The intestine exerts important functions in the digestion and absorption of nutrients, and is also the body’s first line of defence against pathogens, preventing luminal micro-organisms from invading the body( Reference Delgado, Grabinger and Brunner 1 , Reference Blikslager, Moeser and Gookin 2 ). However, the intestinal mucosal damage and dysfunction could be induced by many factors such as inflammation and infection( Reference Liu 3 , Reference Liu, Huang and Hou 4 ). Pro-inflammatory cytokines, especially TNF-α, play a central role in intestinal injury( Reference Mitsui, Fukatsu and Yanagawa 5 ). The intestinal integrity and epithelial function could be impaired by overproduction of these cytokines( Reference Wang, Liu and Shi 6 ). To alleviate the inflammation and maintain health and function, the intestine requires a high amount of energy( Reference Wang, Liu and Li 7 ). Thus, energy deficits in intestinal mucosa are closely related to various degrees of injury in the intestine( Reference Wang, Liu and Chen 8 ).
Activation of inflammatory signalling pathways plays a critical role in intestinal damage( Reference Narimatsu, Higashiyama and Kurihara 9 ). Toll-like receptors (TLR) and nucleotide-binding oligomerisation domain proteins (NOD) are important protein families of inflammatory signalling pathways( Reference Takeuchi and Akira 10 , Reference Kim, Zhao and Zheng 11 ). TLR and NOD can be expressed in the intestine by the challenge of lipopolysaccharide (LPS), and then activate downstream signalling pathways that induce innate immune responses( Reference Ahn, Yoon and Park 12 ). NF-κB pathway is one of the downstream signalling pathways that could provoke the expression of genes encoding pro-inflammatory cytokines such as TNF-α ( Reference Doyle, Zhang and Abdel Fattah 13 ). The overproduction of TNF-α can elicit collateral injury in the intestine( Reference Chen, Liu and Wang 14 ).
The normal physiological differentiation and maturation of intestinal epithelial cells leads to their shedding and apoptotic cell death within a few days, without disturbing the epithelial barrier integrity. However, excessive intestinal epithelial cell death induced by inflammation severely impairs the vital functions of this tissue( Reference Delgado, Grabinger and Brunner 1 ). Necroptosis is a programmed cell death identified in recent years and dependent on the activity of the receptor-interacting protein kinase family, which could be activated by LPS( Reference Green, Oberst and Dillon 15 ). Moreover, necroptosis leads to the rupture of cell membranes and further induction of inflammation( Reference Green, Oberst and Dillon 15 ). Therefore, how to inhibit the inflammatory signalling pathways and necroptosis has been the research hotspot nowadays.
Fatty acids with aliphatic tails of six to twelve C atoms are called medium-chain fatty acids (MCFA), which occur naturally as medium-chain TAG (MCT) in milk fat and various feed materials such as coconut, palm oils and Cuphea seed oils( Reference Rossi, Pastorelli and Cannata 16 ). Both MCFA and MCT have special function in metabolism, such as rapid digestion, passive absorption and obligatory oxidation, which make MCFA and MCT of potential use in young animals( Reference Odle 17 ). MCT can be utilised directly by the enterocytes for energy production and thereby help to support the integrity of the intestine in young piglets( Reference Guillot, Vaugelade and Lemarchal 18 ). In addition, MCT have been suggested to improve intestinal health under inflammatory conditions( Reference Bertevello, De Nardi and Torrinhas 19 , Reference Papada, Kaliora and Gioxari 20 ). Furthermore, some research reported that MCT have anti-microbial and antiviral activity in gastric lining and small intestine of pigs( Reference Zentek, Buchheit-Renko and Männer 21 , Reference Messens, Goris and Dierick 22 ).
In view of the foregoing, we hypothesised that dietary MCT addition could reduce the degree of enterocyte necroptosis, as well as production of intestinal pro-inflammatory cytokines via regulating inflammatory signalling pathways, and thus protect intestinal integrity and function. In this experiment, Escherichia coli LPS was injected to establish the model of endotoxaemia( Reference Crosslan, Constantin-Teodosiu and Gardiner 23 ). In addition, the weanling piglet model was used, which is a good animal model for human nutrition research( Reference Dunshea and Cox 24 , Reference Spurlock and Gabler 25 ). The aim of this study was to investigate whether MCT could mitigate LPS-induced intestinal injury, and to explore its molecular mechanism(s).
Methods
Animal care and diets
The experiment and animal care were conducted in compliance with the guidelines established by Animal Care and Use Committee of Wuhan Polytechnic University. A total of twenty-four weanling crossbred castrated barrows (Duroc×Large White×Landrace, 35 (sem 1) d of age, 9·09 (sem 0·23) kg initial body weight (BW)) were used for this study. Piglets were individually housed in stainless steel metabolic cages (1·80×1·10 m2) with access to feed and water in an environmentally controlled house. The ambient temperature was maintained at 22–25°C. The maize–soyabean meal-type basal diet (Table 1) was formulated in accordance with the National Research Council requirements for all nutrients( 26 ).
Table 1 Ingredient composition of experimental diets (%, as-fed basis)
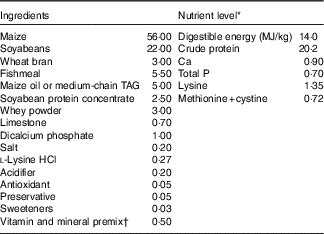
* The nutrient level was the analysed value, except digestible energy, which is the calculated value.
† Premix supplied per kg diet: retinyl acetate, 2700 µg; cholecalciferol, 62·5 µg; dl-α-tocopheryl acetate, 20 mg; menadione sodium bisulfite complex, 4 mg; riboflavin, 5·22 mg; D-calcium-pantothenate, 20 mg; niacin, 26 mg; vitamin B12, 0·01 mg; Mn (MnSO4.H2O), 40 mg; Fe (FeSO4.H2O), 75 mg; Zn (ZnSO4.7H2O), 75 mg; Cu (CuSO4.5H2O), 100 mg; I (CaI2), 0·3 mg; Se (Na2SeO3), 0·3 mg.
Experimental design
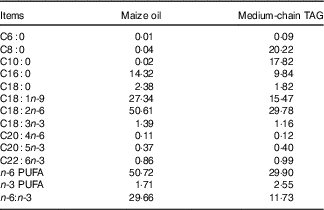
The piglets were randomly allotted to one of four treatments in a 2×2 factorial arrangement that included diet type (5 % maize oil v. 4 % MCT+1 % maize oil; the maize oil was purchased from Xiwang Food Co., Ltd and the MCT was purchased from Huacheng Feed Co., Ltd; the fatty acid composition of the maize oil and MCT, maize oil- and MCT-supplemented diets was shown in Table 2 and Table 3, respectively) and immune stress (0·9 % NaCl solution v. E. coli LPS (E. coli serotype 055:B5; potency≥5 000 000 endotoxin units/mg; Sigma Chemical)). BW and feed consumption were measured on day 1 and day 21. Feeders were removed from the pens at 20.00 hours on the 20th day of the experiment. On the morning of day 21, the piglets in challenged groups were injected intraperitoneally with E. coli LPS at 100 μg/kg BW, and the piglets in the control group were injected with the equivalent amount of 0·9 % NaCl solution. The dose of LPS was chosen in accordance with our previous studies( Reference Liu, Huang and Hou 4 , Reference Pi, Liu and Shi 27 ).
Table 2 Analysed fatty acid profile of the maize oil or medium-chain TAG (%, of total fat)
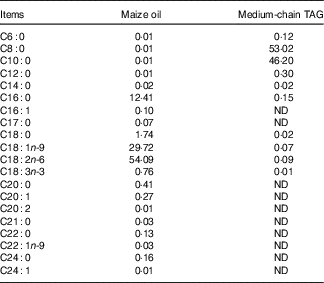
ND, not detected.
Blood and intestinal sample collections
At 4 h after injection with saline or LPS, blood samples (8 ml) were obtained from the anterior vena cava into the heparinised vacuum tube. Plasma was isolated by centrifugation of the blood samples at 3000 g /min and 4°C for 10 min, and subsequently transferred into 2 microcentrifuge tubes (2 ml). Plasma samples were stored at −80°C for further analysis of TNF-α.
Following blood collection, all piglets were humanely killed by injection of sodium pentobarbital. The 3- and 10-cm segments were cut from the mid-jejunum and mid-ileum according to our previous experiment( Reference Pi, Liu and Shi 27 ). A variety of studies have shown that, within 3–6 h post injection, LPS resulted in intestinal morphologic impairment and dysfunction, which was related with increased production of blood and intestinal pro-inflammatory cytokines( Reference Liu, Huang and Hou 4 , Reference Pi, Liu and Shi 27 , Reference Mercer, Smith and Cross 28 ). Therefore, the time point of 4 h following saline or LPS administration was chosen for experimental measurements. The 3-cm intestinal segments were gently flushed and stored in fresh 4 % paraformaldehyde/PBS for histological analysis( Reference Liu, Huang and Hou 4 , Reference Pi, Liu and Shi 27 ). The 10-cm intestinal samples were opened longitudinally and flushed gently to remove luminal chyme. The mucosa samples were collected via scraping with sterile glass slides, and then rapidly frozen in liquid N2 and stored at −80°C for measurement of fatty acid composition, disaccharidase activities and mRNA and protein expression levels. All intestinal samples were collected within 15 min after slaughter.
Intestinal morphology analysis
The small intestinal segments were cut into small pieces not exceeding 2 mm in length and enclosed into plastic tissue cassettes and then processed over a 19-h period in an automatic tissue processor. Fixed intestinal samples were prepared using conventional paraffin-embedding techniques( Reference Luna 29 ). Samples were sectioned at a 5-μm thickness and stained with haematoxylin–eosin. Villus height and crypt depth were measured at 40× magnification with a microscope (Olympus CX31; Olympus Optical Company). A minimum of ten well-oriented and intact villi were selected. Villus height was measured from the tip of the villus to the villus–crypt junction; crypt depth was defined as the depth of the invagination between adjacent villi( Reference Liu, Huang and Hou 4 ).
Fatty acid composition
Maize oil, MCT, experimental diets and intestinal mucosa samples were analysed for fatty acid profiles using GC (6890 series; Agilent Technologies) according to the American Oil Chemists’ Society method( 30 ).
Intestinal mucosa disaccharidase activities
Disaccharidase activities in the supernatant of intestinal mucosa were determined according to the methods described by Liu et al.( Reference Liu, Huang and Hou 4 ) using the glucose kit (No. A082-1 for lactase, no. A082-2 for sucrase and no. A082-3 for maltase; Nanjing Jiancheng Bioengineering Institute). The protein concentrations of intestinal mucosa were determined using Coomassie Brilliant Blue G-250 reagent with bovine serum albumin as a standard( Reference Zhu, Liu and Chen 31 ).
Plasma TNF-α concentration
Plasma TNF-α concentration was analysed by using a commercially available porcine ELISA kit (no. PTA00; R&D Systems) according to the manufacturer’s instructions. The minimum detectable concentration was 3·7 pg/ml.
Table 3 Analysed fatty acid profile of the diets supplemented with maize oil or medium-chain TAG (%, of total fat)
mRNA abundance analysis
Intestinal total RNA extraction, quantification, RT and real-time PCR were carried out as previously described(
26
). The primer pairs used are shown in Table 4. The expression of the target genes relative to housekeeping gene (glyceraldehyde-3-phosphate dehydrogenase (GAPDH)) was analysed by the
![]() $2^{{{\minus}\Delta \Delta C_{T} }} $
method(
Reference Livak and Schmittgen
32
). The results reflected that GAPDH did not show any difference among four treatments. Relative mRNA expression level of each target gene was normalised to the control group (the piglets fed maize oil diet and injected with 0·9 % NaCl solution).
$2^{{{\minus}\Delta \Delta C_{T} }} $
method(
Reference Livak and Schmittgen
32
). The results reflected that GAPDH did not show any difference among four treatments. Relative mRNA expression level of each target gene was normalised to the control group (the piglets fed maize oil diet and injected with 0·9 % NaCl solution).
Table 4 Primer sequences used for real-time PCR

GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IRAK1, IL-1 receptor-associated kinase; MyD88, myeloid differentiation factor 88; NOD1, nucleotide-binding oligomerisation domain protein 1; NOD2, nucleotide-binding oligomerisation domain protein 2; RIP1, receptor-interacting protein 1; RIP2, receptor-interacting protein 2; RIP3, receptor-interacting protein 3; TLR4, toll-like receptor 4; TRAF6, TNF receptor-associated factor 6.
Protein abundance analysis
The analysis of protein abundance in intestinal mucosa was carried out according to the method described previously( Reference Pi, Liu and Shi 27 ). Specific primary antibodies included rabbit anti-claudin-1 (1:1000) from Invitrogen (no. 51–9000; Invitrogen Technology Inc.), mouse anti-heat-shock protein 70 (HSP70) (1:2000) from Stressgen (no. SPA810; Stressgen Biotechnology Corp.) and mouse anti-β-actin (1:10 000) from Sigma Aldrich (no. A2228; Sigma Aldrich Inc.). The relative abundance of target proteins (claudin-1 and HSP70) was expressed as the target protein:β-actin protein ratio.
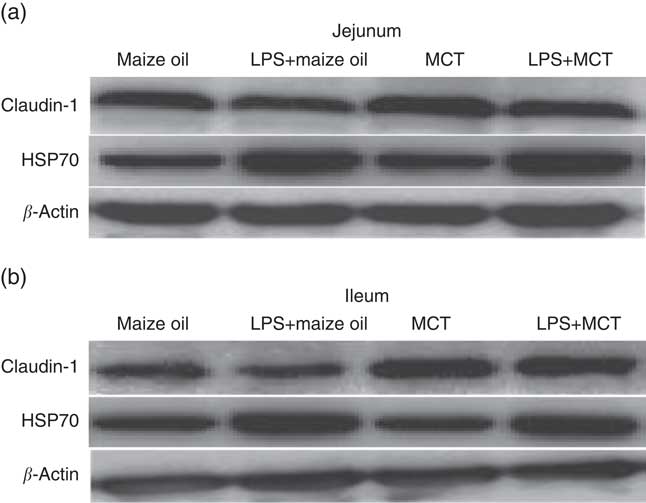
Fig. 1 Effects of medium-chain TAG (MCT) supplementation on protein expressions of claudin-1 and heat-shock protein 70 (HSP70) in jejunum (a) and ileum (b) in weanling pigs. The bands were the representative Western blot images of claudin-1 (22 kDa), HSP70 (70 kDa) and β-actin (42 kDa). LPS, lipopolysaccharides.
Statistical analysis
The growth performance data were analysed using Student’s t test of SAS (SAS Institute Inc.). Other data were analysed as a 2×2 factorial design using the GLM procedure of SAS. The individual piglet was the experimental unit for all parameters. The factors in the model included diet type, LPS injury and their interaction. Least squares means were derived for all treatments and were compared using the PDIFF (adjusted Tukey) and STDERR options of SAS. Results are expressed as least squares means with their standard error of the mean. When significant diet type×LPS treatment or a trend for diet type×LPS treatment occurred, post hoc testing was performed using LSD multiple comparison tests. The results were considered significant at P<0·05 and a trend between 0·05 and 0·10.
Results
Growth performance
There was no difference in average daily gain, average daily feed intake and feed conversion of piglets fed diet supplemented with maize oil or MCT (P>0·10; Table 5).
Table 5 Effects of medium-chain TAG supplementation on growth performance of weanling piglets (Mean values with their pooled standard error of the mean; n 12 (one piglet per pen))
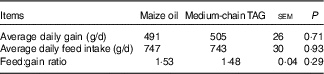
Intestinal mucosa fatty acid composition
LPS challenge decreased n-6 PUFA (P=0·08) and n-3 PUFA (P<0·01) levels and increased n-6:n-3 PUFA ratio in the jejunum (P<0·05; Table 6). Supplementation with MCT decreased n-6 PUFA content (P<0·01) and n-6:n-3 PUFA ratio (P<0·01) and increased n-3 PUFA levels in the jejunum (P<0·01). There was interaction between diet type and LPS challenge in the ileal n-6 and n-3 PUFA level and n-6:n-3 PUFA ratio of the piglets (P<0·05). The pigs fed MCT had higher n-3 PUFA level and lower n-6 PUFA level and n-6:n-3 PUFA ratio in ileum compared with pigs fed maize oil diets among LPS-treated pigs (P<0·05).
Table 6 Effects of medium-chain TAG supplementation on intestinal mucosa fatty acid composition after lipopolysaccharides (LPS) challenge in weaning pigs (%, of total fat) (Mean values with their pooled standard error of the mean; n 6 (one piglet per pen))
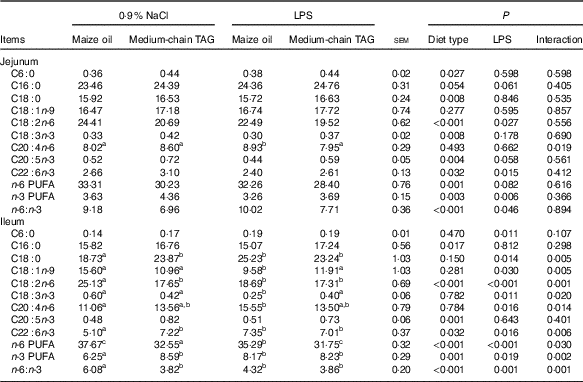
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Intestinal morphology
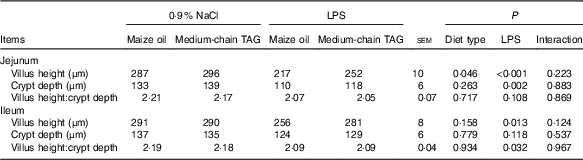
No interaction between diet type and LPS challenge was observed for villus height, crypt depth and height:crypt depth ratio (VCR) (P<0·10; Table 7). The piglets challenged with LPS had decreased villus height in jejunum and ileum (P<0·05), crypt depth in the jejunum (P<0·01) and VCR in the ileum (P<0·05). Supplementation with MCT increased villus height in the jejunum compared with the piglets fed maize oil diet (P<0·05).
Table 7 Effects of medium-chain TAG supplementation on intestinal morphology after lipopolysaccharides (LPS) challenge in weanling pigs (Mean values with their pooled standard error of the mean; n 6 (one piglet per pen))
Intestinal disaccharidase activity
LPS challenge reduced the activities of maltase in jejunum and ileum (P<0·05), and lactase and sucrase in ileum (P<0·05; Table 8). The piglets fed MCT diet had improved activities of sucrase and maltase in jejunum and ileum (P<0·05) compared with piglets fed maize oil diet.
Table 8 Effects of medium-chain TAG supplementation on intestinal disaccharidases activities after lipopolysaccharides (LPS) challenge in weanling pigs (U/mg protein) (Mean values with their pooled standard error of the mean; n 6 (one piglet per pen))
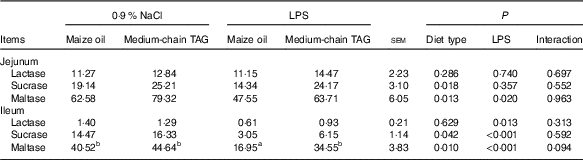
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Intestinal claudin-1 and heat-shock protein 70 protein expression and plasma TNF-α concentration
After LPS challenge, HSP70 expression was increased both in jejunum and in ileum (P<0·01) (Table 9; Fig. 1). Supplementation with MCT increased the expression of HSP70 protein in the jejunum (P<0·05), as well as claudin-1 protein both in jejunum and ileum (P<0·05). The plasma TNF-α concentration of the piglets challenged with LPS increased (P<0·01). There was also interaction between diet type and LPS challenge in the plasma TNF-α concentration of the piglets (P<0·05). The pigs fed MCT had lower plasma TNF-α concentration compared with pigs fed maize oil diets among LPS-treated pigs (P<0·05).
Table 9 Effects of medium-chain TAG supplementation on plasma TNF-α concentration, and intestinal claudin-1 and heat-shock protein 70 (HSP70) protein expressions after lipopolysaccharides (LPS) challenge in weanling pigs (Mean values with their pooled standard error of the mean; n 6 (one piglet per pen))
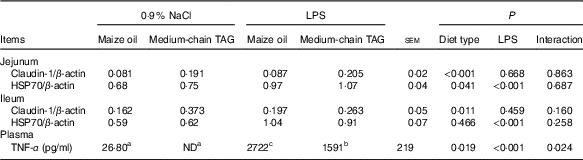
ND, not detected.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Intestinal mRNA expression of the key genes in toll-like receptors 4 and nucleotide-binding oligomerisation domain proteins pathways
Relative to saline piglets, the LPS pigs had higher mRNA abundance of TLR4, NOD1, NOD2, receptor-interacting protein 2 (RIP2) and TNF-α (P<0·05; Table 10) in the jejunum. Furthermore, the LPS pigs had increased mRNA abundance of TLR4, myeloid differentiation factor 88, tumour necrosis factor receptor-associated factor 6 (TRAF6), NF-κB, NOD1, NOD2, RIP2 and TNF-α (P<0·05) in the ileum. Compared with maize oil, supplementation with MCT decreased mRNA expression of TLR4, TRAF6, NF-κB, NOD2 and RIP2 (P<0·10) in the jejunum. In addition, there was an interaction between diet type and LPS challenge in mRNA expression of TRAF6, NOD2 and RIP2 (P<0·05) in the ileum. The pigs fed MCT had decreased ileal expression of TRAF6, NOD2 and RIP2 compared with pigs fed maize oil diets when treated by LPS (P<0·05).
Table 10 Effects of medium-chain TAG supplementation on mRNA expression of key genes in toll-like receptor 4 (TLR4) and nucleotide-binding oligomerisation domain proteins (NOD) in intestine after lipopolysaccharides (LPS) challenge in weanling pigs (Mean values with their pooled standard error of the mean; n 6 (one piglet per pen))
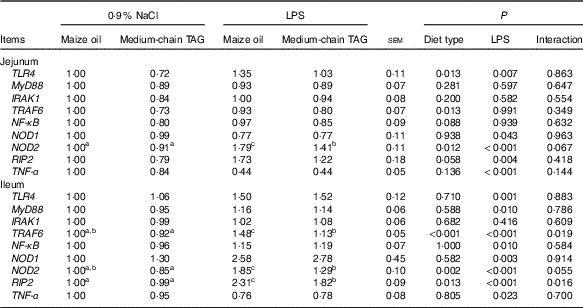
TLR4, toll-like receptor 4; MyD88, myeloid differentiation factor 88; IRAK1, IL-1 receptor-associated kinase; TRAF6, tumour necrosis factor receptor-associated factor 6; RIP2, receptor-interacting serine/threonine-protein 2.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Intestinal mRNA expression of the key genes in necroptosis pathways
The piglets challenged with LPS had higher mRNA expression of RIP1, RIP3 and mixed-lineage kinase domain-like protein (MLKL) both in the jejunum and in the ileum (P<0·05; Table 11). There was also interaction between diet type and LPS challenge in mRNA expression of RIP3 and MLKL in jejunum and ileum (P<0·01). The pigs fed MCT had decreased jejunal and ileal expression of RIP3 and MLKL compared with pigs fed maize oil diets when treated by LPS (P<0·05).
Table 11 Effects of medium-chain TAG supplementation on mRNA expression of key genes in necroptosis in intestine after lipopolysaccharides (LPS) challenge in weanling pigs (Mean values with their pooled standard error of the mean; n 6 (one piglet per pen))
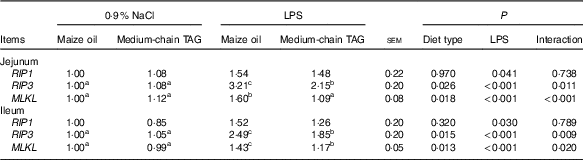
RIP1, receptor-interacting protein 1; RIP3, receptor-interacting protein 3; MLKL, mixed-lineage kinase domain-like protein.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Discussion
In this study, we explored the effect of MCT supplementation on intestinal integrity and function after a 4-h E. coli LPS challenge in a piglet model. As the results show, dietary MCT supplementation reduced the degree of enterocyte necroptosis, as well as production of intestinal pro-inflammatory cytokines via regulating inflammatory signalling pathways such as TLR4 and NOD, and thus protected intestinal integrity and function in piglets after LPS challenge.
The weanling piglets fed maize oil or MCT diets had no difference in growth performance during the former 21 d (before LPS challenge). The effect of MCT on the weanling piglet performance was not consistent with the previous research( Reference Hanczakowska, Świątkiewicz and Natonek-Wiśniewska 33 , Reference Price, Lin and van Heugten 34 ). The additive dose of MCT, individual difference of the piglets or the feeding time could lead to the conflicting results.
The trend of intestinal mucosal fatty acid composition is similar to the fatty acid proportion in diets. In agreement with our study, the previous reports showed that the dietary fatty acid proportion directly affects the body fatty acid composition( Reference Kouba, Enser and Whittington 35 ). Supplementation with MCT decreased the ratio of n-6:n-3 PUFA in diet, which led to the decreased ratio of n-6:n-3 PUFA in jejunum and ileum. n-3 PUFA play a positive role in body health such as anti-inflammatory action; however, n-6 PUFA has the opposite function( Reference Horrobin 36 ). In our study, the increased portion of n-3 PUFA in the intestinal mucosa of the piglets fed diet supplemented with MCT might provide a potential way to alleviate intestinal inflammation induced by LPS.
Villus height, crypt depth and VCR are key indicators reflecting gross intestinal morphology( Reference Liu, Huang and Hou 4 ). Many studies have shown that LPS challenge can result in a variety of morphologic alterations in the intestine such as submucosal oedema, haemorrhage, mucosal necrosis and epithelial lifting, reduced villus height and increased crypt depth( Reference Liu 3 , Reference Mercer, Smith and Cross 28 , Reference Touchette, Carroll and Allee 37 ). In this experiment, the piglets challenged with LPS had reduced villus height both in jejunum and in ileum, which indicates that LPS-induced intestinal morphologic injury. The jejunal villus height of the piglets fed MCT increased compared with pigs fed maize oil diets. The increased villus height would directly affect the nutrient absorption capability in the intestine as it would increase the absorptive and surface area. The finding in this experiment coincides with research done by Czernichow et al.( Reference Czernichow, Galluser and Cui 38 ) and Chwen et al.( Reference Chwen, Foo and Thanh 39 ), who found a positive association between supplementation of MCT and villus height.
Intestinal epithelial barrier is important in the maintenance of gut homoeostasis by preventing the penetration of luminal bacteria and dietary allergens into the mucosa( Reference Blikslager, Moeser and Gookin 2 ). Intestinal epithelial barrier integrity is maintained by cohesive interactions between cells via forming tight junctions( Reference Travis and Menzies 40 ). Claudin-1 is a key protein in tight-junction formation, and it determines permeability characteristics in many tissues especially the intestine( Reference Travis and Menzies 40 ). Generally, the increased expression of claudin-1 is associated with improved function of the epithelial barrier( Reference Pinheiro, Pacheco and Alvarenga 41 ). The disaccharides cannot be absorbed directly and they must be hydrolysed to monosaccharides by disaccharidases such as lactase, maltase and sucrase in intestine mucosa( Reference Weightac, Jonesac and Horna 42 ). Therefore, the disaccharidase activities are involved in the energy supply of the organism, and can mirror intestinal digestive function( Reference Weightac, Jonesac and Horna 42 ). In the present study, the activities of lactase, sucrase and maltase in the ileum were all reduced by LPS challenge, which is in accordance with previous studies( Reference Wang, Liu and Li 7 ). The data indicate that injection of LPS caused intestinal digestive injury in weanling piglets. Unexpectedly, LPS had no significant effect with the claudin-1 expression. Parts of previous studies showed that LPS challenge reduced the expression of claudin-1( Reference Chen, Liu and Wang 14 ). However, another part of researches demonstrated that LPS had no significant effect with claudin-1 expression( Reference Wang, Liu and Shi 6 , Reference Wang, Zhang and Wu 43 ). The reason for discrepancy may be the different animals or feeding environment. In our study, supplementation with MCT in the diets caused higher expression of claudin-1 and increased activities of sucrase and maltase in both jejunum and ileum compared with maize oil diet. These results demonstrated that MCT improved intestinal barrier and digestive function. Different from long-chain TAG, MCT has special function in metabolism, such as rapid digestion, passive absorption and obligatory oxidation( Reference Odle 17 ). Therefore, MCT can be used directly by the enterocytes for energy production, which could support the integrity of the intestine in weanling piglets( Reference Guillot, Vaugelade and Lemarchal 18 ).
We hypothesised that MCT exerted its beneficial effect on the intestine by reducing intestinal inflammatory response. TNF-α, as an intestinal pro-inflammatory cytokine, is an important marker of inflammation in intestine( Reference Liu, Huang and Hou 4 ). It is rapidly induced in the intestinal mucosa upon initial activation of immune cells, and is important for the further acceleration of the inflammatory response( Reference Zhu, Liu and Chen 44 ). In contrast, HSP70 is a key anti-inflammatory factor that can inhibit the expression of inflammatory pathway genes such as NF-κB( Reference Siggers, Siggers and Boye 45 , Reference Noti, Corazza and Mueller 46 ). Generally, the expression of HSP70 increases when the animals were under inflammation or stress. In this study, LPS challenge resulted in higher TNF-α concentration in plasma and higher expression of HSP70 in jejunum and ileum, which demonstrates that the piglets challenged with LPS were under inflammation. Supplementation with MCT reduced the expression of HSP70 in the jejunum and the concentration of TNF-α in plasma, which supports the notion that MCT is effective in reducing intestinal inflammatory response in weanling piglets.
To explore the deep molecular mechanism by which MCT might alleviate the intestinal inflammatory response, the role of inflammatory signalling pathways was determined. TLR and NOD are key protein families of inflammatory signalling pathways. They play a central role in detection of invading pathogens and induction of innate antibacterial and inflammatory responses. Current research has demonstrated that activation of TLR and NOD signalling is associated with multi-layered inflammatory intestinal diseases( Reference Satoshi, Rebecca and John 47 , Reference Leaphart, Cavallo and Gribar 48 ). Activation of these intracellular signalling pathways further leads to expression and release of pro-inflammatory cytokines such as TNF-α and then motivates the expression of anti-inflammatory cytokines such as HSP70( Reference Mansi and Garabet 49 ). In this experiment, the key genes such as TLR4, TRAF6 and NF-κB in the TLR4 signalling pathway, as well as NOD2 and RIP2 in the NOD signalling pathway, were all reduced in the piglets fed MCT compared with the piglets fed maize oil diet. These results demonstrate that MCT supplemented in diets could alleviate the intestinal inflammatory response by inhibition of TLR4 and NOD2 signalling pathways. Some previous reports showed that MCT could ameliorate inflammation in mice or humans, which is in accordance with our results( Reference Naso, Porto and Fillmann 50 , Reference Geng, Zhu and Xie 51 ).
Necroptosis as a recently identified form of programmed cell death, similar to necrosis, is characterised by the release into the extracellular milieu of immunogenic cytosol content, which leads to the activation of inflammatory signalling pathways such as TLR( Reference Li, Wang and Yu 52 ). Simultaneously, the activated inflammatory signalling pathways are intimately interconnected with cell death pathways including necroptosis( Reference Pasparakis and Vandenabeele 53 ). Therefore, necroptosis and inflammatory signalling pathways were interacted to result in more severe intestinal epithelial injury. RIP1, RIP3 and MLKL are key mediators in the necroptosis signalling pathway( Reference Gunther, Neumann and Neurath 54 ). In this study, LPS-challenged piglets had higher mRNA expression of RIP1, RIP3 and MLKL in jejunum and ileum, which demonstrates that LPS-induced epithelial cell necroptosis. This is in agreement with previous reports( Reference Negroni, Cucchiara and Stronati 55 , Reference Wen, Ling and Yang 56 ). Supplementation with MCT reduced the mRNA expression of RIP3 and MLKL in jejunum and ileum of the piglets, especially under LPS challenge. Thus far, little evidence shows the effect of additives on necroptosis. MCT may alleviate necroptosis of epithelial cell directly or indirectly through inhibiting inflammatory signalling pathways such as TLR4 or NOD.
In summary, dietary MCT supplementation improves intestinal morphology, disaccharidase activities and barrier function in piglets after LPS challenge. The beneficial effects of MCT on the intestine may be closely related to (1) decreasing expression of intestinal pro-inflammatory cytokine through inhibiting TLR4 and NOD signalling pathways; and (2) decreasing enterocyte necroptosis via suppressing mRNA expression of RIP3 and MLKL.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (31772615, 31422053 and 31372318), State’s Key Project of Research and Development Plan (2016YFD0501210) and the Project of the Hubei Provincial Department of Education (T201508).
The authors’ contributions are as follows: Y. L. designed the research; Y. L., S. C., H. W., Z. T., S. W. and J. Z. conducted the research; Y. L., X. W. and X. X. analysed data; Y. L. and X. X. wrote the paper; Y. L., H. Z. and C. W. edited and revised the manuscript; Y. L. had primary responsibility for final content. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.















