Acute inflammation, a necessary process of the body's natural response to tissue injury, helps to heal wounds and promote tissue regeneration( Reference Keibel, Singh and Sharma 1 – Reference Warnberg, Gomez-Martinez and Romeo 4 ). A chronic, low-grade inflammatory state results when this process of inflammation is not controlled properly( Reference Warnberg, Gomez-Martinez and Romeo 4 ). Chronic inflammation has been shown to be associated with cancer( Reference Terzić, Grivennikov and Karin 5 , Reference Elinav, Nowarski and Thaiss 6 ) and CVD( Reference Pearson, Mensah and Alexander 7 – Reference Ridker, Rifai and Stampfer 10 ) such as CHD( Reference Danesh, Collins and Appleby 11 ) and myocardial infarction( Reference Ridker, Rifai and Stampfer 10 ). The major inflammatory markers that are implicated in these chronic diseases are IL-6( Reference Ridker, Rifai and Stampfer 10 ), fibrinogen( Reference Danesh, Collins and Appleby 11 ), high-sensitivity C-reactive protein (hs-CRP)( Reference Danesh, Collins and Appleby 11 ) and leucocyte count( Reference Danesh, Collins and Appleby 11 ).
Dietary factors also have been associated with inflammation. The Western-type diet, which is high in red meat, high-fat dairy products and refined grains, is associated with higher levels of CRP, IL-6 and fibrinogen( Reference Johansson-Persson, Ulmius and Cloetens 12 , Reference King, Egan and Geesey 13 ). In contrast, the Mediterranean diet, which is high in whole grains, fruit and green vegetables, and fish and low in red meat and butter, with moderate alcohol consumption and olive oil intake, is associated with lower levels of inflammation( Reference Estruch, Martinez-Gonzalez and Corella 14 ). Diets high in fruit and vegetables are associated with lower levels of CRP( Reference Esmaillzadeh, Kimiaga and Mehrabi 15 ). Specific nutrients also have been consistently shown to be associated with lower levels of inflammation. These include complex carbohydrates( Reference Kitabchi, McDaniel and Wan 16 ), n-3 PUFA( Reference Ferrucci, Cherubini and Bandinelli 17 ), fibre( Reference Ma, Griffith and Chasan-Taber 18 ), moderate alcohol intake( Reference Avellone, Di Garbo and Campisi 19 ), vitamin E( Reference Bertran, Camps and Fernandez-Ballart 20 ), vitamin C( Reference Wannamethee, Lowe and Rumley 21 ), β-carotene( Reference Erlinger, Guallar and Miller 22 ) and Mg( Reference King, Mainous and Geesey 23 ).
The dietary inflammatory index (DII) was developed to provide a means for estimating the overall inflammatory potential of the diet( Reference Shivappa, Steck and Hurley 24 , Reference Cavicchia, Steck and Hurley 25 ). The DII is based on an extensive literature search incorporating cell culture, animal and epidemiological studies of the effect of diet on inflammation. DII scoring is not dependent on subjective evaluation of the diet or recommendations of intake. Because it is based on the literature that links diet to inflammation, the DII is not limited to micronutrients and macronutrients, but also incorporates commonly consumed components of the diet including flavonoids, spices and tea. Previously, the DII has been shown to predict CRP levels( Reference Cavicchia, Steck and Hurley 25 , Reference Shivappa, Steck and Hurley 26 ). Using the Asklepios Study, we tested the hypothesis that higher DII scores, indicating a more pro-inflammatory diet, are associated with increased systemic inflammation, as shown by increased levels of inflammatory markers.
Methods
Study design
Briefly, the Asklepios Study was a longitudinal population-based study conducted in Belgium, with baseline data collected in October 2002. The primary objective of the study was to explore the interplay between ageing, diet, cardiovascular haemodynamics and inflammation. A total of 2524 healthy volunteers aged between 35 and 55 years were recruited. Subjects were randomly sampled from the twinned communities of Erpe-Mere and Nieuwerkerken in Flanders, Belgium. Inclusion criteria were as follows: male or female volunteers aged 35–55 years at study initiation; living in the communities of Erpe-Mere or Nieuwerkerken. Exclusion criteria were as follows: the presence of clinical atherosclerosis; major comorbidity; diabetes mellitus; pregnancy; atrial fibrillation; irregular heart cycle; inability to give informed consent. More details about the inclusion and exclusion criteria used can be found in the methods and baseline characteristics of the Asklepios Study( Reference Rietzschel, De Buyzere and Bekaert 27 ).
For the present study, only baseline data on diet, inflammatory markers and covariates were used to perform a cross-sectional analysis. After excluding thirty-seven participants who did not complete the FFQ, there were 2487 subjects with evaluable data, of whom 1200 were men and 1287 were women. Data on demographic characteristics were obtained using a self-administered questionnaire. Anthropometric measurements and blood samples were collected, and the levels of inflammatory markers were determined( Reference Rietzschel, De Buyzere and Bekaert 27 ). Basic clinical data assessment and routine biochemical assays were performed as described previously( Reference Rietzschel, De Buyzere and Bekaert 27 ). In summary, five markers of inflammation were measured: hs-CRP; leucocyte count; fibrinogen; homocysteine; IL-6.
Dietary intake and dietary inflammatory index
Participants were asked to complete a semi-quantitative FFQ that included questions on their habitual daily consumption of twenty-five food items during the past year( Reference Hoebeeck, Rietzschel and Langlois 28 ). This FFQ was based on an existing FFQ used in this population and on a short FFQ (i.e. sixty items) developed by Willett( Reference Cade, Thompson and Burley 29 , Reference Willett 30 ). Participants were asked to indicate how often they consumed each item in a list of frequencies (every day; 5–6 d/week; 2–4 d/week; 1 d/week; 1–3 times/month; never or less than once a month), and to indicate approximate portion size.
FFQ-derived dietary information was used to calculate DII scores for all of the subjects, as described in detail elsewhere( Reference Shivappa, Steck and Hurley 24 , Reference Cavicchia, Steck and Hurley 25 ). Briefly, dietary data for each study participant were first linked to a regionally representative global database that provided a robust estimate of means and standard deviations for each of the food parameters considered (i.e. foods, nutrients and other food components such as flavonoids)( Reference Shivappa, Steck and Hurley 24 ). A z-score was derived by subtracting the ‘standard global mean’ from the amount reported, and then this value was divided by the standard deviation. To minimise the effect of ‘right skewing’ (a common occurrence with dietary data), this value was then converted to a centred percentile score, which was then multiplied by the respective inflammatory effect score of the food parameters (derived from a literature review and scoring of 1943 ‘qualified’ articles) to obtain the subject's food parameter-specific DII score. All of the food parameter-specific DII scores were then summed to create the overall DII score for each subject in the study. For the current FFQ, data were available for a total of seventeen food parameters (carbohydrate, protein, total fat, fibre, cholesterol, saturated fat, monounsaturated fat, polyunsaturated fat, n-6 fatty acid, thiamin, riboflavin, vitamin B12, Fe, Mg, Zn, vitamin A and vitamin C). A description of the validation work of the DII score, based on both dietary recalls and a structured questionnaire, the 7 d dietary recall that is similar to an FFQ, is available elsewhere( Reference Shivappa, Steck and Hurley 26 ). Thus far, the DII has been found to be associated with inflammatory cytokines, including CRP and IL-6( Reference Shivappa, Steck and Hurley 26 , Reference Wirth, Burch and Shivappa 31 , Reference Wood, Shivappa and Berthon 32 ), the glucose intolerance component of the metabolic syndrome, the increased odds of asthma and FEV1 (reduced forced expiratory volume in 1 min), inflammatory markers in shift workers, and colorectal, prostate and pancreatic cancers( Reference Wirth, Burch and Shivappa 31 – Reference Shivappa, Bosetti and Zucchetto 38 ).
Statistical analyses
All markers of inflammation were analysed as categorical variables using conventional cut-off points. As recommended by the Centers for Disease Control and Prevention (CDC) and the American Heart Association, we dichotomised hs-CRP at the level of 3 mg/l( Reference Pearson, Mensah and Alexander 7 ), categorised homocysteine at the level of 15 μmol/l( Reference Welch and Loscalzo 39 ) and fibrinogen at the level of 4·5 g/l, considering measurements greater than this level as indicative of higher CVD risk. IL-6 was categorised at a detection level of 1·6 pg/ml( Reference Hoebeeck, Rietzschel and Langlois 28 ). As there were no clear cut-off values for leucocyte count, it was not analysed.
All statistical analyses were carried out using the SAS® statistical software package (version 9.3; SAS Institute, Inc.). Comparisons of baseline characteristics by sex were made by χ2 tests for categorical variables and by two-sample t tests for continuous variables. BMI was categorised as normal ( < 25 kg/m2), overweight (25–30 kg/m2) and obese (>30 kg/m2). Physical activity is expressed as metabolic equivalents (METS). Analyses were carried out using multivariable logistic regression, adjusting for energy, age, sex, BMI, smoking status, education level, use of non-steroidal anti-inflammatory drugs, blood pressure, use of oral contraceptives, lipid-lowering drugs, anti-hypertensive therapy and physical activity.
Results
Table 1 shows the baseline characteristics of the study participants and the mean DII scores for both sexes. Women had lower DII scores than did men ( − 1·01 v. 0·90), indicating that women consume a more anti-inflammatory diet than men. Women were more educated, less likely to be obese and more likely to be current smokers compared with men. Women had higher CRP levels; however, other inflammatory markers did not differ by sex. Table 2 presents the distribution of characteristics, various food groups and inflammatory markers across the tertiles of the DII. Tertile 3 had a higher number of current smokers and males than did tertile 1. Participants in tertile 3 had a lower consumption of anti-inflammatory food groups such as vegetables, fish and fruit, and had a higher consumption of pro-inflammatory foods such as sugar-sweetened soft drinks. Participants in tertile 2 had a higher consumption of meat than those in tertile 1; however, participants in tertile 3 had a lower consumption of meat. Participants in tertile 3 had higher levels of IL-6 and homocysteine.
Table 1 Characteristics of the Asklepios Study population and mean dietary inflammatory index (DII) scores (Number of participants and percentages; mean values and standard deviations; medians and interquartile ranges (IQR))

hs-CRP, high-sensitivity C-reactive protein.
* ANOVA was used for continuous variables and χ2 test for categorical variables.
† The sum does not add up to the total because of some missing values.
Table 2 Description of population characteristics across the tertiles of the dietary inflammatory index (Number of participants and percentages*; mean values and standard deviations†)

* Categorical variables.
† Continuous variables.
‡ The t test was used for continuous variables and χ2 test for categorical variables.
Analysis of inflammatory markers as categorical variables
Multivariable-adjusted analysis showed positive associations between the DII and the inflammatory markers IL-6 (OR 1·19, 95 % CI 1·04, 1·36) and homocysteine (OR 1·56, 95 % CI 1·25, 1·94). For each unit increase in the DII, the odds of having IL-6 >1·6 pg/ml and homocysteine >15 μmol/l increased by 19 and 56 %, respectively. The DII was not found to be associated with hs-CRP (>3 mg/l) and fibrinogen (>4·5 g/l) (Table 3).
Table 3 Associations between the dietary inflammatory index and inflammatory markers as categorical variables
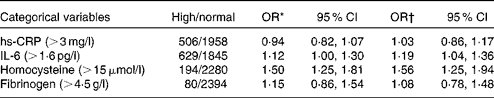
hs-CRP, high-sensitivity C-reactive protein.
* Adjusted for age.
† Adjusted for energy, age, sex, BMI, smoking status, education level, use of non-steroidal anti-inflammatory drugs, blood pressure, use of oral contraceptives, anti-hypertensive therapy, lipid-lowering drugs and physical activity.
Discussion
The results from the present study indicate that a diet with predominantly pro-inflammatory food parameters such as cholesterol and saturated fat, and relatively poor in anti-inflammatory food parameters such as fruit and vegetables, increased inflammation in the study participants as evidenced by the increased levels of IL-6, homocysteine and leucocyte count. Overall, the results of the present study are consistent with the hypothesis that diet modulates inflammation. The inference is that through this process of modulating inflammation, there is an effect on chronic diseases such as several cancers and CVD.
Previous results from the Asklepios Study have shown that adherence to Flemish food-based dietary guidelines results in lower inflammation( Reference Hoebeeck, Rietzschel and Langlois 28 ). The present results are in accordance with the findings from previous studies that have found a relationship between diet and inflammatory markers( Reference Dai, Miller and Bremner 40 – Reference Chrysohoou, Panagiotakos and Pitsavos 44 ).
We found an independent positive association between adherence to the pro-inflammatory diet (increasing DII score) and the inflammatory markers IL-6, and homocysteine, but not CRP and fibrinogen. This is consistent with the observations from previous studies showing that IL-6 is a more sensitive indicator of CVD such as atherosclerosis and cardiovascular risk than are hs-CRP and fibrinogen( Reference Ridker, Rifai and Stampfer 10 , Reference Cesari, Penninx and Newman 45 ). IL-6 promotes atherosclerosis, by stimulating the endothelial synthesis of cellular adhesion molecules, procoagulant effects, and stimulation of hepatic hs-CRP synthesis( Reference Ridker, Rifai and Stampfer 10 , Reference Cesari, Penninx and Newman 45 ). Leucocytosis has consistently been shown to be an independent risk factor and prognostic indicator of future cardiovascular outcomes, regardless of disease status. Mechanisms that link leucocytosis to CHD occur through the mediation of inflammation, resulting in proteolytic and oxidative damage to the endothelial cell that plug the microvasculature, induce hypercoagulability and promote infarct expansion( Reference Madjid, Awan and Willerson 46 ). Hyperhomocysteinaemia is also known to play an important role in the causation of CVD( Reference Refsum, Ueland and Nygård 47 ). The results from animal and in vitro experimental studies have shown blood homocysteine levels to be positively associated with vascular and platelet damage( Reference Refsum, Ueland and Nygård 47 – Reference Harker, Slichter and Scott 49 ). Homocysteine is not a commonly studied inflammatory marker; however, previous research( Reference Oudi, Aouni and Mazigh 50 , Reference Refsum, Ueland and Nygård 51 ) has shown that homocysteine can be considered as an inflammatory marker and higher levels of homocysteine tend to be strongly positively correlated with inflammatory markers related to an increased risk of CVD.
The present study has several limitations. Although the FFQ is typically used to investigate habitual (long-term) dietary intakes in large-scale surveys, its closed structure with limited response options limits its ability to detect between-person variations; this is in contrast to open-ended methods such as food records or 24 h dietary recalls. In addition, the FFQ relies on the respondents' memory and their capabilities to interpret those questions on frequency and quantity of consumption. However, the important strengths of the FFQ are its low respondent burden and cost, and the fact that, at least theoretically, it gives information about the respondents' usual or habitual dietary intakes( Reference Willett 30 ). Another limitation of this design is that no cause–effect relationships can be inferred from these cross-sectional data, and a single measure of diet notably reduces at least the precision (and, probably, the accuracy) of our estimates. Also, it is possible that multiple testing may have resulted in chance associations being declared significant.
In the DII validation study( Reference Shivappa, Steck and Hurley 26 ), sensitivity analysis was conducted to compare DII scores calculated from multiple (up to 15/person) 24 h dietary recalls with those calculated from 7 d dietary recalls (providing data on twenty-eight food parameters). We found that the ability to predict CRP was not attenuated when using the more limited list available with the 7 d dietary recalls( Reference Shivappa, Steck and Hurley 26 ). In the present study, the DII was calculated using the data on just seventeen food parameters derived from the FFQ, the shortest list on which we have published thus far. This could explain the absence of an association between the DII and CRP in the present study. However, despite the large reduction in the number of food parameters, we still were able to predict various inflammatory markers successfully.
Conclusion
Chronic inflammation appears to play a key role in the development of CVD and certain cancers. The results from the present study suggest that eating a diet high in sugar, saturated fat and other pro-inflammatory foods promote inflammation, which may increase the risk of a variety of chronic diseases. The next logical step would be to use the DII to predict CVD outcomes, such as atherosclerosis, and indicators of CVD including intimal thickening, plaque formation and cardiac output in the Asklepios Study.
Acknowledgements
The authors thank the Asklepios Study staff for assistance in the data preparation. The authors acknowledge all the participants from the Asklepios Study who voluntarily contributed to the study.
The Asklepios Study was partly funded by an FWO research grant (G.0427.03). J. R. H. was supported by an Established Investigator Award in Cancer Prevention and Control from the Cancer Training Branch of the US National Cancer Institute (NCI) (K05 CA136975). This research received no other specific grant from any funding agency, commercial or not-for-profit sectors. The FWO and NCI had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: N. S. was involved in the calculation of the DII in the dataset, performed all the analyses and drafted the first version of the manuscript; I. H. helped with the analyses, data acquisition, interpretation of the data, and critical revision of the manuscript; E. R. R., M. L. D. B., M. L., E. D. and A. M. contributed to the data interpretation and drafting of the manuscript; J. R. H. provided expertise and oversight throughout the process. All the authors approved the final version.
All authors declare that there is no conflict of interest.






