Since December 2019, the world has been grappling with escalating cases of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infections leading to previously unknown Coronavirus Disease – COVID-19. By April 15, 2021 – there were nearly 140 million confirmed cases of infection with the SARS-CoV-2 and almost 3 million deaths from COVID-19 (for up-to-date data, see World Health Organization, WHO 2020).
Within a few months of the initial SARS-CoV-2 infections detected in Wuhan, physicians in charge of ill patients observed that the disease involved multiple organs besides the lungs, including the heart, liver, gut, peripheral nerves, and the brain (Yang et al., Reference Yang, Yu, Xu, Shu, Xia, Liu and Shang2020). A retrospective observational case series of 214 consecutive hospitalized patients with laboratory-confirmed diagnosis of SARS-CoV-2 (Mao et al., Reference Mao, Jin, Wang, Hu, Chen, He and Hu2020) showed that neurological involvement was frequent (in 36% of 214 patients and in 45% of those with severe disease vs. 30% in those with non-severe disease). Various cerebrovascular events (e.g., ischemic stroke, intracerebral hemorrhage, cerebral venous sinus thrombosis) are described as the most prominent COVID-19-associated neurological symptoms. This is followed by inflammatory CNS syndromes (e.g., encephalitis, encephalomyelitis). Peripheral neurological disorders (e.g., Guillain–Barré) and variants are less common (Frontera et al., Reference Frontera, Mainali, Fink, Robertson, Schober, Ziai and Study2020; Paterson et al., Reference Paterson, Brown, Benjamin, Nortley, Wiethoff, Bharucha and Zandi2020; Varatharaj et al., Reference Varatharaj, Thomas, Ellul, Davies, Pollak and Tenorio2020). SARS-Cov-2 may change the risk of stroke through an enhanced systemic inflammatory response, hypercoagulable state, and endothelial damage in the cerebrovascular system (Abootalebi et al., Reference Abootalebi, Aertker, Andalibi, Asdaghi, Aykac, Azarpazhooh and Zand2020). Frequent but typically less severe neurological symptoms include headache, dizziness, anosmia, and ageusia (Frontera et al., Reference Frontera, Mainali, Fink, Robertson, Schober, Ziai and Study2020; Helms et al., Reference Helms, Kremer, Merdji, Clere-Jehl, Schenck, Kummerlen and Meziani2020). Anosmia and ageusia are reported even in patients whose presentation is not severe enough to warrant hospital admission or who are otherwise asymptomatic (Gane, Kelly, & Hopkins, Reference Gane, Kelly and Hopkins2020). In some cases, the involvement of the nasal epithelium may only reflect local inflammation. However, trafficking of viral particles and protein, in addition to SARS-CoV-2 RNA to the CNS cannot be excluded (Meinhardt et al., Reference Meinhardt, Radke, Dittmayer, Franz, Thomas, Mothes and Heppner2021).
Across the pool of retrospective studies on COVID-19, new-onset psychosis, affective disorders, altered mental status including agitation, and dysexecutive symptoms have also been reported (Helms et al., Reference Helms, Kremer, Merdji, Clere-Jehl, Schenck, Kummerlen and Meziani2020). Some of these neuropsychiatric symptoms were linked to premorbid status (e.g., dementia), while others represented de novo symptoms (Varatharaj et al., Reference Varatharaj, Thomas, Ellul, Davies, Pollak and Tenorio2020). Among the emerging prospective studies of COVID-19, one key finding is the relatively high prevalence of PTSD, depressive, and anxiety symptoms (Bo et al., Reference Bo, Li, Yang, Wang, Zhang, Cheung and Xiang2020; Xiang et al., Reference Xiang, Yang, Li, Zhang, Zhang, Cheung and Ng2020). While such high prevalence may be associated with pandemic stress and higher anxio-depressive symptoms across the community (Ettman et al., Reference Ettman, Abdalla, Cohen, Sampson, Vivier and Galea2020), the possibility of immune-related or direct SARS-CoV-2 brain impact cannot be excluded at this stage (Troyer, Kohn, & Hong, Reference Troyer, Kohn and Hong2020). PTSD is known to occur in patient groups who undergo severe and critical illness, especially ICU survivors, those who are intubated and mechanically ventilated, and ultimately those that experience delirium (Marra, Pandharipande, & Patel, Reference Marra, Pandharipande and Patel2017). An association between delirium and PTSD has been described recently in COVID-19 (Kaseda & Levine, Reference Kaseda and Levine2020). Depression, anxiety, and PTSD can be associated with various neuropsychological deficits (Marcopulos, Reference Marcopulos, Morgan and Ricker2018), which will complicate the differential diagnosis of long-term neurocognitive effects of COVID-19 (Kaseda, & Levine, Reference Kaseda and Levine2020). Finally, the rate and extent of recovery (chronic effects of COVID-19 on the CNS and the newly recognized “Long-COVID” also newly known as Post-Acute Sequelae of SARS-CoV-2 infection (PASC)), and potential increased risk for long-term neurodegenerative effects and neuropsychological sequelae are yet to be investigated (De Felice, Tovar-Moll, Moll, Munoz, & Ferreira, Reference De Felice, Tovar-Moll, Moll, Munoz and Ferreira2020; Wilson & Jack, Reference Wilson and Jack2020).
SARS-CoV-2 neuropathogenic mechanisms are thought to be multifactorial, including possible direct and indirect effects of the virus in the CNS (Frontera et al., Reference Frontera, Mainali, Fink, Robertson, Schober, Ziai and Study2020; Koralnik & Tyler, Reference Koralnik and Tyler2020). Evidence for the presence of SARS-CoV-2 RNA in the CNS and associated morphological changes (such as thromboembolic ischemic infarction of the CNS), specifically in the brain stem, has been shown (Meinhardt et al., Reference Meinhardt, Radke, Dittmayer, Franz, Thomas, Mothes and Heppner2021). Viral load of 5.0–59.4 copies per cubic millimeter was also reported in the brain sections from the medulla oblongata, the frontal lobes, and olfactory nerves, which is obtained from 16 patients who died with COVID-19 (Solomon et al., Reference Solomon, Normandin, Bhattacharyya, Mukerji, Keller, Ali and Sabeti2020). Inconsistencies in the detection of SARS-CoV-2 in the CNS remain. This may be due to the dynamics of the infection in relation to when samples were obtained, and/or the fact that viral load and neural infectivity have a nonlinear relationship (Yi et al., Reference Yi, Nam, Yun, Gim, Joe, Kim, Kim, Han and Lee2020).
In the acute phase, progressive respiratory involvement can lead to Acute Respiratory Distress Syndrome (ARDS), which is itself associated with a high risk of hypoxia and concomitant cognitive and psychiatric sequelae; this represents as one of the main indirect pathways to brain damage in COVID-19 (Ellul et al., Reference Ellul, Benjamin, Singh, Lant, Michael, Kneen and Solomon2020; von Weyhern, Kaufmann, Neff, & Kremer, Reference von Weyhern, Kaufmann, Neff and Kremer2020; Wu et al., Reference Wu, Xu, Chen, Duan, Hashimoto, Yang and Yang2020). Acute hypoxic injuries were detected in the cerebrum and cerebellum in 18 patients who died with COVID-19, with loss of neurons in the cerebral cortex, hippocampus, and cerebellar Purkinje cell layer (Solomon et al., Reference Solomon, Normandin, Bhattacharyya, Mukerji, Keller, Ali and Sabeti2020).
Severe forms of COVID-19 illness requiring intensive care unit (ICU), intubation, and ventilation may be associated with further immune, inflammatory, and vascular brain damage. Secondary effects such as ICU delirium and possible long-term cognitive disorders are further observed and may be related to CNS invasion, inflammation, other organ failure, and induction of sedatives (Kotfis et al., Reference Kotfis, Williams Roberson, Wilson, Dabrowski, Pun and Ely2020).
The picture, course, and long-term consequences of COVID-19 are modified by many factors. Serious health complications and the death toll from infection are greater among older individuals (>60 years), those with underlying medical conditions (including hypertension, obesity, chronic lung disease, diabetes, and cardiovascular disease). COVID-19 may also have a distinct course and impact in patients with preexisting neurological, psychiatric, and immune conditions including schizophrenia (Fonseca et al., Reference Fonseca, Diniz, Mendonça, Malinowski, Mari and Gadelha2020; Kozloff, Mulsant, Stergiopoulos, & Voineskos, Reference Kozloff, Mulsant, Stergiopoulos and Voineskos2020), mild cognitive impairment, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis (Matías-Guiu et al., Reference Matías-Guiu, Gomez-Pinedo, Montero-Escribano, Gomez-Iglesias, Porta-Etessam and Matias-Guiu2020), and HIV-associated neurocognitive disorder (Levine, Sacktor, & Becker, Reference Levine, Sacktor and Becker2020). Poverty, living in densely populated neighborhoods of lower socioeconomic status, a higher prevalence of comorbid diseases, and poor accessibility to healthcare facilities and services are further risk factors for contracting the virus, as well as negative health outcomes (Bialek et al., Reference Bialek, Bowen, Chow, Curns, Gierke, Hall and Wen2020; Laurencin & McClinton, Reference Laurencin and McClinton2020; Public Health England, 2020; Raifman & Raifman, Reference Raifman and Raifman2020).
The above data indicate that as a result of many pathological factors and mechanisms associated with COVID-19, people recovering from that disease may experience cognitive, emotional, and behavioral problems that require a referral to neuropsychology and/or neuropsychiatry services. It is not known how long these problems may persist, but for a certain number of COVID-19 survivors, it may even be a lifelong impairment, significantly influencing everyday life.
Neuropsychologists have already signaled urgent needs for developing research as well as clinical practice services for COVID-19 survivors (Postal et al., Reference Postal, Bilder, Lanca, Aase, Barisa, Holland and Salinas2021; Sozzi et al., Reference Sozzi, Algeri, Corsano, Crivelli, Daga, Fumagalli and Balconi2020; Wilson, Betteridge, & Fish, Reference Wilson, Betteridge and Fish2020). These studies and mounting evidence from neurological studies (Taquet et al., Reference Taquet, Geddes, Husain, Luciano and Harrison2021) support the hypothesis that COVID-19 may lead to neurocognitive disorders. One study included a sample of over 84,000 individuals who were coincidently participating in another study amid the COVID-19 pandemic (Hampshire et al., Reference Hampshire, Trender, Chamberlain, Jolly, Grant, Patrick and Mehta2020). This UK study revealed that individuals who recovered from suspected or confirmed COVID-19 performed significantly worse on tests in multiple cognitive domains compared to people who did not suffer from COVID-19. This deficit was evident in hospitalized COVID-19 survivors, but also among individuals who did not receive hospital treatment. However, the study had significant methodological limitations in determining what may have been due to COVID-19 versus any other causes of impairment. Zhou et al. (Reference Zhou, Yang, Wang, Hu, Zhang, Zhang and Huang2020) in China, Wuhan, examined cognitive functions (i.e., attention, memory, processing speed, executive functions, and perceptual abilities) in 29 hospitalized patients who recovered from COVID-19 and 29 closely matched controls. They found impairment of sustained attention in the clinical group and a significant relationship between reaction time and inflammatory level as indicated by C-reactive protein.
Almeria, Cejudo, Sotoca, Deus, and Krupinski (Reference Almeria, Cejudo, Sotoca, Deus and Krupinski2020) described cognitive disorders in 35 patients (aged 20–60) with confirmed COVID-19, without any previous neurological or psychiatric diseases. The patients were examined in-person, for 10–31 days after hospital discharge, using a set of standardized neuropsychological tests. Individuals presenting with headache, anosmia, dysgeusia, diarrhea, and those who required oxygen therapy had lower scores in memory, attention, and executive function tests as compared to asymptomatic patients. Marked disorders (scores 2 SD below appropriate norms, controlling for age and education) were noted in the domains of memory, attention, and semantic fluency [in 2 patients (5.7%)], in working memory and mental flexibility [3 (8.6%)], and phonetic fluency [4 (11.4%)]. Anxiety and depression indicators were significantly related to subjective cognitive complaints.
Finally, an Australian study (Darley et al., Reference Darley, Dore, Cysique, Wilhelm, Andresen, Tonga and Masters2020) conducted in a community sample (only 10% hospitalized) found a low rate of neurocognitive impairment (9%) 2 months after recovering from COVID-19 illness on the Cogstate Test Battery measuring visual learning, speed of processing, attention/working memory, and executive functions. However, 24% showed impairment on the NIH Toolbox Odor Identification test, and this was associated with neurocognitive impairment. Further, there was an association between moderate-to-severe initial neurological symptoms and continued subtle neurocognitive changes. Because this is a prospective study, it will be important to assess how these results evolve on longitudinal testing.
In response to the urgent needs associated with possible neuropsychological consequences of COVID-19, we formed the NeuroCOVID International Neuropsychology Taskforce in April 2020, with the goal of developing recommendations for harmonized standard neuropsychological methods and procedures/protocols to determine the prevalence, pattern, and incidence of neurological and neuropsychological symptoms associated with COVID-19 in adults. The use of similar, harmonized assessment methods will help to combine data on COVID-19 from different sources. As of April 2021, the group has 107 members from 19 countries (see Figure 1).
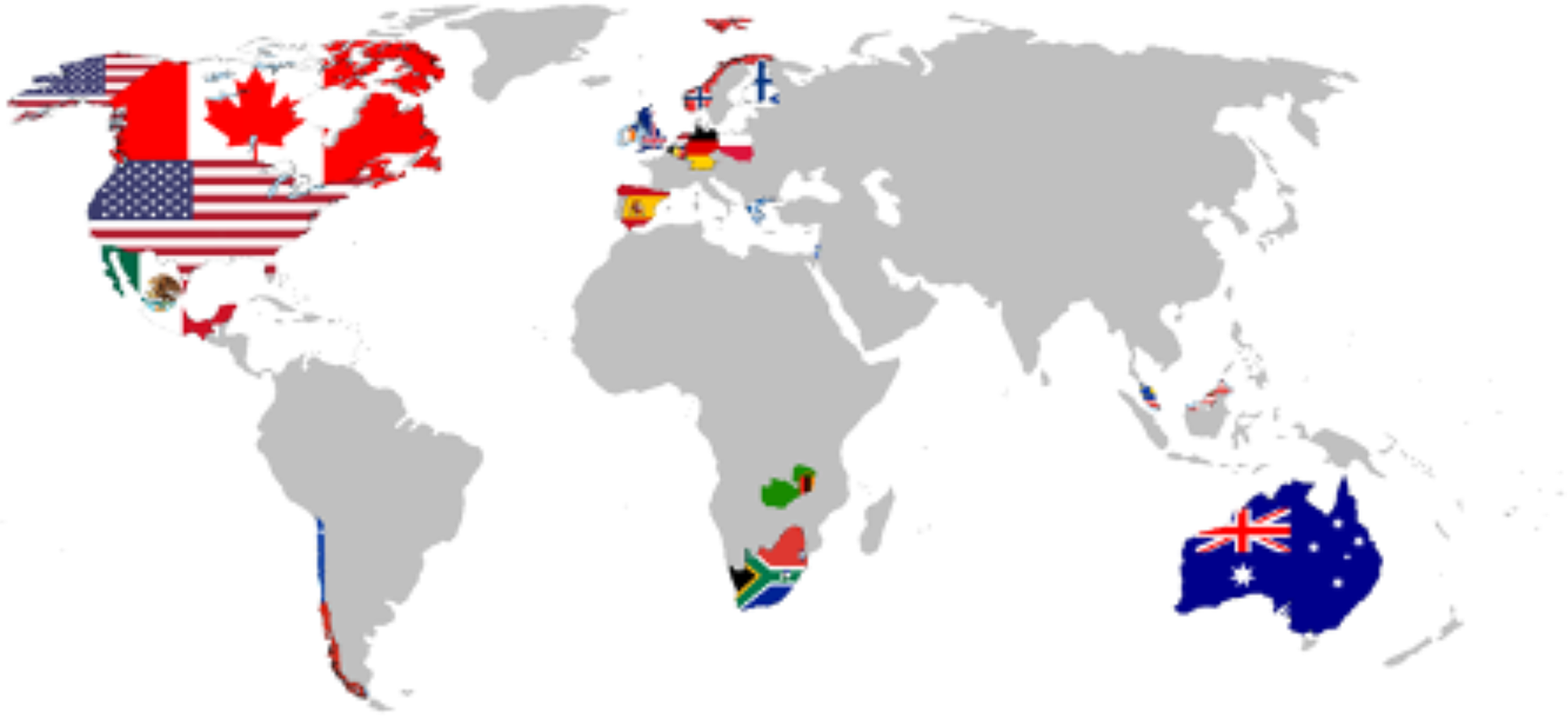
Fig. 1. The Taskforce international representation.
Taskforce includes 107 members from the following countries: USA (52 Members), Australia (15), Poland (7), Canada (5), Netherlands (5), South Africa (4), UK (4), Spain (2), Belgium (2), Norway (2), Chile (1), Finland (1), Germany (1), Greece (1), Israel (1), Malaysia (1), Mexico (1), Zambia (1), Portugal (1) [numbers correct as of February–April 27, 2021].
Neuropsychological knowledge and methods can play a key role in understanding the prevalence, profile, and nature of COVID-19 neurological and psychiatric symptoms. They may also contribute to the development of clinical management and facilitate the development of rehabilitation guidelines for patients with COVID-19-related neurological disorders worldwide. There are currently no definitive standards for neuropsychological (i.e., cognition, motor functions, global-, mental-, and psychosocial health, olfaction, and taste) assessment of patients with COVID-19. A lack of standard will lead to disparate results, which will be difficult to interpret as the methods and procedures will not be comparable and have unreliable associations with disease processes and biomarkers. This could result in inconsistent management guidelines, inadequate policies, and poor outcomes for patients.
COVID-19 is a new disease. It is complex as different (both direct infection and indirect) mechanisms, may be responsible for neuropsychological dysfunctions. The range and severity of neurological symptoms are varied and potentially affect the entire neuraxis (Paterson et al., Reference Paterson, Brown, Benjamin, Nortley, Wiethoff, Bharucha and Zandi2020). Developing research protocols that appreciate this complexity will have important clinical repercussions. The social lockdowns make standard in-person neuropsychological assessment practice difficult or impossible, even in countries with developed neuropsychological services. While awaiting a global vaccine and its rollout, neuropsychologists adapted to the COVID-19 pandemic by modifying their services and adapting their assessments using telehealth – audio or video conferencing technologies (Bilder et al., Reference Bilder, Postal, Barisa, Aase, Cullum, Gillaspy and Woodhouse2020; Matchanova et al., Reference Matchanova, Babicz, Medina, Rahman, Johnson, Thompson and Woods2020; Postal et al., Reference Postal, Bilder, Lanca, Aase, Barisa, Holland and Salinas2021). This adaptation also necessitates a shift in standard methods of neuropsychological research of patients infected with COVID-19.
Since COVID-19 is a global pandemic, we must develop harmonized methods and procedures that are globally relevant and promote health equity just as we strived to do for HIV infection. Our recommendations must be applicable across various settings and work in low-middle and high-income countries. Building capacity to address such diverse objectives is fully embraced as one of the major goals of these recommendations.
To provide standard and harmonized neuropsychological methods and procedures for research in patients with COVID-19 infection and potential translation to clinical practice, we apply the following selection criteria:
-
A) Methods appropriate for measuring the consequences of COVID-19, in order to:
-
Measure the range and severity of COVID-19-associated neuropsychological dysfunctions (i.e., direct and indirect causes of COVID-19-associated neurological and psychiatric symptoms).
-
Differentiate neuropsychological impairment from psychological distress.
-
Measure consequences at different phases of disease (acute/infectious, subacute, chronic) that fit the requirements of longitudinal study design.
-
Consider premorbid and comorbid effects, performance validity, and other factors that may affect neuropsychological performance in a manner specific to patients with COVID-19.
-
-
B) Methods and procedures adaptable to the pandemic social lockdown, and patients’ quarantine status, or patient’s hospitalization and alertness status (e.g., ICU vs. ambulatory):
-
Telehealth, computerized, remote/online, pen, and pencils assessments options.
-
Screening strategies, medium-size evaluation, comprehensive assessment options.
-
-
C) Methods and procedures appropriate for international purposes:
-
Selection of tests with evidence for cross-cultural validity or widely available instruments.
-
Guidelines or other considerations to promote valid cross-cultural test translation/adaption, as well as data fidelity.
-
To facilitate the implementation of the recommendations, the context in which each harmonization level could be used is described. Issues pertinent to required training level for administration and scoring, assessment timeline, guidance to support (remote) data fidelity, norms, and impairment definitions are also described.
To address our aims, we propose three levels of harmonization of neuropsychological examination methods and procedures in COVID-19. Each level of harmonization covers a different level (from minimal, medium to comprehensive) of neurocognitive, mental and psychosocial functions, and other important factors for describing medical and demographic characteristics. Harmonization Level 3 (HL 3) was designed to represent a close equivalent to clinical practice. All procedures recommended in the current work involve human participants and thus they should be conducted in accordance with the ethical standards of the relevant institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
RECOMMENDATIONS FOR HARMONIZATION LEVEL 1
General Assumptions
Harmonization Level 1 (HL1) is focused on research and clinical contexts requiring brief screening either remotely or in-person, adaptable to various health settings and the health/infectious status of the patient. It is also based on tools that have global applications and are inexpensive for cognition, sensation, and mental health; with administration requiring minimum training. HL1 is designed to fit a baseline assessment to a potential prospective longitudinal observational study; it can also serve as a stand-alone cross-sectional study design. The recommendations of an exact set of measures and variables will facilitate data merging for international comparisons and global epidemiological data.
Recommendations for Application
Patient’s infectious status
Eligible participants include SARS-CoV-2 seropositive patients (see WHO case definition at WHO 2021) in the early phase of the disease, including asymptomatic individuals), as well as more advanced stages of the disease including patients presenting progressive respiratory involvement and focal/systemic inflammation. In these phases, it is very important to take into account the medical history to assess whether remote or bedside testing should be conducted at all. HL1 should only be conducted when a patient is fully able to participate in testing.
ICU status
Can be in ICU, any exams require personal protective equipment (PPE) in ICU settings and depend on local capacity to handle exams in ICU.
Time of testing for hospitalized patients
Assessment of cognition should be completed around the time of discharge, ideally before.
Quarantine status
Can be in quarantine or no quarantine.
Patient’s alertness status
Test should only be completed when the patient is fully able to do the testing via a brief assessment of CNS symptoms.
Setting
Telehealth, in-person with PPE. Considering pandemic-related limitations in research and clinical activities, the HL1 protocol can avoid in-person face-to-face contact through the use of remote assessment methods. Thus, HL1 facilitates studying participants in the infectious phase who are (self-)quarantined, isolated, or hospitalized.
Testing type
Brief/screen.
Level of required training for administration and scoring
Minimal.
Control group
SARS-CoV-2-negative individuals can be recruited as the control group. Control group should be matched on demographics, health characteristics, quarantine, and hospitalization setting.
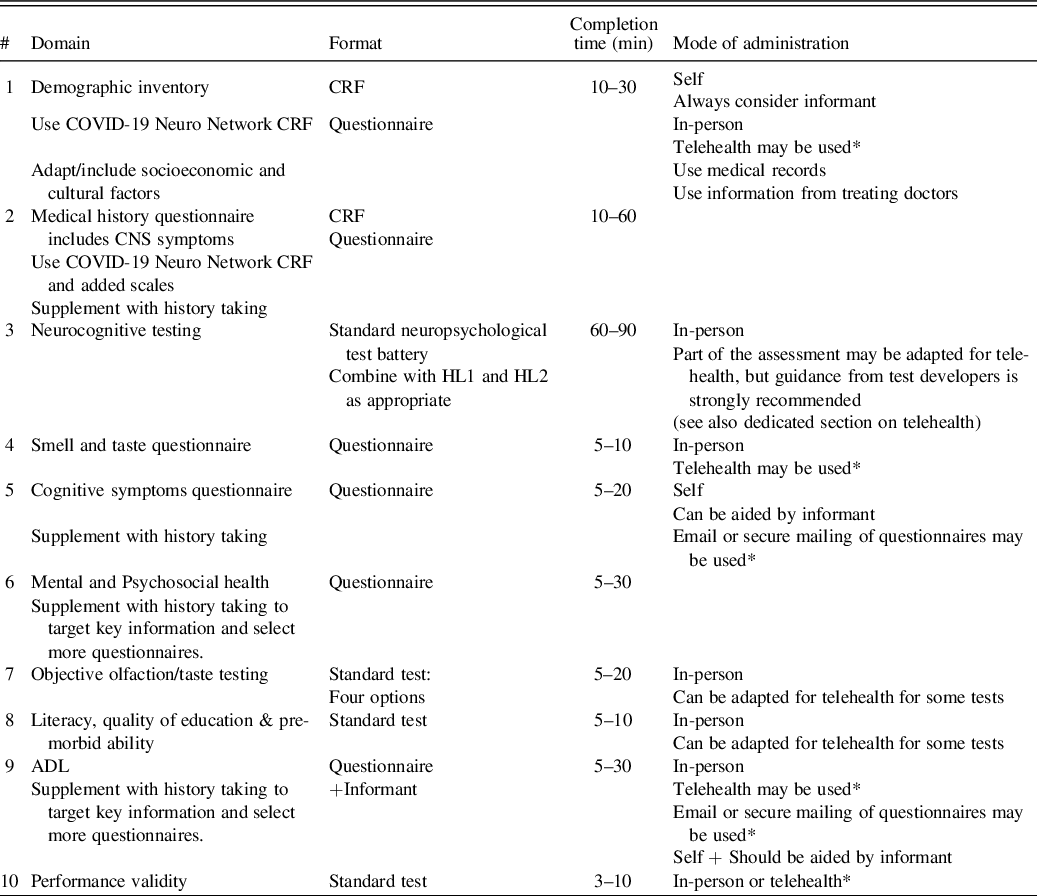
Table 1 summarizes the HL1 protocol.
Table 1. Harmonization Level 1 protocol

NB: the protocol material is available in Supplementary Material 1.
* Some information may be filled out by participants/patients and their informants at their convenience within 3 days of the screen exam.
** We also recommend the Brief Test of Adult Cognition Telephone (BTACT; Tun & Lachman, Reference Tun and Lachman2006) as a potential alternative to the MOCA-5/T-MoCA as the standard screening test. The BTACT is composed of the Rey Auditory Verbal Learning Test (RAVLT), Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) Digit Span Backwards, Category Fluency test, Red/Green test, Number series, and Backward counting. The BTACT was originally developed to monitor the effects of aging on cognition; thus, it assesses wider neurocognitive function than dementia screening tools (Bodien et al., Reference Bodien, McCrea, Dikmen, Temkin, Boase, Machamer and Investigators2018; Dams-O’Connor, Landau, Hoffman, & St De Lore, Reference Dams-O’Connor, Landau, Hoffman and St De Lore2018). The administration time is 15–20 min. The BTACT has high validity with other pen & paper tests and has good reliability, hence the in-person test version can be alternatively used when possible. The testing procedure includes accuracy checks and time of completion. There are four alternate versions (1 original + 3 alternatives) to minimize practice effects on repeated assessments. The subtests examine episodic memory, working memory, reasoning, verbal fluency, and executive function and there is an option to calculate a composite score. The English, Spanish, and French versions of the BTACT have been normed (Lachman, Agrigoroaei, Tun, & Weaver, Reference Lachman, Agrigoroaei, Tun and Weaver2013). Importantly, the BTACT can be accessed and used for free with permission from the developer (versions A–B contact Dr. Lachman; versions C–D contact Dr. Silverberg).
Recommended Measurement Methods
Demographic inventory and medical history questionnaires
Refer to the material provided in Supplementary Material 1 either via link access or copy of the material when authorized. We recommend the use of the Case Report Form (CRF) developed by the COVID-Neuro Network (access to the CRF requires a registration at Brain Infectious Global COVID-Neuro Network, 2021). We strongly recommend completing all the demographic and medical data sections of the CRF. The laboratory data sections are optional. This CRF includes CNS symptoms using the Glasgow Coma Scale (GCS) and the Modified Rankin Score, which is provided in Supplementary Material 1. We recommend documenting acute confusion states using the Confusion Assessment Method (CAM), which is also included in Supplementary Material 1.
Neurocognitive screens
Montreal Cognitive Assessment 5-Minute Protocol (MoCA-5, Wong et al., Reference Wong, Nyenhuis, Black, Law, Lo, Kwan and Mok2015) is the short form of the Montreal Cognitive Assessment (MoCA), which was originally developed to screen for vascular cognitive impairment and dementia (Nasreddine et al., Reference Nasreddine, Phillips, Bedirian, Charbonneau, Whitehead, Collin and Chertkow2005; O’Driscoll & Shaikh, Reference O’Driscoll and Shaikh2017; Wong et al., Reference Wong, Nyenhuis, Black, Law, Lo, Kwan and Mok2015), but later research covers various other neurological conditions (Hebert, Day, Steriade, Tang-Wai, & Wennberg, Reference Hebert, Day, Steriade, Tang-Wai and Wennberg2017; Phabphal & Kanjanasatien, Reference Phabphal and Kanjanasatien2011; Rodrigues, Gouveia, & Bentes, Reference Rodrigues, Gouveia and Bentes2020). The four items of the shortened protocol cover attention, verbal learning, and memory (with delayed recall), executive functions/language, and orientation. The advantage of the test is that it could be used in teleneuropsychology. A shortcoming is that visuospatial abilities would not be assessed. The full form has been translated and validated in 27 languages with most of them having norms provided (Mast & Gerstenecker, Reference Mast, Gerstenecker and Lichtenberg2010). MoCA-5 is also available with alternative versions in English, French, Italian, and Chinese. Its cultural sensitivity among racial and ethnic minorities has been researched (Milani, Marsiske, Cottler, Chen, & Striley, Reference Milani, Marsiske, Cottler, Chen and Striley2018; Milani, Marsiske, & Striley, Reference Milani, Marsiske and Striley2019; O’Driscoll & Shaikh, Reference O’Driscoll and Shaikh2017). The test is freely accessible, though test users are recommended to complete an official online training and certification in order to administer and interpret the MoCA and its various short forms.
While the MoCA-5 could be a preferred choice for quick screening in-person or in teleneuropsychology, domains such as attention and executive functions are abbreviated. A solution would be to consider the 22-point telephone Montreal Cognitive Assessment (T-MoCA, suggested cutoff = 18/19; Pendlebury et al., Reference Pendlebury, Welch, Cuthbertson, Mariz, Mehta and Rothwell2012), sometimes referred to as the “Blind MoCA” (Wittich et al., Reference Wittich, Phillips, Nasreddline and Chertkow2010). It essentially removes the visually related items from the full MoCA, and thus could cover the rest of the cognitive domains in all the languages in which MoCA has been validated. Its limitations, as pointed out by the test co-developers, are the lack of published validations with remote testing and norms for key groups of interest (Phillips et al., Reference Phillips, Chertkow, Pichora-Fuller and Wittich2020). However, recent evidence supports the validity of remote administration of the TMoCA in an ethnically and economically diverse US community cohort (Katz et al., Reference Katz, Wang, Nester, Derby, Zimmerman, Lipton and Rabin2021).
Alternatively, we recommend The Brief Test of Adult Cognition Telephone (BTACT; Tun & Lachman, Reference Tun and Lachman2006), though it is important to note that this tool has been recommended only for research. See legend of Table 1 for further details.
Cognitive symptoms
The Patient’s Assessment of Own Functioning (PAOFI) is a well-validated self-report questionnaire (Chelune, Heaton, & Lehman, Reference Chelune, Heaton, Lehman, Goldstein and Tarter1986). The PAOFI covers cognitive domains such as memory function, language, and communication, sensory, and perceptual function, use of hands, and also provides a summary score. The PAOFI has been translated into multiple languages (HIV Neurobehavioral Research Program, 2020 see also Supplementary Material 1).
Smell/taste questionnaire
This is a very brief set of questions adapted from The Smell and Taste component of the National Health and Nutrition Examination Survey (NHANES) 2013–2014, which can be easily adapted/translated. The questionnaire is provided in Supplementary Material 1.
Mental and psychological health questionnaires
This step includes Global health assessment with the widely used – MOS 36-Item Short-Form Health Survey (SF-36) and the assessment of psychological health using the Depression, Anxiety, and Stress (DASS-21) short form. Both instruments have been translated and adapted in many languages.
RECOMMENDATION FOR HARMONIZATION LEVEL 2
General Assumptions
Harmonization Level 2 (HL2) can be used as a first follow-up assessment for post-acute infection. The set of measurement methods proposed at this level enables a more in-depth examination equivalent to a medium-size research battery, which would also enable collaborative projects. Clinically, it could also serve as a more in-depth screen. This harmonization level also incorporates some flexibility for the tests’ administration mode (telehealth and in-person) and attempts to minimize the testing duration. At this level, harmonization is achieved by recommending a set of selected tools, and recommending the coverage of specific cognitive, sensory, global, and psychosocial domain areas. Additionally, the availability of adaptations/translation and cross-cultural validity is documented. At this level, objective olfaction testing is also recommended.
Recommendations for Application
Patient’s infectious status
Negative (HL2 testing is deferred until the patient has recovered). Eligible participants are no longer infectious as proven with a SARS-CoV-2-negative result. HL2 should only be conducted when the patient is fully able to participate in testing.
Time of testing for hospitalized patients
Assessment of cognition should be completed close to the time of discharge.
Quarantine status
Quarantine or no quarantine
Patient’s antibody status
Documented if possible
Patient’s alertness status
As for a standard neuropsychological assessment
Setting
Telehealth by video call, remote online testing, in-person/in-clinic (PPE), maximizing ventilation (e.g., open windows).
Testing type
5–20 min screens; 3–10 min questionnaires
Level of required training for administration and scoring
Closely follow the available manual guidelines and use supervised administration training when indicated
Control group
SARS-CoV-2-negative individuals (no history of a positive test) can be recruited as the control group. Control group should be matched on demographics, health characteristics, quarantine, and hospitalization setting.
Table 2 summarizes the HL2 protocol.
Table 2. Harmonization Level 2 protocol

NB: the protocol material is available in Supplementary Material 2.
* Some information may be filled out by participants/patients and their informants at their convenience within 3 days of the neuropsychological exam.
Recommended Measurement Methods
The aim of HL2 is to examine the effects of COVID-19 on neurocognition, olfaction, taste, and psychological well-being in greater detail. HL2 can assist in providing a more robust estimate of the potential disease-related neurocognitive impairment prevalence, but it cannot be considered a comprehensive assessment. HL2 is a medium-size assessment, with remote options (although with some caveats for cognitive computerized testing). Where a longitudinal study has used HL1 as a study screen, HL2 outcome scores may be adjusted for performance on HL1. For participants who are unable to perform computerized neurocognitive testing (e.g., because of lack of appropriate hardware), HL1 assessment protocol is recommended. At HL2, options for remote completion of questionnaires are also proposed. We recommend clearly documenting the role of any informant in assisting questionnaire completion. We also recommend that the examiner dedicates some time with the participant/patient over the phone or face to face to clarify any responses on these questionnaires as appropriate. Finally, at this level, we recommend the inclusion of performance validity tests (see Supplementary File 5 for further guidance). COVID-19 is a widespread condition affecting a wide range of people. Ensuring that measurements of cognitive performance are valid is therefore essential.
Demographic inventory and medical history questionnaires
We recommend using the same protocol as for HL1 and complementing the basic demographic data with a more extensive testing of premorbid abilities. See also Supplementary Material 2.
Neurocognitive testing
The cognitive domains of interest include those affected in a wide range of neurological and psychiatric conditions to capture potential direct COVID-19 effects on the brain as well as potential indirect effects: (1) Attention/working memory; (2) Executive function; (3) Motor function; (4) Processing speed; and (5) Learning and memory. HL2 remote testing is possible through online self-administration, but we recommend checking with the test providers whether this will fit your study population. In case of conflict with national health guidelines on telehealth, or wide variability of Internet access and hardware suitability in your study population, we advise that you conduct the neurocognitive testing in person. The other option is to repeat the HL1 protocol via telephone, and the rest of the HL2 protocol using telehealth or in-person assessment. Thus, using some flexibility in your protocol, you may be able to conduct a minority of tests/questionnaires in person and use telehealth for the remainder. The rationale for the neurocognitive test selection includes (i) tools that are widely used with well-developed training manuals; (ii) tools used internationally that have several language versions with evidence of cross-cultural validity and for some use in resource-limited settings; (iii) and tools that have good criterion validity and test–retest reliability. Construct validity for standard neuropsychological tests was not retained as a selection criterion, but is documented in Supplementary Material 2. The computerized format was primarily chosen to facilitate test administration (including by trained nonspecialists), integrated data capture, and automatic scoring. The computerized format also facilitates multi-site studies. Lastly, we considered the availability of large normative datasets for optimal interpretation of performance. Supplementary Material 2 includes detailed information about the four neurocognitive computerized tests, all available on tablets/iPad: Test My Brain (TMB); Cogstate Computerized Battery; NeuroScreen; and the NIH Toolbox Cognition and Motor Batteries.
Literacy, quality of education, and premorbid ability and additional neuropsychological measures
Literacy, quality of education, and premorbid abilities can be documented via a demographic interview to which standard tests of reading or reasoning may be added. Careful consideration of the person’s native language and level of education is needed to interpret test performance. The study scope might require additional neuropsychological tests, which we have also documented in Supplementary Material 2. The Grooved Pegboard Test could be used for motor functions or, alternatively, the 9-hole Pegboard Test is part of the recommended NIH Toolbox – Motor.
Cognitive symptoms
Use HL1 protocol or consider other options provided in Supplementary Material 2. Specific consideration should be given to the timelines covered by these questionnaires, which may not fit the timeline of an acute infection with a range of recovery such as COVID-19.
Smell/taste questionnaire
We recommend the longer version questionnaire adapted from the Taste and Smell component of the NHANES 2013–2014, which can be easily adapted/translated. This is provided in Supplementary Material 2.
Objective smell/taste testing
Olfactory disturbances are commonly observed in COVID-19. Therefore, at HL2, we recommend the objective testing of olfaction because it is well established that self-report is not reliable, although this may not be the case for an acute infection such as COVID-19. We recommend the use of standard tests, which were selected based on their validity to determine anosmia and ageusia at various levels of granularity, the availability of good normative data, and some evidence of cross-cultural adaptation. Test details and access are described in Supplementary Material 2. The quickest olfaction tests may be adapted to remote online testing using a webcam, plus mailing of the scratch and sniff cards.
Mental and psychosocial health questionnaires
We recommend using the HL1 protocol and, time permitting, adding a wider array of mental and psychosocial health questionnaires (see Supplementary Material 2 for details). As per current mental health literature in COVID-19, symptoms of PTSD, anxiety, depression, and fatigue may be the most important to screen. Careful consideration of mental health risk is needed if sending psychological questionnaires remotely; the scoring should be immediately interpreted using remote technologies to flag and follow-up with patients at high risk of distress.
Activities of Daily Living (functional) assessment (ADL)
It may be useful to assess Instrumental Activities of Daily Living (IADL), particularly for hospitalized cohorts, which typically have more severe COVID-associated neurological symptoms. Indeed, it is important to document the everyday functioning relevance of any acquired neurocognitive impairment. This also represents as the first step toward rehabilitation strategies when needed. Traditional tools for IADL assessment are based on a set of predetermined activities, which may not be relevant to some individuals, depending on their gender, age, educational status, and specific activity engagement (Sikkes, de Lange-de Klerk, Pijnenburg, Scheltens, & Uitdehaag, Reference Sikkes, de Lange-de Klerk, Pijnenburg, Scheltens and Uitdehaag2009). Traditional IADL measures also have low cross-cultural validity and poor psychometric properties for both criterion validity of IADL impairment and detection of decline upon repeated testing (Sikkes et al., Reference Sikkes, de Lange-de Klerk, Pijnenburg, Scheltens and Uitdehaag2009). We, therefore, recommend the use of recent instruments, which have addressed some of these challenges (see Supplementary Material 2). These new instruments also have screening versions and several language versions and offer methods for developing cross-culturally validated versions (Dubbelman et al., Reference Dubbelman, Verrijp, Facal, Sánchez-Benavides, Brown, van der Flier and Sikkes2020; Jutten et al., Reference Jutten, Harrison, Lee Meeuw Kjoe, Opmeer, Schoonenboom, de Jong and Sikkes2018).
RECOMMENDATIONS FOR HARMONIZATION LEVEL 3
General Assumptions
HL3 is akin to a standard, in-person comprehensive neuropsychological assessment for which we recommend a set of standard neuropsychological tests including performance validity tests. Objective olfaction and taste testing is also recommended.
Recommendations for Application
Patient’s infectious status
HL3 testing is deferred until the patient recovers. Eligible participants are no longer infectious as proven with a SARS-CoV-2-negative result.
Time of testing for hospitalized patients
Assessment of cognition should be completed close to the time of discharge.
Quarantine status
No quarantine.
Patient’s antibody status
Should be documented if possible.
Patient’s alertness status
As for a standard neuropsychological assessment.
Setting
In-patient, in-clinic face-to-face (no/partial PPE), telehealth may be used for parts of the assessment.
Testing type
Comprehensive, 2–4 h sessions with breaks as appropriate.
Level of required training for administration and scoring
Clinical Neuropsychology training, psychometricians.
Control group
SARS-CoV-2-negative individuals (no history of a positive test) can be recruited as the control group. Control group should be matched on demographics, health characteristics, quarantine, and hospitalization setting.
Sanitary considerations for an in-person examination
Mask and gloves should be used when appropriate in a dedicated room which would be disinfected after each patient. Test materials would also need to be disinfected (see Postal et al., Reference Postal, Bilder, Lanca, Aase, Barisa, Holland and Salinas2021).
Table 3 summarizes the HL3 protocol.
Recommended Measurement Methods
The aim of HL3 is to examine in more detail the permanent, long-term, and transient characteristics of COVID-19 effects on neurocognitive functions. Such a comprehensive assessment is critical to establish a solid rehabilitation strategy in patients with moderate-to-severe neurological/neuropsychological symptoms. HL3-in-person assessment comprises a selection of well-known standard neuropsychological tests, in addition to the olfactory and taste tests described in HL2. The primary cognitive domains of interest at HL3 are common to HL2, and so HL2 and HL3 may be combined when desirable. HL3 remote testing options represent as a more robust estimate of the potential disease-related neurocognitive impairment prevalence at this stage of the disease than HL2 testing. If a study has used HL1 as a screen or even HL2, HL3 outcome scores can be adjusted for previous performance. For participants who are unable to undergo computerized neurocognitive testing, the HL1 over-the-phone assessment protocol can be repeated. At HL3, depending on the level of physical and possible cognitive difficulties a participant/patient may experience, some or all questionnaires may be done at home, but we recommend clearly documenting the role of any informant in assisting their completion. We also recommend that the examiner dedicates time with the participant/patient face to face to clarify any responses on these questionnaires as appropriate. For this more extensive assessment, we strongly recommend the inclusion of performance validity tests (see Supplementary File 5). For greater ecological validity, we also recommend the use of prospective memory tests which can have a closer relationship to functional changes compared to other cognitive domains (Woods et al., Reference Woods, Iudicello, Moran, Carey, Dawson and Grant2008, Reference Woods, Weinborn, Velnoweth, Rooney and Bucks2012).
Demographic inventory and medical history questionnaires
In line with the harmonization aim of our recommendations, we advise using HL1/2 protocols and supplementing as appropriate (e.g., depending on your study/patient population) with a more extensive assessment of demographics, socioeconomic and cultural factors. Please consult Supplementary Material 2, where you will also find suggestions on the assessment of premorbid abilities.
Neurocognitive testing
Supplementary Material 3 presents a detailed description of a standard neuropsychological battery. This covers core domains for HL1 and HL2 and goes well beyond to cover the complex neurological syndromes that have been described whether directly due to COVID-19 or due to associated and underlying comorbidities. Addition of specific tests is warranted for other patient populations who have also been diagnosed with COVID-19 (e.g., Parkinson’s disease).
Literacy, quality of education, and premorbid ability
Use HL2 protocol and see Supplementary Material 2.
Cognitive symptoms
Use HL1 protocol and consider other options provided in Supplementary Material 2. The history taken prior to the comprehensive assessment is important to consider and to allow a nuanced interpretation of responses to the questionnaires (particularly with regard to symptom timelines).
Smell/taste questionnaire
Use HL2 protocol.
Objective smell/taste testing
Use HL2 protocol. The short or long version of the proposed assessments could be used, depending on your study questions (e.g., focusing on perception rather than cognition), time constraints, and participant/patient engagement and fatigue.
Mental and psychosocial health questionnaires
We recommend including HL1 and HL2 protocols (see Supplementary Material 2). Depending on study-related factors (e.g., focusing on mental health more than cognition; different study populations) or patient-related factors (e.g., time constraints, engagement, and fatigue), you may select more targeted mental and psychosocial health questionnaires.
IADL
For HL3, ADL assessment is strongly recommended. Consult HL2 information above and Supplementary Material 2.
HARMONIZATION LEVELS: CROSS-CULTURAL AND DISPARITIES ISSUES
The recommendations attempt to deal a priori with the international aspect of the epidemic. In this section, we, therefore, provide guidance on how best to use the recommended tests across diverse populations. Although our recommended tests are used internationally, cross-cultural appropriateness and availability of tests are crucial. Within local contexts, tests should be selected and administered considering the background characteristics of the target population to avoid violating fairness in testing (Aghvinian et al., Reference Aghvinian, Santoro, Gouse, Joska, Linda, Thomas and Robbins2020; International Test Commission, 2019). Cultural and sociodemographic factors (sex, age, education, ethnicity, socioeconomic status) impact neuropsychological test performance (Brickman, Cabo, & Manly, Reference Brickman, Cabo and Manly2006). Neuropsychological tests must therefore be culturally appropriate with regard to language use and test stimulus items. Age- and education-appropriate norms are necessary to determine whether a person’s performance falls outside the normal range (Fernandez, Reference Fernandez2019; Mitrushina, Boone, Razani, & D’Elia, Reference Mitrushina, Boone, Razani and D’Elia2005). Where such normative data are not available, a well-matched control sample is required (Casaletto & Heaton, Reference Casaletto and Heaton2017). These issues are particularly pertinent in low- and middle-income countries (LMIC) where few neuropsychological measures have been adapted and validated, and normative data are scarce.
Key issues for the implementation of the current recommendations across settings include access to human resources and expertise, technological and socioeconomic considerations, and availability and adaptation of study measures. Access to human resources and expertise varies between and within countries. Where there is a lack of expert- and human resources (e.g., trained neuropsychologists) in LMIC, clinical, or general psychologists may be involved. Laypeople can also be trained to do assessments under supervision by a psychologist, allowing the delegation to less specialized health care- or lay-workers, particularly when combined with automated, easy-to-use tests that can be performed on a phone or a tablet (e.g., NeuroScreen; Magidson et al., Reference Magidson, Gouse, Psaros, Remmert, O’Cleirigh and Safren2017; Robbins et al., Reference Robbins, Gouse, Brown, Ehlers, Scott, Leu and Joska2018). Mental health screening must similarly be supervised by a clinical/neuropsychologist or psychiatrist familiar with the local setting. Distance supervision applies in locations without direct access to specialists, in line with the current Taskforce guidelines.
Access to technology and connectivity also varies across settings and the use of mobile health applications must be viewed in light of available resources. In LMIC access to computers or tablets may be limited, for example, but access to smartphones is ubiquitous. The high cost of mobile data in some settings may limit the ability to complete online assessments. These issues must be carefully considered during study design. Availability and affordability of study measures vary across countries and so, if possible, tests that are in the public domain should be used. With regard to test use and adaptation, if the recommended tools have not been adapted, existing original or adapted tests that measure the same construct should be used. If no adapted/validated measure is available, best practice guidelines for test adaptation and translation should be followed (e.g., a committee/team translation or forward- and back translation (Harkness & Schoua-Glusberg, Reference Harkness, Schoua-Glusberg and Harkness1998; Vallejo-Medina et al., Reference Vallejo-Medina, Gómez-Lugo, Marchal-Bertrand, Saavedra-Roa, Soler and Morales2017). Test publishers must be contacted timeously for permission to translate tests. All measures (e.g., mental health, medical history, etc.) must be translated and adapted to reflect the regional language and cultural practices.
The recommended neuropsychological measures included in HL 1–3 to a large degree reflect the neuropsychological test battery widely used in HIV studies, both in high-income countries (HIC) and LMIC (Kabuba, Anitha Menon, Franklin, Heaton, & Hestad, Reference Kabuba, Anitha Menon, Franklin, Heaton and Hestad2017; Nyamayaro, Chibanda, Robbins, Hakim, & Gouse, Reference Nyamayaro, Chibanda, Robbins, Hakim and Gouse2019), suggesting the applicability of tests across diverse settings. There are, however, some considerations to keep in mind when selecting tests for the purpose of describing the neurocognitive presentation associated with COVID-19. In particular, tests that measure cognitive constructs and global levels of functional capacity must be culturally valid. Below, we provide further comments on particular measures from HL 1–3 that may require cross-cultural adaptation in some settings (Table 4); for computerized tests, see also Supplementary Material 2.
Table 4. Cross-cultural considerations and recommendations for instruments proposed in HL 1–3

HARMONIZATION LEVELS: NORMS, IMPAIRMENTS RATINGS, AND REPEATED NEUROPSYCHOLOGICAL TESTING
Practitioners should carefully follow the standard scoring instructions and guidance for interpretation of all the tests, using standard materials obtained from accredited test providers. Practitioners are responsible for determining whether the tools we have recommended are either in the public domain and thus free to use and reproduce, or whether the tools need to be purchased from accredited providers, some of which require specific qualifications to access.
Norms
We recommend the use of published nationally representative normative data appropriate to your study sample age, education/SES, and sex, in addition to race/ethnicity and rural/urban living when possible. When nationally representative normative data are not available, we recommend using a demographically and geographically comparable control sample and, if capacity and expertise permits, developing norms.
Controls
Data collection in a local, demographically representative, and healthy control group is recommended. The published norms should be checked in your local sample to assess if they “work”, that is, whether they correct for demographic effects. Depending on the study question, controls may also be from a clinical comparison group, for example, patients who have been through ICU.
Neurocognitive impairment levels
In research studies, methods to determine levels of cognitive impairment (e.g., as cutoff scores on screens, or normative standard scores on one or more neuropsychological tests) should be clearly described and linked to a well-established nomenclature of performance levels. Extra attention should be given to computerized cognitive tests, and associated literature using those tests to determine standard levels of deficits. Reporting the level of “neurocognitive impairment” in controls is advised for transparency and better interpretation of the burden of the disease in COVID-19 samples.
Smell/taste impairment levels
The current recommendations include both questionnaires and smell/taste tests. The suggested measures are commonly used in general as well as clinical populations. They can be used to quantify smell loss or describe the severity of alteration caused by COVID-19. While we selected tests with available norms in several countries, it is still possible that these norms may not be appropriate for your population. In this case, we recommend that you compare results from your SARS-CoV-2-positive sample to a demographically comparable SARS-CoV-2-negative or asymptomatic control group, and/or longitudinally in order to track within-patient changes across the stages of the disease. Diagnosing impairment should be done with caution and follow the standard impairment grading of the original norms.
Repeated Neuropsychological testing
COVID is an evolving condition that starts with an acute infection phase, whether symptomatic or not. It is likely that a large part of the forthcoming research will be longitudinal to assess disease recovery on several occasions. Because of this, longitudinal data analysis and considerations of issues associated with neuropsychological repeated testing will be critical to characterize the neurocognitive complications of COVID-19. Please consult Supplementary File 6 for further guidance.
Consideration of contributing, confounding, and incidental medical, psychological, lifestyle, and demographic factors (beyond the norms corrections)
We advise carefully documenting any preexisting (e.g., systemic, immune, neurological, or psychiatric) or comorbid (e.g., stroke, hypoxia, lung disease) conditions to determine to what extent they may impact on neurocognitive, sensorimotor, and psychosocial health. Besides traditional demographics, it is important to note whether the participant is literate or may have been diagnosed with a learning disability. These various factors should be carefully documented, and their effects tested as appropriate.
Feedback reports for research
It is advised to produce individual feedback reports when conducting a research study. Such reports should ideally be sent to the participant’s doctor of choice so that the information is interpreted in the relevant clinical context. Reports should provide a detailed description of tests/questionnaires administered, modality of testing (in-person; remote: over-the-phone/computer-based), and involvement of the research/clinical personnel (personnel present in-person/remotely; or self-administered by the participant/informant). For remote assessment, reports should additionally provide information on the testing platform and describe nonstandard administration procedures and related limitations (e.g., limited understanding of participant’s vision, hearing or level of familiarization with testing devices). Research reports employing (at least a part of) the currently recommended neuropsychological protocols are welcome to include a citation of this paper. However, the description of the testing protocol should still be provided in order to enable comparisons with other sites. Additionally, references can be made directly to the Harmonization Levels 1–3 (basic/full; in-person/remote) and selected measures. Citation will further enhance the visibility of the original research papers and support building comparable databases for future meta-analysis and between-site data sharing. Clinical reports should further describe the potential impact of the administration procedure, and its alterations, on the proposed diagnosis and (if applicable) recommended treatment.
GUIDELINES FOR HOME/REMOTE ASSESSMENT TO SUPPORT DATA FIDELITY AND TELEHEALTH CONSIDERATIONS
Although there are no formal published standards for remote assessment and telehealth in neuropsychology, several national organizations have issued professional practice guidelines in recent months (American Psychological Association, 2020a; Interorganizational Practice Committee, 2020; The British Psychological Society, 2020). Key points from these guidelines are summarized in Supplementary Material 4. The APA has published a useful (though US-focused) checklist to help practitioners prepare for clinical sessions with these considerations in mind (American Psychological Association, 2020b). Practitioners must adhere to test publisher rules regarding copyright and sharing of materials (e.g., Pearson Assessment, 2020).
A meta-analysis of teleneuropsychology administration compared with in-person (Brearly et al., Reference Brearly, Shura, Martindale, Lazowski, Luxton, Shenal and Rowland2017) found that the difference between videoconference and in-person performance was very small (Hedges g = −0.03), and not statistically or clinically significant. Results were less consistent in patients aged over 75 and in situations with slower Internet connection speed. The authors concluded that videoconference administration of verbally mediated tasks by qualified professionals using existing norms was supported, and the use of visually dependent tasks may also be considered, but motor-based tasks require further investigation. Lastly of interest, is a newly proposed telehealth battery, which greatly overlaps with our HL3 recommendations, but also include testing of prospective memory (Matchanova et al., Reference Matchanova, Babicz, Medina, Rahman, Johnson, Thompson and Woods2020).
CONCLUDING REMARKS AND FUTURE DIRECTIONS
The long-term impact for COVID-19 survivors in the months and years post-recovery is, as yet, unknown; however, there are suggestions that based on the prevalence of critical illness alone, post-COVID-19 long-term cognitive impairment will be significant in some patients (Needham, Chou, Coles, & Menon, Reference Needham, Chou, Coles and Menon2020). Neuropsychologists will benefit from approaching assessment and rehabilitation of individuals after COVID-19 from a holistic point of view, considering cognition, emotional functioning, behavior, and potential socioeconomic pandemic impact as interacting variables that impact functional independence, quality of life, and emotional well-being. It is with this framework in mind that the current recommendations have been prepared.
The NeuroCOVID Neuropsychology International Taskforce will promote these recommendations through our research and collaborations. The group anticipates that the recommendations will facilitate multi-site and international collaborations and we encourage colleagues from HIC to develop studies that assist research in LMIC when appropriate. Implementation research regarding the acceptability, usability, and validity of the recommendations will be critical to their uptake and the Taskforce welcomes feedback on potential improvements and adjustments to inform refinement of the recommendations. It is important to note that these recommendations apply only to adult research and practice; analogous recommendations for neuropsychological research with children infected with SARS-CoV-2 are urgently needed.
The Taskforce will start the development of a minimum dataset and associated code book protocol, including a proposal for protocol registration. This minimum dataset will first be based on the lowest common denominator as developed in the recommendations (i.e., HL 1) and we hope to then include other harmonization levels. This effort will include secure online data storage and good practice guidelines for the participating sites. It is planned that individual researchers will access the database after contacting the coordinators and proposing the analysis to be conducted. Funding will be sought for the development and maintenance of the database.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/S1355617721000862.
ACKNOWLEDGMENTS
The authors would like to thank all members of the NeuroCOVID International Taskforce. New members are welcome, and membership enquiries can be sent to Dr. Cysique (lcysique@unsw.edu.au) and Dr. Łojek (emilia@psych.uw.edu.pl) by email. The group works from the Slack platform at: https://neurocovidinssig.slack.com/?redir=%2Fgantry%2Fclient#/. The Taskforce is also represented at the International Neuropsychological Society (INS) as a Special Interest Group (https://www.the-ins.org/sigs/). The statement that the opinions/data/perspectives belong solely to the authors and the NeuroCOVID International Taskforce and may not necessarily represent the viewpoint of INS. We thank Dr. Susan McPherson for bringing to our attention about the question of performance validity tests.
FINANCIAL SUPPORT
-
Dr. Cysique is supported for her NeuroCOVID research by the Peter Duncan Neuroscience Unit, St. Vincent’s Applied Medical Research Centre, Sydney, Australia, and the Alfred Hospital in Melbourne, Australia.
-
Drs. Łojek, Hansen, Holas, and Malinowska are supported by the Faculty of Psychology, Warsaw University, Poland.
-
Dr. Muñoz-Moreno is supported by the Fundació Lluita contra la SIDA, Germans Trias i Pujol University Hospital, Barcelona, Catalonia, Spain.
-
Mr. Theodore C.K. Cheung is supported by the Ontario Graduate Scholarship, Canada.
-
Dr. Gouse is supported by the Fogarty International Center 1K43TW010361–01.
-
Dr. Silva (A.R.) is supported by the Portuguese Agency of Science and Technology.
CONFLICTS OF INTEREST
The authors have nothing to disclose.
APPENDIX 1. AUTHORS’ CONTRIBUTIONS
The Taskforce is coordinated from a dedicated Slack platform. All members have access to the Welcome and Literature/Resources channels, and all are able to share, propose, and access the material, including the manuscript as it evolved via a Slack-Google Drive link. The SIG co-chairs have administrative access to Slack. SIG members are required to become INS members to join, as per INS policies. For the current recommendations, members were asked to self-nominate for leading manuscript sections. More than one member could be a section author. The co-chairs led the synthesis from all the co-authors and all co-authors have reviewed the paper, including two senior researchers who are native English speakers.