intertidal species of the Clinidae and Gobiidae were caught using baited traps; and estuarine species of the Gobiidae and Mugilidae were collected using sein and cast nets. Fish collections took place on the south coast of South Africa at the coastal towns of Chintsa East (32°50ʹ12″S, 28°7ʹ1″E), Kenton-on-Sea (Kariega River Estuary) (33°36ʹ32″S, 26°39ʹ16″E) and Boknes (33°43ʹ32″S, 26°34ʹ60″E) (Figure 1, Table 1). Sampling was also done at 2 sites in the Garden Route National Park, Tsitsikamma Storms River Mouth (34°1ʹ15″S, 23°52ʹ43″E) and in the Groot River West Estuary, Natures Valley (33°58ʹ49″S, 23°34ʹ2″E) (Figure 1, Table 1). Following collection, fishes were identified using the keys in Smith’s Sea Fishes (Smith and Heemstra, Reference Smith and Heemstra2003). As mullet species are particularly difficult to identify on morphology alone, DNA from fin clips of each sampled specimen was barcoded using the Cytochrome c oxidase subunit I marker following Thieme et al. (Reference Thieme, Bogorodsky, Alpermann, Whitfield, Freitas and Durand2022), and resulting sequences were compared to available sequences in GenBank using the Basic Local Alignment Search Tool (BLAST, https://blast.ncbi.nlm.nih.gov/Blast.cgi). The fish used in this study formed part of a larger project on the parasites of South African marine fishes (see Vermaak et al., Reference Vermaak, Kudlai, Yong and Smit2023), wherein fish were humanely killed using the Standard Operating Procedure (SOP no. NWU-00267-17-A5) of blunt force trauma followed by cervical transection. A maximum of 0.4 ml of blood was drawn from the caudal vein using a hypodermic needle (Ethics nos. NWU-00372-16-A5, NWU-01265-23-A9). Thin blood smears were prepared. Each blood smear was air-dried, then fixed with absolute methanol and allowed to air-dry and subsequently stained for 20 min in a 10% dilution of Giemsa stain (Sigma-Aldrich, Steinheim, Germany). For genetic analysis, the remainder of the blood drawn was preserved in a 2-mL microtube containing 96% molecular-grade ethanol (Pretorius et al., Reference Pretorius, Smit, Schaeffner and Cook2021).
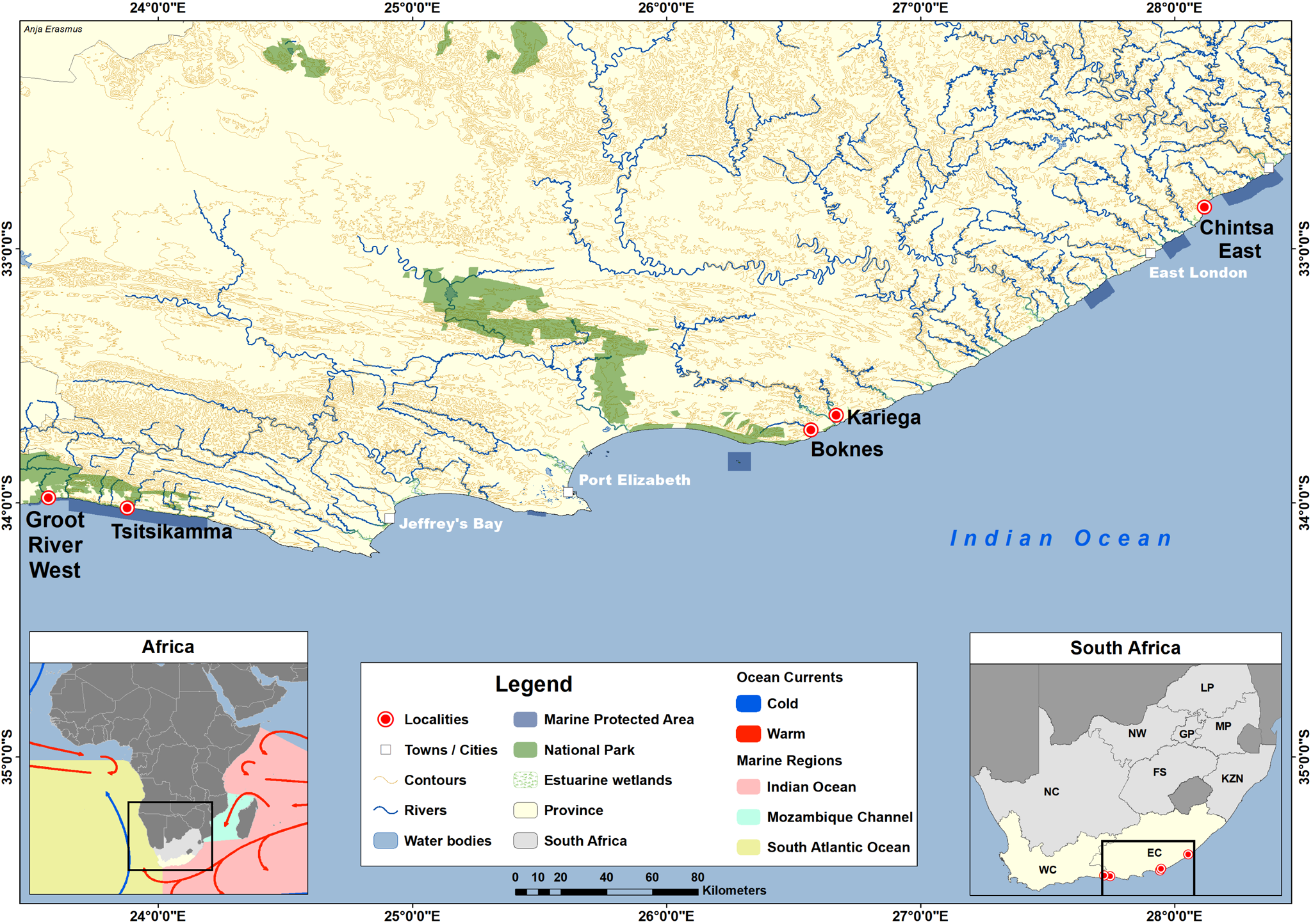
Figure 1. Map showing the sampling locations. Chintsa East, Tsitsikamma Storms River Mouth, Boknes, Kariega River Estuary and Groot River West Estuary.
Table 1. Study sites on the south coast of South Africa with year of collection, details of the fish species samples, number and size of collected fishes, as well as the prevalence of trypanosome infections
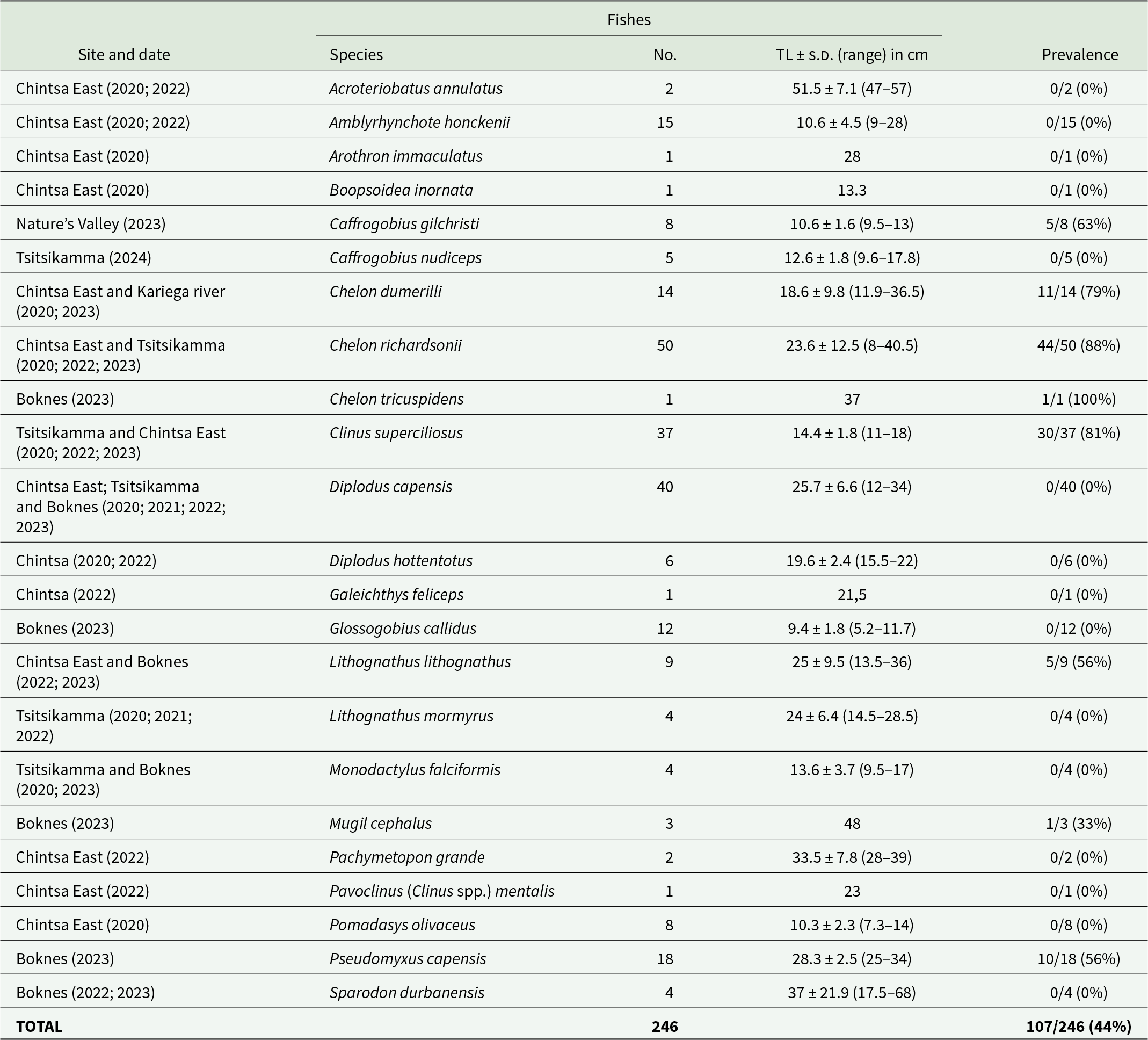
No., number of fishes sampled; TL, total length; s.d., standard deviation.
Morphological characterization
Giemsa-stained blood smears were screened for blood parasites using a Nikon Eclipse Ni microscope (Nikon, Amsterdam, Netherlands) at 100–400× magnification and immersion oil was added for photography and detailed study at 1000× magnification. When trypanosomes were observed, they were examined, photographed and measured with a fixed digital camera (DS-Fi3) using the NIS-Elements BR Ver. 4.60 camera analysis software (Nikon, Tokyo, Japan). Measurements included the distance from the mid-nucleus to the posterior tip of the flagellate (MP), the distance from the mid-nucleus to the anterior end (MA), the nuclear length (NL) and the distance from the posterior tip of the flagellate to the kinetoplast (PK) following Hayes et al. (Reference Hayes, Lawton, Smit, Gibson and Davies2014) and Pretorius et al. (Reference Pretorius, Smit, Schaeffner and Cook2021) (Table 2). Additionally, the nuclear index (NI) was calculated by dividing MP by MA (Dias and de Freitas, Reference Dias and de Freitas1943). Body width, with the undulating membrane [BW(UM)] and at the nucleus [BW(N)], flagellum length (FL) and total body length (TBL) were measured following Pretorius et al. (Reference Pretorius, Smit, Schaeffner and Cook2021). Measurements are presented in micrometres (μm) as range (mean ± standard deviation). The aim was to measure at least 20 trypanosome specimens per host species, however, if less than 20 in total were on the slides, all of them were measured.
Table 2. Morphometrics of all the trypanosomes collected from various host fishes at the study sites
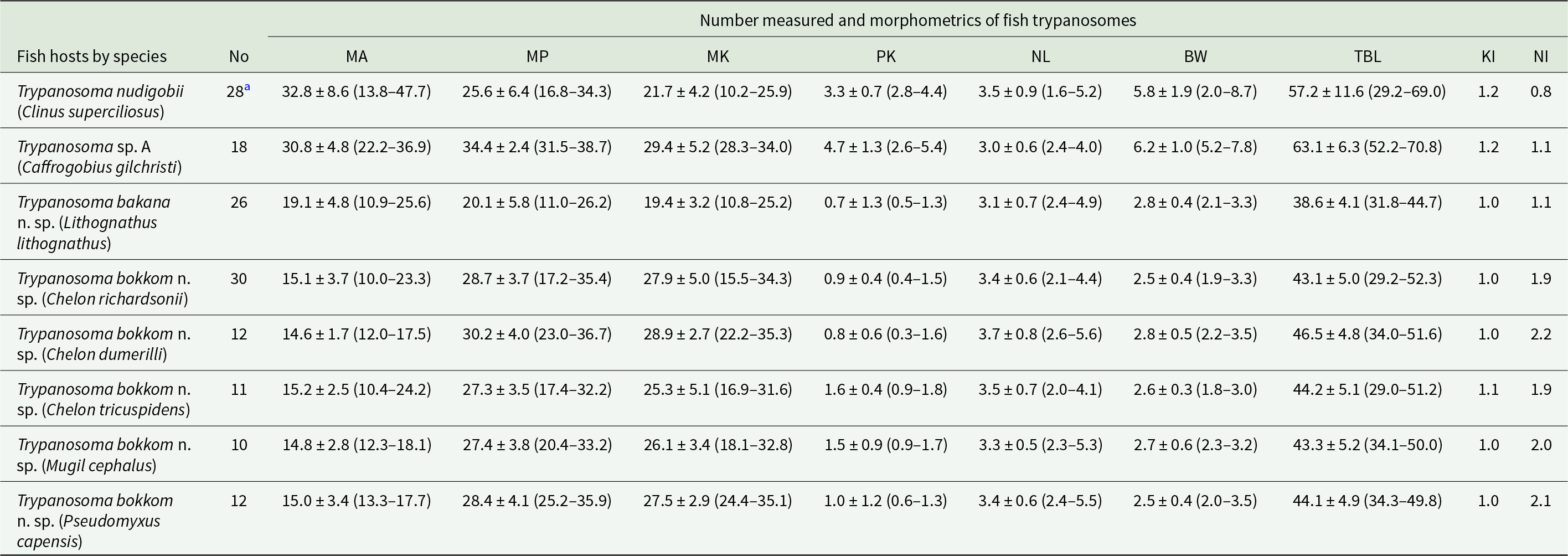
a Include specimens from fish collected at Tsitsikamma and Chintsa East.
No., number measured; MA, mid-nucleus to anterior region; MP, mid-nucleus to posterior region; MK, mid-nucleus to kinetoplast; PK, posterior region to kinetoplast; BW, body width; TBL, total body length; nuclear index (NI) = MP/MA; kinetoplast index (KI) = MP/MK).
A non-metric multidimensional scaling (nMDS) biplot (PRIMER v7) was constructed to illustrate the correlation of trypanosome measurements across different species. The data matrix was compiled, with rows representing species and columns representing quantitative measurements. The stress value indicates whether the data were compressed or manipulated to show a specific result. Stress values close to 1 suggest a reduction in plotting accuracy, while values closer to 0 demonstrate that the dataset was not compressed or manipulated. A distance matrix was calculated from this data using Euclidean metric to quantify dissimilarities. The nMDS analysis utilized the distance matrix to reduce dimensionality, typically to 2 dimensions for visualization. The coordinates from the nMDS output were extracted and plotted on a scatter plot to generate the biplot. This visualization showed the spatial relationships between species based on their measurements, allowing for the assessment of similarities and differences. Additionally, features or vectors were highlighted to indicate the correlation of specific measurements with the dimensions of the nMDS plot, and the stress value was evaluated to ensure the adequacy of the dimensional reduction. A stress value below 0.10 is ideal, showing a reliable representation, while values between 0.10 and 0.20 are acceptable but may have some distortion. Values above 0.20 suggest a poor fit, indicating that the nMDS may not adequately capture the data’s relationships.
DNA extraction, amplification and sequencing
Whole blood of hosts with trypanosome-positive blood smears were used for DNA extraction using the KAPA Express Extract Kit (Kapa Biosystems, Cape Town, South Africa) following the manufacturer’s instructions specific for animal blood. The 18S rRNA gene was selected as the target because it is widely used for classifying ectotherm trypanosomes and has the highest number of reference sequences currently available of species in the genus Trypanosoma (Jordaan et al., Reference Jordaan, du Preez and Netherlands2023). For the polymerase chain reaction (PCR), the resulting supernatant obtained from the DNA extraction served as the template. PCRs were carried out in final volumes of 25 μL. The reaction mixture consisted of 12.5 μL of Thermo Scientific DreamTaq PCR master mix (2 ×) (2 × DreamTaq buffer, 0.4 mM of each dNTP, 4 mM MgCl2), 1.25 μL of each primer (10 μM) and at least 25 ng of DNA. PCR-grade nuclease-free water was used to adjust the final reaction volume. A nested PCR was followed, which utilized primary PCR primers SLF (5’-GCTTGTTTCAAGGACTTAGC-3’) and S762.2 (5’-GACTTTTGCTTCCTCTAATG-3’) and for the secondary PCR, 2 rounds were conducted using different primer sets, namely B (5’-CGAACAACTGCCCTATCAGC-3’) and I (5’-GACTACAATGGTCTCTAATC-3’), and S825 (5’-ACCGTTTCGGCTTTTGTTGG-3’) and SLIR (5’-ACATTGTAGTGCGCGTGTC-3’). The thermal cycling conditions were as follows: for the primary PCR, an initial single cycle (×1) of 95°C denaturation for 5 min, 50°C annealing for 2 min, 72°C extension for 4 min; followed by 35 cycles of 94°C denaturation for 30 sec, 52°C annealing for 30 sec, 72°C extension for 2 min 20 sec, followed by a final 72°C extension for 7 min. For the secondary PCR (B & I), the same thermal profile was followed, but with an annealing temperature of 60°C instead of 52°C. Finally with the last set of primers (S825 & SLIR) an initial single cycle (×1) of 95°C annealing for 3 min; followed by 35 cycles of 95°C denaturation for 30 sec, 57°C annealing for 30 sec, 72°C extension for 1 min, followed by a final 72°C extension for 7 min. To verify the presence of DNA amplicons, visualization was done using a 1% agarose gel electrophoresis. Positive PCR products were sent to Inqaba Biotechnical Industries (Pty) Ltd for purification and sequencing.
Phylogenetic analysis
Sequence data for each isolate were assembled, and chromatogram-based contigs were generated and trimmed (1278–1506 bp) using Geneious Prime® 2024.0.7 (Kearse et al., Reference Kearse, Moir, Wilson, Stones-Havas, Cheung, Sturrock, Buxton, Cooper, Markowitz, Duran, Thierer, Ashton, Meintjes and Drummond2012). To identify the closest congeners to the sequences obtained in the present study, the sequences were subjected to a BLAST search (Altschul et al., Reference Altschul, Gish, Miller, Myers and Lipman1990) followed by an alignment with congeners using the MUSCLE (Edgar 2004) alignment tool in Geneious Prime®. Relevant information regarding the country in which each Trypanosoma has been found, the host it was recorded in and an accession number for each trypanosome, respectively are provided in Table 3. The uncorrected p-distance and number of base differences per site between sequences were calculated under the ‘pairwise deletion’ option using MEGA11 (Supplementary Table 1) (Tamura et al., Reference Tamura, Stecher and Kumar2021). This analysis included 83 unique nucleotide sequences and the most suitable nucleotide substitution model (GTR + I + G) was determined using jModelTest (Guindon and Gascuel, Reference Guindon and Gascuel2003; Darriba et al., Reference Darriba, Taboada, Doallo and Posada2012). A Bayesian Inference (BI) analysis of the alignment (2564 bp including gaps) was done using MrBayes 3.2.7 (Ronquist et al., Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012) through the online computational resource CIPRES (Miller et al., Reference Miller, Pfeiffer and Schwartz2010) with parameters with 4 category Gamma distribution. Markov Chain Monte Carlo chains were run for 10 000 000 generations, sampling every 100 generations. The ‘burn-in’ parameter was set at 25% discarded. A Maximum Likelihood (ML) analysis was implemented using PhyML 3.0 (Guindon et al., Reference Guindon, Dufayard, Lefort, Anisimova, Hordijk and Gascuel2010), and 1000 rapid bootstrap inferences were run on the ATGC Montpellier Bioinformatics Platform (available from http://www.atgc-montpellier.fr/phyml/, Guindon et al., Reference Guindon, Dufayard, Lefort, Anisimova, Hordijk and Gascuel2010). Phylogenetic trees obtained from the respective analyses were visualized using FigTree v. 1.4.4 (Rambaut, Reference Rambaut2012).
Table 3. List of marine Trypanosoma species used in the phylogenetic analyses of this study, with associated host, country, references and GenBank accession numbers
Results
General observations of trypanosomes in the fish blood
A total of 246 fishes belonging to 23 species in 9 families were collected and screened for trypanosomes. Individuals of 8 of the 23 species were infected with trypanosomes (Table 1). The infected species were the klipfish [Clinus superciliosus) (Linnaeus)], prison goby [Caffrogobius gilchristi (Boulenger)], white steenbras [Lithognathus lithognathus (Cuvier)], South African mullet [Chelon richardsonii (Smith)], grooved mullet [Chelon dumerili (Steindachner)], striped mullet [Chelon tricuspidens (Smith)], flathead grey mullet (Mugil cephalus Linnaeus) and freshwater mullet [Pseudomyxus capensis (Valenciennes)]. All 8 infected species had unidentified leeches in their mouth or on their gills that could potentially serve as vectors of these trypanosomes.
Description and diagnosis of stages found in the fish blood
Diagnosis
Phylum: Euglenozoa Cavalier-Smith, 1981
Class: Kinetoplastea Honigberg, 1963, emend. Vickerman, 1976
Subclass: Metakinetoplastina Vickerman, 2004
Order: Trypanosomatida Kent, 1880
Family: Trypanosomatidae Doflein, 1951
Genus: Trypanosoma Gruby, 1843
Trypanosoma nudigobii Fantham 1919
Host from the present study: Clinus superciliosus (Linnaeus)
Other hosts: Caffrogobius nudiceps (type host), Blennophis anguillaris, Clinus agilis, Clinus cottoides, Clinus taurus, Parablennius cornutus (Hayes et al., Reference Hayes, Lawton, Smit, Gibson and Davies2014).
Localities from the present study: Chintsa East, Eastern Cape, South Africa (32°50ʹ12″S, 28°7ʹ1″E) and Tsitsikamma Storms River Mouth, Eastern Cape, South Africa (34°1ʹ15″S, 23°52ʹ43″E).
Other localities: Kalk Bay (type locality), St. James (Fantham, Reference Fantham1919), Tsitsikamma and Koppie Alleen (Hayes et al., Reference Hayes, Lawton, Smit, Gibson and Davies2014).
Site of infection: Peripheral blood
Vector: The leech, Zeylanicobdella arugamensis Silva (see Hayes et al., Reference Hayes, Smit, Seddon, Wertheim and Davies2006, Reference Hayes, Lawton, Smit, Gibson and Davies2014)
Present study
Voucher material: Three peripheral blood smears deposited in the parasite collection of the National Museum, Bloemfontein, South Africa, under accession numbers NMB P 1085, NMB P 1086 and NMB P 1087.
Representative DNA sequences: The sequence data specifically associated with T. nudigobii have been submitted to GenBank: nuclear 18S rRNA (nu 18S) seven partial sequences PV344731 to PV344737.
Morphology: Twenty-eight trypanosomes were measured: total body length 57.2 ± 11.6 (29.2–69.0); body width 5.8 ± 1.9 (2.0–8.7); nucleus length 3.5 ± 0.9 (1.6–5.2); nucleus width 1.4 ± 0.4 (1.0–2.2); mid-nucleus-to-anterior-body-end distance 32.8 ± 8.6 (13.8–47.7); mid-nucleus-to-posterior-body-end distance 25.6 ± 6.4 (16.8–34.3); undulating membrane width 2.2 ± 0.8 (1.2–4.5); number of undulations 15.7 ± 2.1 (13–18) and kinetoplast width 0.9 ± 0.1 (0.7–1.2). Free flagellum length 7.3 ± 1.6 (4.5–9.7). Body index 10.4 ± 2.1 (7.4–14.3); nucleus index 0.8 ± 0.2 and nucleus position as a percentage 56.7 ± 7.1 (47.3–69.2%) (Table 2). The body stains bluish purple in colour with uniform density (Figure 2A–F). The undulating membrane stains lighter purple with a colourless and transparent outer edge. The nucleus stains light pink and is oval-shaped, extending the width of the body. It is positioned parallel to the body and present in the posterior half. The kinetoplast is distinct, stains dark purple in colour and is typically positioned close to the posterior end, on the edge of the body and posterior to the undulating membrane. The flagellum is visible in most of the individuals. The individuals are mostly positioned in a curly manner with the posterior and anterior ends bent.
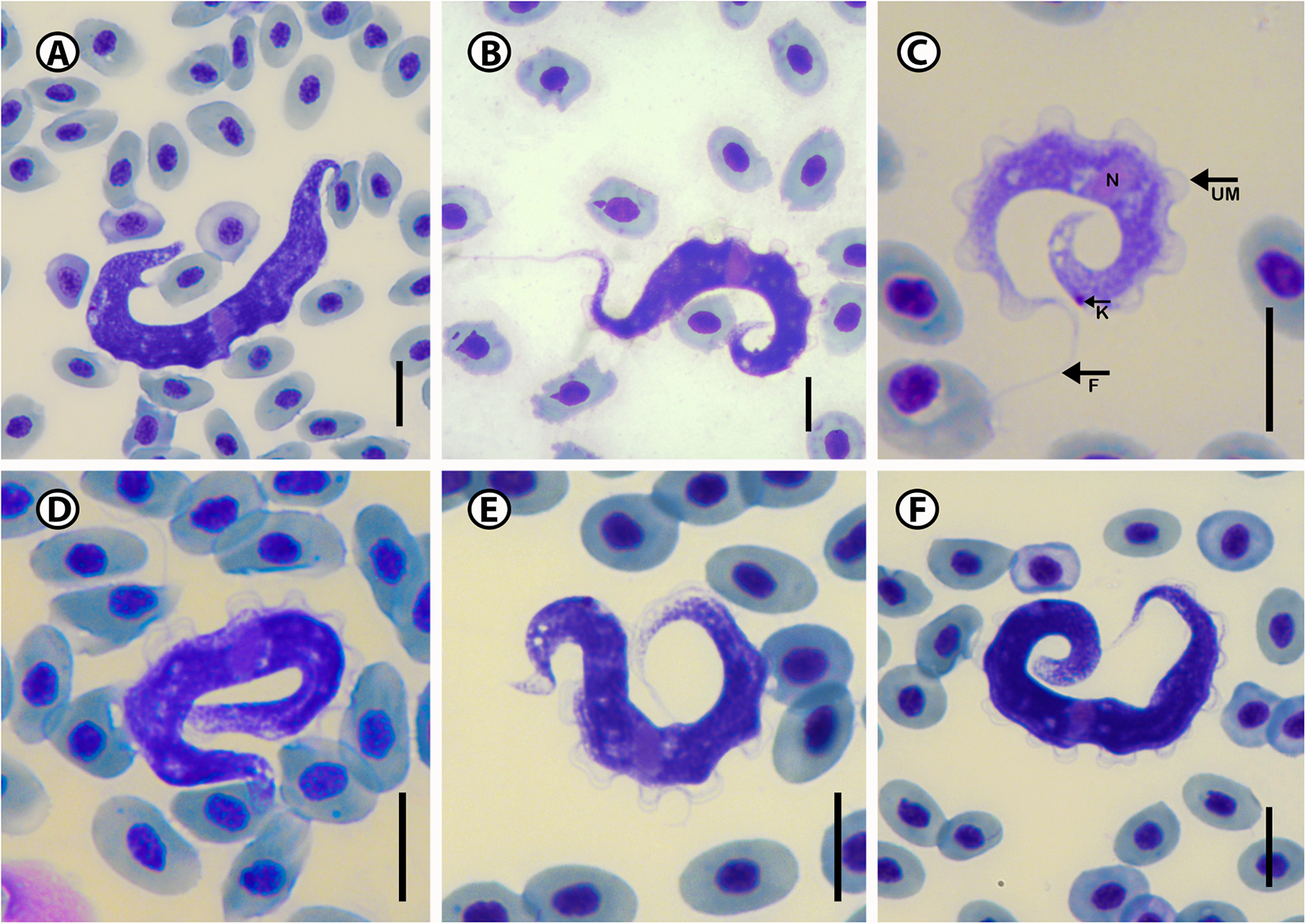
Figure 2. Photomicrographs (A–F) of Trypanosoma nudigobii (Fantham, Reference Fantham1919) from Clinus superciliosus (Linnaeus). Scale bar = 10 µm. N, nucleus; UM, undulating membrane; K, kinetoplast; F, free flagellum.
Remarks
Consequently, and due to the identical nucleotide sequences at both locations, we are clearly dealing with the same species of trypanosome from both these localities and the same host species. The present trypanosome from Cl. superciliosus at both sites agrees with the original morphological description of T. nudigobii by Fantham (Reference Fantham1930) as well as the redescription provided by Hayes et al. (Reference Hayes, Lawton, Smit, Gibson and Davies2014). The latter is expected as it also includes specimens from Cl. superciliosus collected from Tsitsikamma. In general, it shares common features such as a long, slender body, a free flagellum and an undulating membrane. Although the T. nudigobii described by Fantham (Reference Fantham1930) is larger in size, our specimens are proportionality relative to the dimensions presented in the redescription of this species by Hayes et al. (Reference Hayes, Lawton, Smit, Gibson and Davies2014) (73.3 ± 11.0 vs. 57.2 ± 11.6). Our trypanosomes are the same length as the small trypanosomes (25.2–46.3) redescribed from Clinus cottoides Valenciennes, but not as large as the big trypanosomes (55.1–97.7) from Cl. supercilious reported in Hayes et al. (Reference Hayes, Lawton, Smit, Gibson and Davies2014) (Figure 2A and D). Free flagella, especially in large form, were challenging to stain, with some trypanosomes, especially the large ones, appearing to be without a free flagellum (Figure 2A). However, when comparing the body width and nucleus length and orientation, it’s the same as the description by Hayes et al. (Reference Hayes, Lawton, Smit, Gibson and Davies2014), the posterior region appeared thicker than the anterior, and both extremities were attenuated, often reflexed, or curled. The kinetoplast, referred to as the parabasal body by Fantham (Reference Fantham1930), is consistently positioned away from the posterior end with long PK regions (6–9 µm for T. blenniclini, and 10 µm for T. capigobii). This is one of the differences between the originally described T. nudigobii and the current redescription by Hayes et al. (Reference Hayes, Lawton, Smit, Gibson and Davies2014). Additionally, the posterior ends of the trypanosomes did not taper and were rounded. The undulating membrane was generally well developed (Figure 2B and C), with larger trypanosomes exhibiting 8–10 waves close to the body. The nucleus of normal trypomastigote forms of T. nudigobii, as described by Fantham (Reference Fantham1930), is rounded or oval. Parasitaemia in the blood of Cl. superciliosus was relatively high, a similar finding to that of Hayes et al. (Reference Hayes, Lawton, Smit, Gibson and Davies2014), with 5–6 trypanosome individuals per slide.
Molecular characterization
DNA was extracted from 10 samples of infected fish. Following PCR, 7 samples were positive with the correct amplicons, yielding a success rate of 70%. All the trypanosome sequences were from the host Cl. superciliosus, on which the morphological characterization was based. The sequences generated from this host were 99–100% identical to each other. According to GenBank, the best match for these sequences was Trypanosoma pleoronectidium (Karlsbakk and Nylund, Reference Karlsbakk and Nylund2006) with a similarity of 97.97%, and Trypanosoma rajae (Kefil and Grellier, 2018) with a similarity of 97.95%.
Description of Trypanosoma sp. A
Host: Caffrogobius gilchristi (Boulenger) (syn. Gobius gilchristi)
Voucher material: Three peripheral blood smear deposited in the parasite collection of the National Museum, Bloemfontein, South Africa, under accession number NMB P 1088, NMB P 1089 and NMB P 1990.
Locality: Groot River West Estuary, Natures Valley, Eastern Cape, South Africa (34°1ʹ15″S, 23°52ʹ43″E).
Site of infection: Peripheral blood
Vector: Unknown
Representative DNA sequences: The sequence data associated with Trypanosoma sp. A have been submitted to GenBank: nuclear 18S rRNA (nu 18S) partial sequence PV344729 and PV344730.
Description
Eighteen trypanosomes were measured, yielding the following results: total body length 63.1 ± 6.3 (52.2–70.8); body width 6.2 ± 1.0 (7.8–5.2); nucleus length 3.0 ± 0.6 (2.4–4.0); nucleus width 2.2 ± 1.4 (1.2–2.1); mid-nucleus-to-anterior-body-end distance 30.8 ± 4.8 (22.2–36.9) and mid-nucleus-to-posterior-body-end distance 34.4 ± 2.4 (31.5–38.7) and kinetoplast width 1.1 ± 0.5 (0.8–2.3). Body index 10.4 ± 2.3 (8.0–13.1); nucleus index 1.1 ± 0.2 and nucleus position as a percentage 48.6 ± 3.8 (42.4–53.7%) (Table 2). The body stains purple in colour with striae in the cytoplasm of varying density (Figure 3A–F). The undulating membrane stains lighter purple with a colourless and transparent outer edge, which is difficult to visualize in some of the individuals. The nucleus stains light pink and is narrow and elongated. It is typically oriented with its long axis transversely, running across the body of the organism and present in the middle of the body. The kinetoplast has a round form and stains dark purple in colour and is positioned on the posterior end of the body with a kinetoplast index of 1.3 and PK distance of 3.3 ± 0.7 (2.8–4.4). The free flagellum is a short, thin extension of the anterior end and may be absent in some of the specimens.
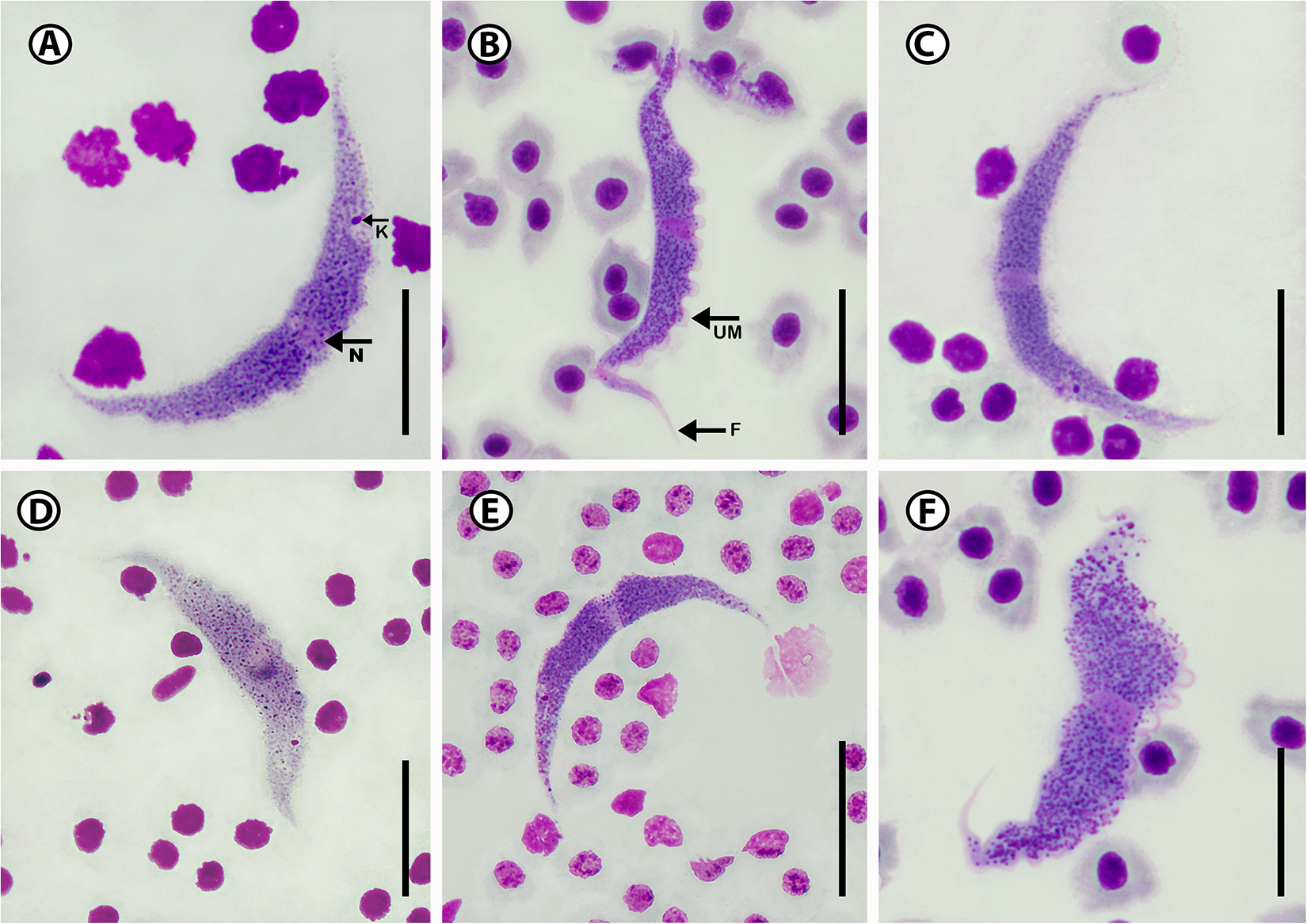
Figure 3. Photomicrographs of Trypanosoma sp. A (A–F) from Caffrogobius gilchristi (Boulenger). Scale bar = 10 µm. N, nucleus; UM, undulating membrane; K, kinetoplast; F, free flagellum.
Remarks
Trypanosoma sp. A is characterized by having a half-moon shape body. Morphologically the closest trypanosome described in relation to Trypanosoma sp. A is the species previously reported and described from the Caffrogobius species in South Africa, namely T. nudigobii and T. capigobii (Table 4). Fantham (Reference Fantham1919) described T. nudigobii from Caffrogobius nudiceps. This species is 60–85 µm long and has a body width of 6.6–7.5 µm. This is similar to Trypanosoma sp. A, with a total body length ranging from 52.2 to 70.8 µm and a body width ranging from 5.2 to 7.8 µm. Trypanosoma capigobii is smaller than Trypanosoma sp. A, with a body length ranging from 42 to 60 µm and body width of 2–4.4 µm. Both Trypanosoma sp. A and T. nudigobii have short flagellums, their membrane is close to their body and the nuclei are oval. However, this species is not similar to T. nudigobii redescribed by Hayes et al. (Reference Hayes, Lawton, Smit, Gibson and Davies2014). Individuals of Trypanosoma sp. A differ from those of T. nudigobii [redescribed by Hayes et al. (Reference Hayes, Lawton, Smit, Gibson and Davies2014)] in their overall elongated body with a small and oval-sized nucleus positioned centrally. Trypanosoma sp. A can also be differentiated from other described marine species by being overall stretched out and half-moon shaped (Figure 3A and E) compared to the curled shape of T. nudigobii (Figure 2C). The undulating membrane is not clearly visible which makes counting of the waves challenging. Further, Trypanosoma sp. A has an overall larger body size than T. nudigobii (63.1 ± 6.3 (52.2–70.8) vs. 57.2 ± 11.6 (29.2–69.0). No discernible difference in stain colour or density between the anterior and posterior regions was observed. Based on the above morphological comparison, it now seems quite possible that this species (Trypanosoma sp. A) represents the true T. nudigobii, and that the synonymizing of T. nudigobii, T. capigobii and T. blenniclini by Hayes et al. (Reference Hayes, Lawton, Smit, Gibson and Davies2014) was erroneous. However, in order to make a conclusive recommendation regarding the synonymy proposed by Hayes et al. (Reference Hayes, Lawton, Smit, Gibson and Davies2014), molecular data from the type hosts and type localities of all the 3 original species described by Fantham (Reference Fantham1919) are needed. In order not to create more confusion regarding these 3 synonymized species by introducing a fourth name, we refrain from naming the species infecting Ca. gilchristi here until such genetic data from these trypanosomes become available. Parasitaemia in the blood of Ca. gilchristi was relatively low, with 3–4 trypanosome individuals per slide.
Table 4. Comprehensive list of all 32 known marine fish Trypanosoma species described location, reference, vectors and measurements where available
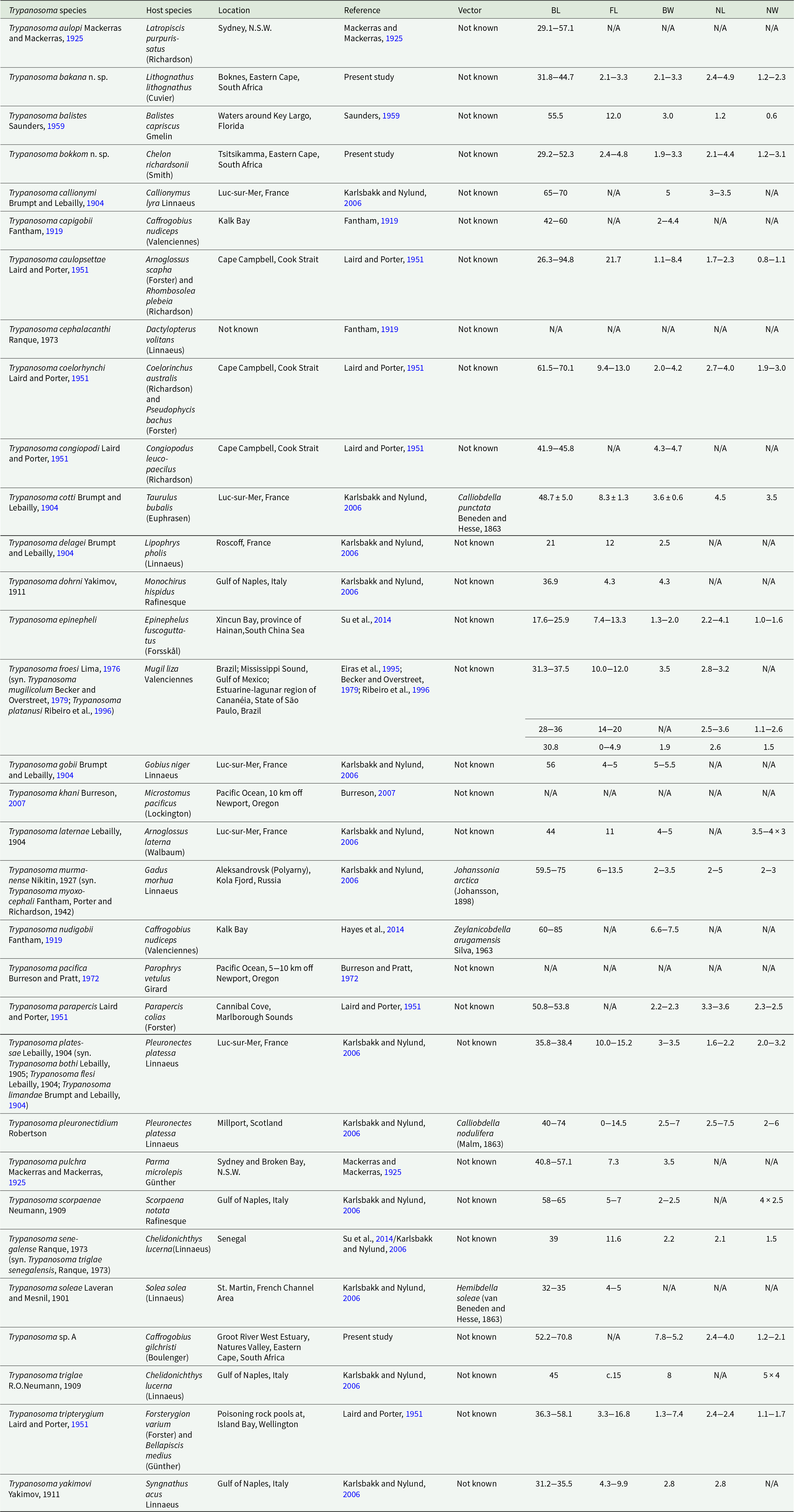
BL, body length; FL, flagellum length; BW, body width; NL, nucleus length; NW, nucleus width.
Molecular characterization
Molecular data were obtained from DNA extracted from 5 samples of infected fish. Of these, 4 samples were positive following PCR for the correct amplicons, resulting in a success rate of 80%. All trypanosome sequences were derived from the host Ca. gilchristi, which was the basis for the morphological characterization. The sequences generated from 2 infected Ca. gilchristi were 100% identical. According to GenBank, the closest match for these sequences was Trypanosoma pleoronectidium (Karlsbakk and Nylund, Reference Karlsbakk and Nylund2006), with a similarity of 97.09%, and Trypanosoma rajae (Kefil and Grellier, 2018), with a similarity of 96.94%.
Description of Trypanosoma bakana n. sp
Type host: Lithognathus lithognathus (Cuvier) (syn. Pagrus lithognathus)
Type material: Hapantotype, 1× peripheral blood smear deposited in the parasite collection of the National Museum, Bloemfontein, South Africa, under accession number NMB P 1079. Other voucher material, 2× peripheral blood smears NMB P 1080 and NMB P 1081. Under Article 73.3.2 of the ICZN.
Type locality: Boknes, Eastern Cape, South Africa (33°43ʹ32″S, 26°34ʹ60″E).
Site of infection: Peripheral blood
Vector: Unknown
Representative DNA sequences: The sequence data specifically associated with T. bakana n. sp. have been submitted to GenBank: nuclear 18S rRNA (nu 18S) partial sequence PV344721.
ZooBank registration: n. sp.: urn:lsid:zoobank.org:act:0808EDA4-84A4-474 F-9AE6-297E66CA9BC5
Etymology: The name ‘Bakana’ is derived from the Khoekhoen language indigenous to the area and means ‘father’s river’. The Bakana river flows into Boknes where the hosts of this trypanosome were collected.
Description
Twenty-six trypanosome specimens were measured: total body length 38.6 ± 4.1 (31.8–44.7); body width 2.8 ± 0.4 (2.1–3.3); nucleus length 3.1 ± 0.7 (2.4–4.9); nucleus width 2.3 ± 1.5 (1.2–2.3); mid-nucleus-to-anterior-body-end distance 19.1 ± 4.8 (10.9–25.6) and mid-nucleus-to-posterior-body-end distance 20.1 ± 5.8 (11.0–26.2); undulating membrane width 2.4 ± 0.6 (1.2–2.8); number of undulations 2.5 ± 0.6 (1–3) and kinetoplast width 1.6 ± 0.9 (0.7–2.5). Free flagellum length 3.7 ± 0.4 (2.1–3.3). Body index 14.2 ± 3.1 (10.8–21.6); nucleus index 1.1 ± 0.6 and nucleus position as a percentage 50.0 ± 13.8 (30.9–69.3%) (Table 2). The body is stained purple in colour with uniform density and has white dots (Figure 4A–F). The undulating membrane stains lighter purple with a darker purple outer edge. The nucleus is oval or round and stains light pink. It is positioned parallel to the body and present in the anterior half. The kinetoplast is distinct, big, stains deep pink in colour and is typically positioned on the posterior end (Figure 4E). The flagellum is short and visible in most of the individuals.
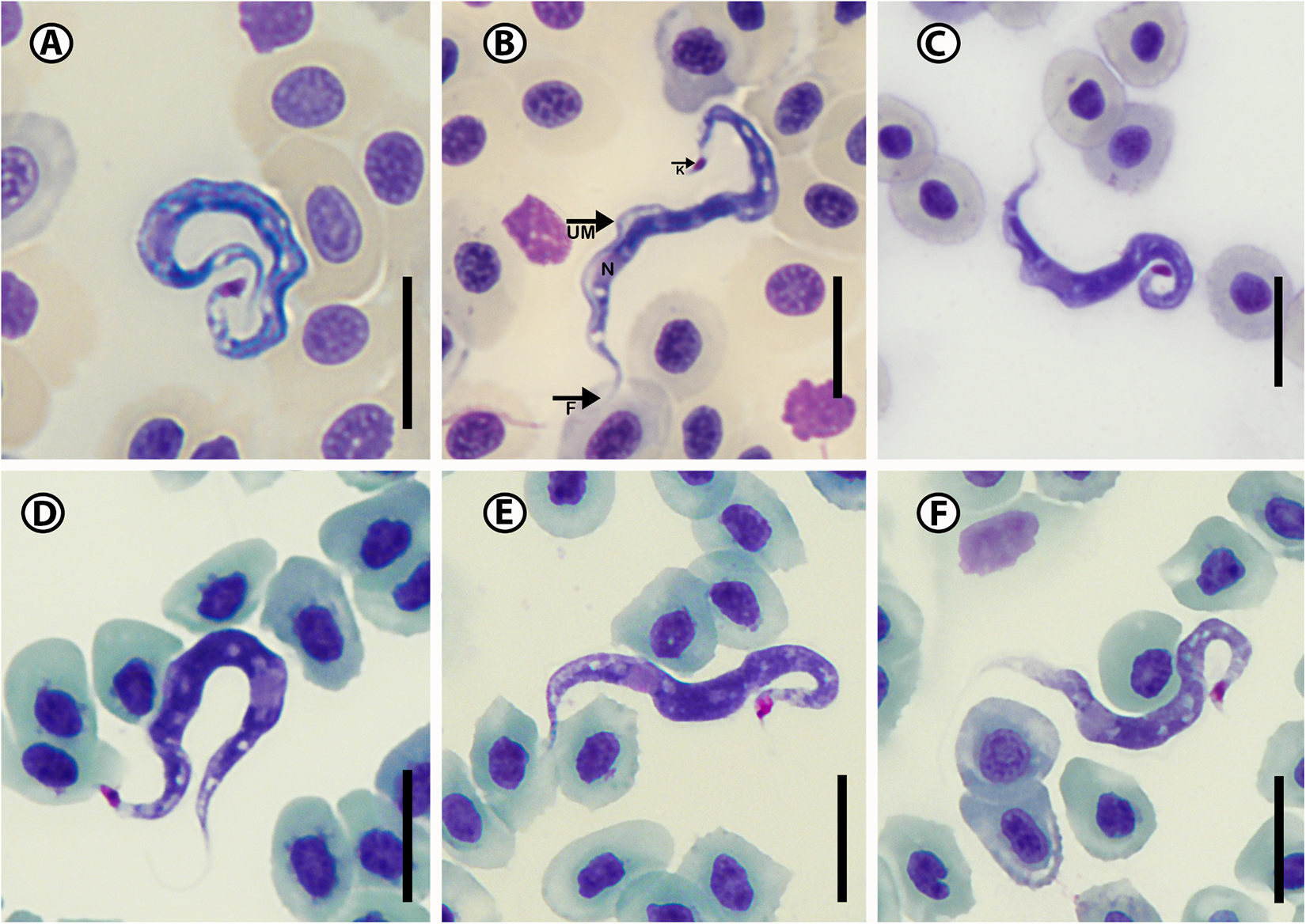
Figure 4. Photomicrographs of Trypanosoma bakana n. sp. (A–F) from Lithognathus lithognathus (Cuvier). Scale bar = 10 µm. N, nucleus; UM, undulating membrane; K, kinetoplast; F, free flagellum.
Remarks
Trypanosoma bakana n. sp. is elongated with an S-shape body (Figure 4B), has a large round or oval nucleus that is positioned towards the anterior end (Figure 4A) and the kinetoplast is located at the tip of the posterior region (Figure 4E). Trypanosoma bakana n. sp. is large and clear in all the images (Figure 4A–F). The posterior region is more likely to present curled while the anterior region does not curl, flagellum is curved (Figure 4D). The undulating membrane composed of 3–4 waves from the anterior region to just past the nucleus, is not as clear as in T. nudigobii but stains light purple and is more visible when compared to that of Trypanosoma sp. A. The posterior region of T. bakana n. sp. is thicker and darker than its anterior region. According to our knowledge, this is the first marine teleost trypanosome described from a host of the Sparidae; however, Fantham (Reference Fantham1919) reported and described a Herpetomonas denticis (Fantham, Reference Fantham1919) from Argyrozona argyrozona (Valenciennes) in South Africa, which could potentially be an early form of a trypanosome. When compared to South African species Trypanosoma bakana n. sp. is overall smaller than both Trypanosoma sp. A and T. nudigobii with a total length of 38.6 ± 4.1 (31.8–44.7). The closest non-South African marine teleost trypanosome species to T. bakana n. sp., based on measurements and an average body length of 39 µm, is Trypanosoma yakimovi (Yakimov, 1911), which was described from the greater pipefish Syngnathus acus Linnaeus in the Gulf of Naples, Italy (Table 4). Trypanosoma yakimovi has a similar nucleus length (2.8 µm) but is smaller in body length (31.2–35.5 µm) compared to T. bakana n. sp. Additionally, T. yakimovi exhibits a curly body shape with a long flagellum (4.3–9.9 µm) and a distinctive undulating membrane. Furthermore, T. yakimovi has a distinctive kinetoplast positioned further away from the posterior end (KI = 1.28) than the kinetoplast of T. bakana n. sp. (see Karlsbakk and Nylund, Reference Karlsbakk and Nylund2006) and has a more centrally positioned nucleus. In contrast, T. bakana n. sp. has a long, S-shaped body with a less distinctive undulating membrane, while its nucleus is located toward the anterior end of the body, and the kinetoplast is large, distinctive and situated at the posterior end (Figure 4). The thickened flagellar end makes T. yakimovi unique among marine fish trypanosomes, but its size and proportions are otherwise similar to T. platessae (Karlsbakk and Nylund, Reference Karlsbakk and Nylund2006). Trypanosoma pulchra (Mackerras and Mackerras, Reference Mackerras and Mackerras1925), described in Sydney, Australia, from the white-ear scalyfin Parma microlepis Günther, is larger in size (40.8–57.1 µm) and has a long free flagellum (7.3 µm), but has a similar body width (3.5 µm) compared to T. bakana n. sp. The nucleus positions differ, and the undulating membrane is more visible than in T. bakana n. sp. Parasitaemia in the blood of L. lithognathus was relatively low, with 5–6 trypanosome individuals per slide.
Molecular characterization
DNA was extracted from the whole blood of 4 infected fish for PCR analysis. Of these, 2 samples tested positive for the correct amplicons, resulting in a success rate of 50%. All the trypanosome sequences originated from the same host specimens, which was used for the morphological characterisation. These sequences showed 97.8% identity with those of Trypanosoma sp. A. The closest matches in GenBank were Trypanosoma pleoronectidium (Karlsbakk and Nylund, Reference Karlsbakk and Nylund2006), with a 97.55% identity, and Trypanosoma murmanense (Karlsbakk and Nylund, Reference Karlsbakk and Nylund2006), with a 97.35% identity.
Description of Trypanosoma bokkom n. sp.
Type host: Chelon richardsonii (Smith) (syn. Mugil richardsonii)
Other hosts: Chelon dumerili (Steindachner), Chelon tricuspidens (Smith), Mugil cephalus Linnaeus and Pseudomyxus capensis (Valenciennes)
Type material: Hapantotype, 1× peripheral blood smear deposited in the parasite collection of the National Museum, Bloemfontein, South Africa, under accession number NMB P 1082. Other voucher material, 2× peripheral blood smears NMB P 1083 and NMB P 1084. Under Article 73.3.2 of the ICZN.
Type locality: Tsitsikamma Storms River Mouth, Eastern Cape, South Africa (34°1ʹ15″S, 23°52ʹ43″E)
Other localities: Chintsa East, Eastern Cape, South Africa (32°50ʹ12″S, 28°7ʹ1″E), Boknes, Eastern Cape, South Africa (33°43ʹ32″ S, 26°34ʹ60″E) and Kariega River, Eastern Cape, South Africa (33°36ʹ32″ S, 26°39ʹ16″E).
Site of infection: Peripheral blood
Vector: No vector data
DNA sequences: The nuclear 18S rRNA (nu 18S) partial sequence data from all the different hosts and localities of T. bokkom n. sp. have been submitted to GenBank: Sequences from trypanosomes from: Ch. richardsonii (PV344723; PV344724), Ch. dumerili (PV344722), Ch. tricuspidens (PV344726), M. cephalus (PV344727) and P. capensis (PV344728).
ZooBank registration: n. sp.: urn:lsid:zoobank.org:act:BDF2BA09-E6AC-4794-91B0-4F85FD22D97C
Etymology: The name ‘bokkom’ refers to whole, salted and dried mullet, a well-known delicacy from South Africa.
Description
In total, 75 specimens were measured from all 5 hosts including 45 specimens infecting the type host. Measurements of specimens from the type host: Total body length 43.1 ± 5.0 (29.2–52.3); body width 2.5 ± 0.4 (1.9–3.3); nucleus length 3.4 ± 0.6 (2.1–4.4); nucleus width 2.5 ± 1.6 (1.2–3.1); mid-nucleus-to-anterior-body-end distance 15.1 ± 3.7 (10.0–23.3) and mid-nucleus-to-posterior-body-end distance 28.7 ± 3.7 (17.2–35.4); undulating membrane width 1.3 ± 0.5 (0.7–1.9); number of undulations 1.8 ± 0.4 (1–10) and kinetoplast width 1.0 ± 0.5 (0.9–1.7). Free flagellum length 3.4 ± 0.6 (2.4–4.8). Body index 17.5 ± 3.2 (13.2–27.7); nucleus index 1.9 ± 0.5 and nucleus position as a percentage 33.9 ± 6.0 (23.9–48.9%) (Table 2). The body stains purple or blue in colour with uniform density (Figure 5A–L). The undulating membrane stains light purple with a colourless and transparent outer edge. The nucleus stains light pink and is stretched. It is positioned parallel to the body and present in the anterior half. The kinetoplast is prominent, large, stains deep pink in colour and is positioned on the posterior end. The flagellum is long (Figure 5A, C, K).
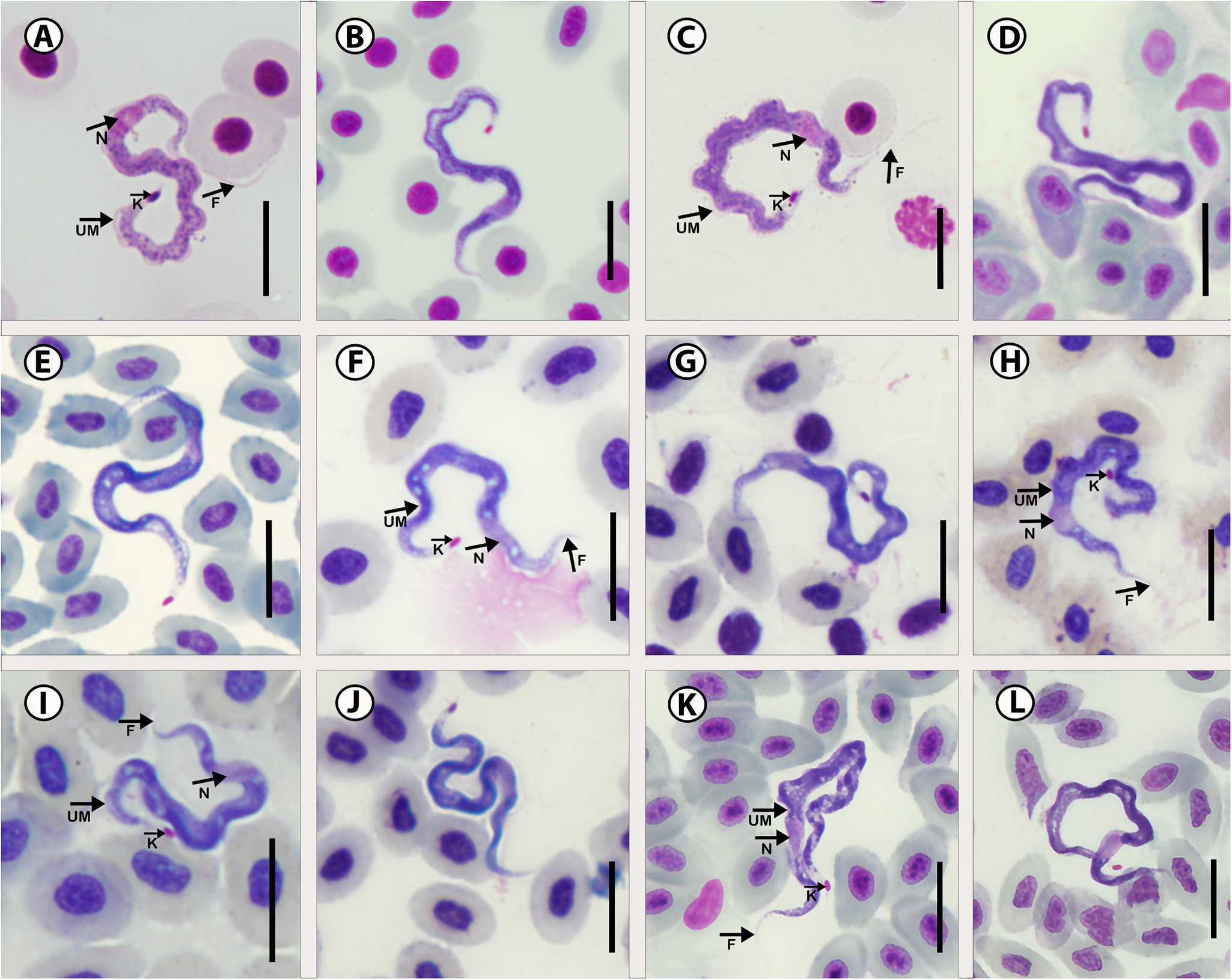
Figure 5. Photomicrographs of Trypanosoma bokkom n. sp. From 5 mullet species. (A–C) Chelon richardsonii (Smith); (D–F) Chelon dumerili (Steindachner); (G,H) Chelon tricuspidens (Smith); (I,J) Mugil cephalus Linnaeus; (K,L) Pseudomyxus capensis (Valenciennes). Scale bar = 10 µm. N, nucleus; UM, undulating membrane; K, kinetoplast; F, free flagellum.
Remarks
Consequently, and due to the identical nucleotide sequences at all the locations, the morphometrics of specimens from these areas were amalgamated in Table 2 as all the trypanosomes of the different mullet species are here considered to be of the same species. Trypanosoma bokkom n. sp. is characterized by having a long, thin and wavy body shape where the anterior and posterior regions tend to curl more than the middle part of the body (Figure 5D). The undulating membrane is not clear and almost absent (Figure 5A and C). The posterior and anterior regions stained lighter with a prominent pink kinetoplast at the very tip of the posterior region. The nucleus is oval or sometimes elongated more in the anterior region and the flagellum is mostly invisible (Figure 5K and L). Trypanosoma bokkom n. sp. is easily distinguishable from T. bakana n. sp., Trypanosoma sp. A and T. nudigobii in the following morphological features. Overall, T. bokkom n. sp. is longer in total body length compared to T. bakana n. sp.; further, T. bokkom n. sp. has a wavy body shape, compared to the half-moon shape of Trypanosoma sp. A. The undulating membrane of T. bokkom n. sp. is not as visible as seen in T. nudigobii. To our knowledge, 3 trypanosome species have been described from mullets of the genus Mugil: Trypanosoma froesi (Lima, Reference Lima1976); Trypanosoma mugilicola (Becker and Overstreet, Reference Becker and Overstreet1979); and Trypanosoma platanusi (Ribeiro et al., Reference Ribeiro, Ranzani-Paiva, Ishikawa, Lopes, Satake, Albuquerque and Carraro1996). Trypanosoma froesi was first recorded by Lima (Reference Lima1976) in Mugil platanus (later changed to Mugil liza Valenciennes) in Brazil. A morphologically similar parasite, T. mugilicola, was described from M. cephalus captured in the Gulf of Mexico by Becker and Overstreet (Reference Becker and Overstreet1979), and T. platanusi, which also resembles T. froesi morphologically, was recorded from M. liza (reported as M. platanus) by Ribeiro et al. (Reference Ribeiro, Ranzani-Paiva, Ishikawa, Lopes, Satake, Albuquerque and Carraro1996) in Brazil. The latter authors were either not aware or did not accept the proposal of Eiras et al. (Reference Eiras, Ranzani-Paiva and Davies1995), who indicated that T. mugilicola and T. platanusi, based on their morphological similarity and with reference to Conroy and Conroy (Reference Conroy and Conroy1984), are the same species as T. froesi. Revisiting the morphology and morphometrics of these 3 species, we agree with Eiras et al. (Reference Eiras, Ranzani-Paiva and Davies1995) and consider T. mugilicola and T. platanusi junior synonyms of Trypanosoma froesi. Trypanosoma bokkom n. sp. can be distinguished from T. froesi in that the latter has a long free flagellum (10.0–12.0 μm) (Table 4), while T. bokkom n. sp. has a shorter flagellum (2.4–4.8 μm), which is more similar in size to the flagellum of T. platanusi (mean 4.87 μm). Furthermore, Trypanosoma bokkom n. sp. differs from T. froesi (reported as T. mugilicola) in the position of the kinetoplast that is at the posterior end in T. bokkom n. sp. and the nucleus that is closer to the anterior end. Other measurements that differ from those of T. bokkom n. sp. include the following from T. froesi (reported as T. platanusi) total body length (27.0–43.5 μm); free flagellum length (2.0–11.8 μm); mid-nucleus to posterior end (17.0–25.0 μm); kinetoplast diameter (0.5–1.2 μm); and kinetoplast to posterior end (1.1–3.0 μm) (see Ribeiro et al., Reference Ribeiro, Ranzani-Paiva, Ishikawa, Lopes, Satake, Albuquerque and Carraro1996). Parasitaemia in the blood of all the parasitized mullet hosts was notably high, with 10–15 trypanosome individuals per slide.
Molecular characterization
DNA was extracted from 30 samples of infected fish for PCR analysis as part of the molecular data collection. Of these, 23 samples were positive for the correct amplicons, yielding a success rate of 77%. To avoid repetition and ensure cost-effectiveness, representatives of each sample from the same host and locality were sent for sequencing. All 6 trypanosome sequences were derived from the same host specimens that served as the basis for the morphological characterization. The sequences obtained from these hosts showed 99.9–100% similarity. The closest matches in GenBank were Trypanosoma pleoronectidium (Karlsbakk and Nylund, Reference Karlsbakk and Nylund2006), with a similarity of 97.08%, and Trypanosoma rajae (Kefil and Grellier, 2018), with a similarity of 96.94% identity.
Swimming behaviour
Another new observation in the description of these above trypanosomes is their swimming behaviour. This behaviour was observed during the screening of a live thick blood smear. The swimming behaviour observed for T. nudigobii during the present study is considered to fall within the intermediate swimming behaviour category as described in Doro et al. (Reference Doro, Jacobs, Hammond, Schipper, Pieters, Carrington, Wiegertjes and Forlenza2019), showing a combination of directional and non-directional movements (see Supplementary Video 1). The swimming behaviour of Trypanosoma sp. A can be defined as being persistent, where parasites travel in a straight line over significant distances (Doro et al., Reference Doro, Jacobs, Hammond, Schipper, Pieters, Carrington, Wiegertjes and Forlenza2019). This represents another clear difference between Trypanosoma sp. A and T. nudigobii, an intermediate swimmer (see Supplementary Video 2). Trypanosoma bakana n. sp. is categorized as a tumbler, with non-directional movement or swimming, travelling no further than their body length (Doro et al., Reference Doro, Jacobs, Hammond, Schipper, Pieters, Carrington, Wiegertjes and Forlenza2019) (see Supplementary Video 3). This swimming behaviour represents an additional clear difference between T. nudigobii, an intermediate swimmer, and Trypanosoma sp. A, a persistent swimmer. According to Doro et al. (Reference Doro, Jacobs, Hammond, Schipper, Pieters, Carrington, Wiegertjes and Forlenza2019), this swimming behaviour is classified as tumbling. Tumbling behaviour in trypanosomes, as detailed by Doro et al. (Reference Doro, Jacobs, Hammond, Schipper, Pieters, Carrington, Wiegertjes and Forlenza2019), is characterized by erratic, non-directional movement where the parasites move only short distances, generally not exceeding their body length (see Supplementary Video 4). This highlights a distinct difference between T. nudigobii, an intermediate swimmer, and Trypanosoma sp. A, which displays persistent swimming behaviour but shares the same tumbling behaviour as T. bakana n. sp. These live appearances of trypanosomes may vary between host individuals. This may potentially also depend on host species, host immune status, nutrients in the blood and stage of trypanosomes and on inapparent plesiomorphy.
Phylogenetic analysis
A final alignment of the 18S rRNA spanning 2581 bp (including gaps) contained 83 unique sequences of various trypanosome species from a range of vertebrate hosts including amphibians, reptiles and freshwater fishes (Table 3). The BI and ML trees obtained during the analyses have similar topologies. Trypanosome species from marine hosts were recovered as sister to all trypanosome species isolated from clawed frogs, freshwater turtles and terrapins from South Africa, a platypus from Australia and freshwater turtles from sub-Saharan Africa. All representative sequences of T. nudigobii form a well-supported clade. The isolate T. nudigobii [KF871790], previously reported by Hayes et al. (Reference Hayes, Lawton, Smit, Gibson and Davies2014) from Cl. superciliosus and Z. arugamensis from Tsitsikamma National Park, nested between 2 subclades from isolates in the current study. The intraspecific genetic divergence calculated as uncorrected p-distances among T. nudigobii isolates ranged from 0.0% to 0.9% and 0–13 bp differences (1316 bp compared; Figure 7; Supplementary Table 1). The 2 sequences obtained of Trypanosoma sp. A were identical and formed a sister taxon to T. bakana n. sp., which is its closest relative. The genetic divergence between Trypanosoma sp. A and T. bakana n. sp. was 1.6%, corresponding to a 21 bp difference (1303 bp compared; see Supplementary Table 1). The relatedness of these 2 species could be due to L. lithognathus and Ca. gilchristi utilizing estuaries as nursery grounds, where the hosts are exposed to similar vectors (Figure 7). This could potentially suggest past host switching, which may explain the close genetic similarity observed between the species. The interspecific divergence between T. nudigobii and T. bakana n. sp. ranged from 2.4% to 3.7%, with 48–52 bp differences (1314 bp compared; see Supplementary Table 1). There were 44–47 bp differences between T. bokkom n. sp. and T. bakana n. sp., with an uncorrected p-distance ranging from 3.1% to 3.5% (1498 bp compared). All 6 isolates of T. bokkom n. sp. formed a sister clade to T. bakana n. sp. and Trypanosoma sp. A (Figure 7). Isolates 1 and 3–6 was identical to each other (see Supplementary Table 1), but isolates 3–5 were not identical to isolate 2 sequenced from Ch. richardsonii from Kariega River, with an uncorrected p-distance of 0.07% and 1 base pair difference. All these mullets occur in the same geographical regions and habitat types, suggesting that they are exposed to similar vectors, explaining the monophyletic clade. Trypanosoma bokkom n. sp. forms part of the marine clade within the aquatic clade and forms a sister clade with T. bakana n. sp. and Trypanosoma sp. A. The sister group to this clade consists of trypanosomes parasitizing marine fish from the Eastern Atlantic [U39580; U39584] and Indo-Pacific [JQ999962] regions.
Discussion
The current study enriched knowledge of marine fish trypanosomes by proposing 4 distinct species from 8 host species of which 3 are new to science (all illustrated in Figure 8). According to Hayes et al. (Reference Hayes, Lawton, Smit, Gibson and Davies2014), T. nudigobii infects a wide range of intertidal fishes (Fantham, Reference Fantham1930; Hayes et al., Reference Hayes, Lawton, Smit, Gibson and Davies2014), with the type host being Caffarogobius nudiceps. In the present study, the Cl. superciliosus was collected inside and outside intertidal zones, from 2 localities and their morphological and molecular characterization revealed that the species parasitizing Cl. superciliosus was morphologically and genetically similar to T. nudigobii. Interestingly Ca. nudiceps (the type host of T. nudigobii) were collected from intertidal pools in Tsitsikamma, but none were found to be infected (see Table 1). This was unexpected, as T. nudigobii was previously reported to infect fish collected by Hayes et al. (Reference Hayes, Lawton, Smit, Gibson and Davies2014) from Tsitsikamma National Park. Furthermore, a congeneric host, Ca. gilchristi were found to be infected with Trypanosoma sp. A, from an estuary, the Groot River West Estuary, which leads us to believe that despite belonging to the same host genus, the difference in habitat exposes hosts to different vectors and consequently to distinct trypanosome species. The third species reported here is T. bakana n. sp. (ex. L. lithognathus) collected from Boknes, and the fourth species, T. bokkom n. sp. collected from 5 mullet species. Morphological and morphometric analyses revealed differences among the 4 trypanosome species, as illustrated in Figure 6. Notably, the trypanosomes in Ca. gilchristi (Trypanosoma sp. A) was the largest. These findings advance our understanding of these parasites, particularly when considering early 20th-century research, which followed a strict host-specificity paradigm. This earlier view suggested that each newly infected host species represented a distinct haemoflagellate species (Lom, Reference Lom, Lumsden and Evans1979; Karlsbakk and Nylund, Reference Karlsbakk and Nylund2006). Recent studies, particularly by Karlsbakk and Nylund (Reference Karlsbakk and Nylund2006), using leech vectors, have shown that a single haemoflagellate species can infect multiple fish species. Furthermore, the concept of host specificity among fish trypanosomes has been further challenged by pairwise genetic distance analyses of 12S rRNA gene sequences from freshwater fish trypanosomes in Europe (Figueroa et al., Reference Figueroa, Mayer, Lom, Dykova, Weller, Peckova and Klein1999), which have failed to support the classification of distinct species for trypanosomes isolated from various host species. The comprehensive review by Karlsbakk and Nylund (Reference Karlsbakk and Nylund2006) on marine fish trypanosomes in the North Atlantic has led to the synonymizing of several species based on consistent size measurements. In some cases, various trypanosome species were delineated within a single host species based solely on minor size variations (Lom, Reference Lom, Lumsden and Evans1979). Karlsbakk and Nylund (Reference Karlsbakk and Nylund2006) emphasize the importance of focusing on individual infections across multiple host species, a practice applied in the case of the 3 novel species described in this study. The nMDS is usually used to show similarity between individuals; however, here differences are shown between the morphometric data of the trypanosome species reported from various hosts. The plot depicted in Figure 6 strongly supports the similarity in morphometrics between individual trypanosomes within a specific host family, but the dissimilarity of trypanosomes between host families reinforces the morphological evidence of 3 distinct species (Dexter et al., Reference Dexter, Rollwagen‐Bollens and Bollens2018).
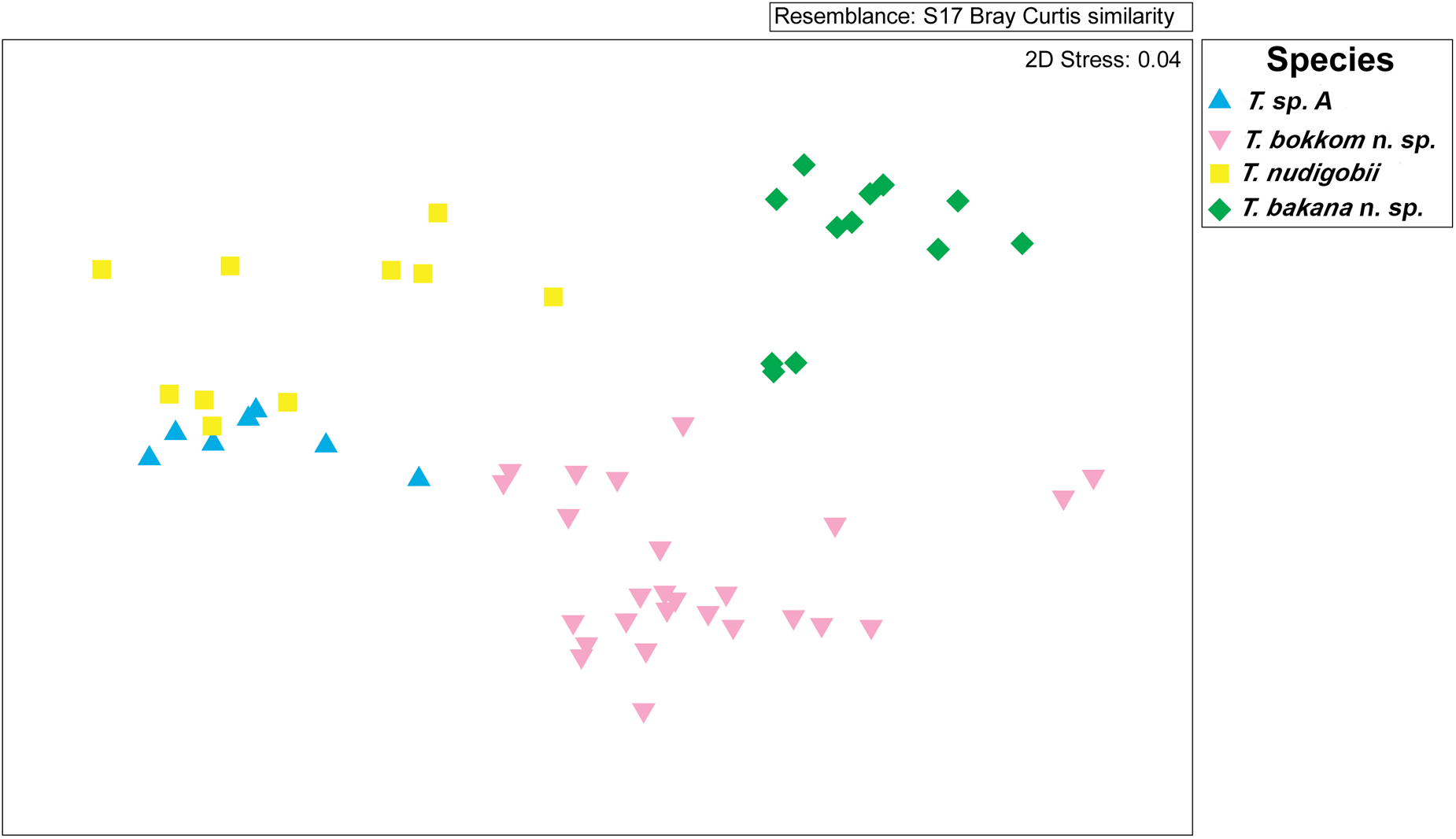
Figure 6. The nMDS biplot indicates the differences in trypanosome measurements across different species.
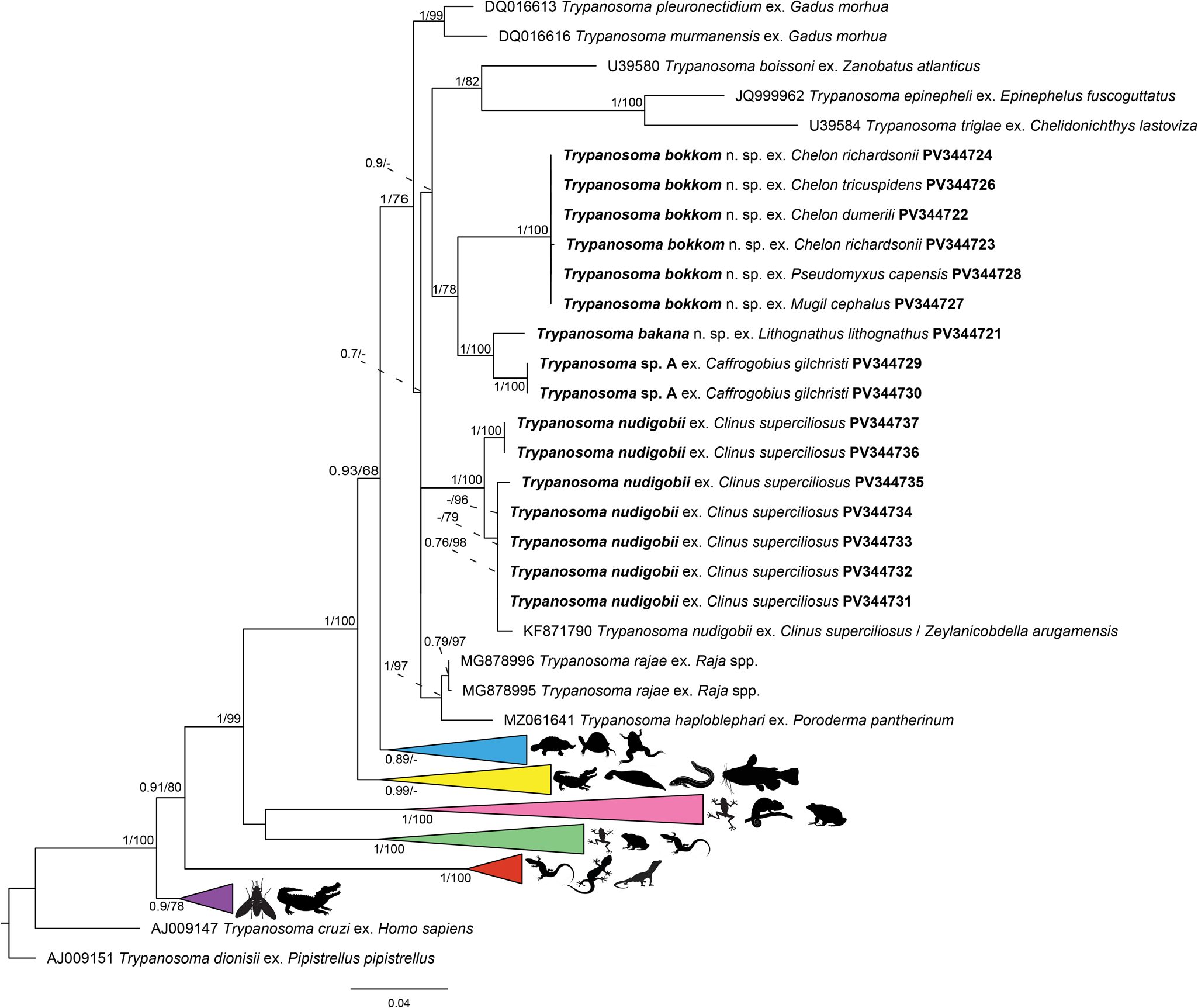
Figure 7. Phylogenetic position of fish trypanosomes from Chintsa East, Tsitsikamma Storms River Mouth, Boknes and Kariega River inferred from the partial 18S rRNA gene region. The outgroups used are Trypanosoma cruzi ex. Homo sapiens and Trypanosoma dionisii ex. Pipistrellus pipistrellus. Pink: Trypanosome sequences from frogs and toads. Green: Trypanosome sequences from frogs, lizards and toads. Black: trypanosome sequences from marine fish. Blue: Trypanosome sequences from clawed frogs, freshwater turtles, platypus and terrapins. Yellow: Trypanosome sequences from caiman, freshwater fish and a freshwater leech. Red: Trypanosome sequences from geckos, lizards and monitors. Purple: Trypanosome sequences from caiman and a tsetse fly.

Figure 8. Line drawings of (A) Trypanosoma sp. A from Caffrogobius gilchristi (Boulenger); (B) Trypanosoma nudigobii from Clinus superciliosus (Linnaeus); (C) Trypanosoma bakana n. sp. From Lithognathus lithognathus (Cuvier); (D) Trypanosoma bokkom n. sp. From Chelon richardsonii (Smith). Scale bar = 10µm.
It is essential to recognize the challenges associated with earlier descriptions, as historical accounts were often brief. However, current knowledge regarding inter- and intraspecific morphological variation among trypanosomes remains incomplete. Variations in fixation, staining, microscopy and measurement tools further complicate these challenges. The morphological description of distinct trypanosome species, or the claim of novel species, remains difficult due to the extensive descriptions based on morphological characterizations across different species and pleomorphism (Kunz, Reference Kunz2002; Hayes et al., Reference Hayes, Lawton, Smit, Gibson and Davies2014; Lemos et al., Reference Lemos, Fermino, Simas-Rodrigues, Hoffmann, Silva, Camargo, Teixeira and Souto-Padrón2015; Zhang et al., Reference Zhang, Liu, Yin, Zhou, Lukeš, Lun and Lai2023). Consequently, researchers have increasingly turned to DNA sequencing to clarify species designations and confidently differentiate between species. Both ML and BI phylogenies define marine fish trypanosomes as a distinct clade (Figure 7). While the overall structure of the ML and BI phylogenetic trees shows some variation, the mullet species are grouped closely in both analyses. All sequences from the present study are part of the marine fish, shark and ray clade. Additionally, the sequences from Trypanosoma sp. A from Ca. gilchristi and T. bakana n. sp. from L. lithognathus were recovered as sister taxa. Each species – Trypanosoma bakana n. sp., Trypanosoma bokkom n. sp. and Trypanosoma sp. A – isolated from L. lithognathus, Ca. gilchristi, and all mullet hosts, respectively, formed their own monophyletic clade. These hosts inhabit estuaries as adults or at some point in their life cycle (Whitfield, Reference Whitfield2023). In contrast, Cl. superciliosus, infected with T. nudigobii, does not inhabit estuaries, suggesting that the vector for this trypanosome species may differ. The majority of sequences from Cl. superciliosus (T. nudigobii) cluster together, with one T. nudigobii sequence from Tsitsikamma and another from Chintsa East clustering below the known sequence of T. nudigobii, showing a bootstrap value of 84%, indicative of high genetic diversity within the host species. However, these sequences are sister to T. nudigobii [GenBank: KF871790], isolated from Cl. superciliosus and the leech Zeylanicobdella arugamensis Silva, 1963. It is important to note that both ML and BI trees exhibit similar branch lengths, with only a few nodes having bootstrap values below 50. This highlights the close relationships among putative trypanosome species infecting marine fish, as evidenced by the 18S rRNA gene region. The combination of morphological and molecular evidence supports the identification of 3 new trypanosome species, indicating a relationship to previously known marine trypanosomes in southern Africa. These findings enhance the knowledge of marine trypanosome diversity within the region.
Understanding the 3 different swimming behaviours described by Doro et al. (Reference Doro, Jacobs, Hammond, Schipper, Pieters, Carrington, Wiegertjes and Forlenza2019) – intermediate swimmers, persistent swimmers and tumblers – adds value to comprehending the dynamics of newly described trypanosome species. While limitations exist, such as the influence of thick blood smears and the timing of screenings, information was still obtained to distinguish between species and warrants further investigation. Modern molecular techniques facilitate the differentiation of closely related or morphologically similar species, and these techniques have also been employed to assess the high levels of pleomorphism found within the same trypanosome species (Pretorius et al., Reference Pretorius, Smit, Schaeffner and Cook2021).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182025000496.
Acknowledgements
The authors are grateful for the assistance of members of the Water Research Group for access to their facilities, as well as to Prof Alan Whitfield, South African Institute for Aquatic Biodiversity, for confirming the identification of the collected goby species. This paper was submitted as part of C le Roux’s MSc degree at the North-West University (supervisor N. J. Smit and co-supervisors C. A. Cook and M. Truter).
Author contributions
C. le Roux and N. J. Smit conceived and designed the study. C. le Roux, N. J. Smit, C. A. Cook and M. Truter conducted data gathering and analyses thereof. C. le Roux, N. J. Smit, C. A. Cook, M. Truter and E. C. Netherlands wrote the article.
Financial support
This work is based on the research supported in part by the National Research Foundation (NRF) of South Africa (PMDS22060819989). Opinions expressed and conclusions arrived at are those of the authors and are not necessarily those of the NRF. MT acknowledges funding from the North-West University (NWU) Postdoctoral fellowship programme and the NRF-Department of Science and Innovation (DSI) Postdoctoral Professional Development Programme (Grant UID 144831).
Competing interests
None.
Ethical standards
This study forms part of an approved North-West University (NWU) Faculty of Natural and Agricultural Research Ethic Committee (FNASREC) project entitled: Biodiversity, systematics and life cycles of freshwater and marine fish trypanosomes in southern Africa (NWU-01265-23-A9) and a NWU Animal Care, Health and Safety in Research Ethics Committee (NWU-AnimCareREC) approved project entitled: Ecology, systematics and evolutionary biology of ectotherm blood parasites (NWU-00372-16-A5). Permit numbers for the collection of materials: SANParks, SMIT-NJ/2023; EDEDEAT, HO/RSH/16/2022; DFE NWU 0-55505; DFE RES2023/26; SANParks, SMIT-NJ/2024 and DAFF, Greyling-RES2022/44, 01/01/2022-31/12/2022.