The prevalence of malnutrition is very high in elderly patients. This has been demonstrated in every healthcare setting (hospitalised, admitted to sub-acute care or living in home-care units)(Reference Vellas, Hunt and Romero1–Reference McWhirter and Pennington4) and particularly in long-term care facilities(Reference Arvanitakis, Beck and Coppens5, Reference Cereda and Pedrolli6). During the stay, nutritional status usually tends to deteriorate(Reference McWhirter and Pennington4). Moreover, also the risk of malnutrition is greater in patients aged over 65 years(Reference Arvanitakis, Beck and Coppens5, Reference Cereda and Pedrolli6). This should be emphasised, particularly in view of the increase in life expectancy. Accordingly, it is simple to appreciate the necessity of validated instruments able not only to detect malnutrition but also to predict nutrition-related complications. Indeed, identifying patients who might benefit from nutritional support could prevent deterioration of malnutrition and reduce length of stay, readmission rates, morbidity and mortality(Reference Kyle, Genton and Pichard7–Reference Correia and Waitzberg9). Simple assessment tools are suggested for clinical and routine use and, according to the European Society of Parenteral and Enteral Nutrition (ESPEN) guidelines(Reference Kondrup, Allison and Elia10) and recent systematic literature reviews, using the Mini Nutritional Assessment (MNA)(Reference Guigoz11, Reference Vellas, Guigoz and Garry12) in elderly patients is recommended and suggested, respectively, in most healthcare settings.
Faced with the difficulties in establishing usual or normal body weight and obtaining standing height in the elderly, the new Geriatric Nutritional Risk Index (GNRI) was recently proposed and investigated for predicting the risk of nutrition-related complications(Reference Bouillanne, Morineau and Dupont13–Reference Cereda, Limonta and Pusani17). The GNRI represents a modification of Buzby's Nutritional Risk Index (NRI) in whose formula, mainly based on albumin, usual body weight was replaced by ideal body weight(Reference Bouillanne, Morineau and Dupont13). The GNRI was presented as a nutrition-related risk index but its correlations with biochemical (albumin, prealbumin, total lymphocytes count) and anthropometric (weight loss, arm circumference, triceps skinfold) indices of nutritional status led to hypothesising a possible use of this new nutritional indicator also in the assessment of nutritional status(Reference Bouillanne, Morineau and Dupont13, Reference Cereda and Vanotti15–Reference Cereda, Limonta and Pusani18), as was previously made for the NRI(Reference Buzby, Knox and Crosby19, Reference Kyle, Pirlich and Schuetz20). Nevertheless, there is some evidence that, as a nutritional marker, albumin seems to better describe disease severity than nutritional status(Reference Cereda and Pedrolli6, Reference Buzby, Knox and Crosby19). Indeed, nutritional assessment tools do not provide the same information. However, the common purpose is to predict the probability of a better or worse outcome due to nutritional factors and a tool's validity should be more consistently discussed in view of this(Reference Guigoz11). Accordingly, it is also necessary to look at sensitivity, specificity and the predictive values. In regard to this purpose, comparisons between tools might be of interest but for the GNRI scant data are available(Reference Cereda and Pedrolli21, Reference Cereda, Zagami and Vanotti22).
Thus, the aim of the present study is to investigate the ability of the GNRI to assess nutritional status and predict the outcome of home-care resident elderly, when compared with the MNA.
Methods
Subjects, anthropometry and biochemistry
The present study was performed in adherence to the principles established with the Declaration of Helsinki. The protocol was approved by the local Ethics Committee. We also obtained written informed consent for every subject included in the study.
Institutionalised elderly living in two different long-term care structures of the province of Como were considered. Baseline data were collected over a 2-month period (February and March 2005). Exclusion criteria were the presence of well-known liver, renal or neoplastic disorders. In this regard, confirmation was obtained through the evaluation of plasma liver enzymes and creatinine levels. Venous blood samples were drawn after 8–12 h fasting with the patient in the recumbent position and further assessed for serum albumin and prealbumin.
Two well-experienced operators (D. L. and C. P.) collected anthropometric data: weight, to the nearest 0·1 kg by the same calibrated scale (Seca 861; Seca, Hamburg, Germany), chair scale or hoist-provided weighing device (for those bedridden); mid-upper arm and calf circumferences, to the nearest 0·5 cm by a flexible tape; triceps skinfold, to the nearest 0·2 mm by a Holtain calliper (Holtain Ltd, Crymych, Dyfed, UK); knee height, to the nearest 0·5 cm by an anthropometric calliper (Metrica, Italy), according to standard procedures previously described(Reference Cereda, Limonta and Pusani23). The mean of three different measurements was taken into account for triceps skinfold, mid-upper arm circumference, calf circumference and knee height. Estimated height (EH) was extrapolated from knee–heel length according to the equations validated by Chumlea et al. (Reference Chumlea, Roche and Steinbaugh24). Mid-arm muscle area, muscle arm circumference and BMI were calculated(Reference Frisancho25). Ideal body weight, necessary for GNRI determination, was derived by using the following equations of Lorentz(Reference Bouillanne, Morineau and Dupont13):
Weight loss and relative percentage were retrospectively obtained from the 3-month-previous weight recorded on the clinical register of every patient.
Mini Nutritional Assessment
Baseline nutritional status was defined and graded according to the MNA. This tool consists of eighteen questions grouped in four rubrics addressing anthropometry (BMI, weight loss, mid-upper arm and calf circumferences), general state (medications, mobility, presence of pressure ulcers, lifestyle, presence of psychological stress or neuropsychological problems), dietary assessment (autonomy of feeding, quality and number of meals, fluid intake) and self-perception about health and nutrition, respectively. After having all the items answered, a maximal score of thirty points is achievable while threshold values are set as follows: adequately nourished, MNA ≥ 24; at risk of malnutrition, MNA = 17-23·5; protein–energy malnutrition, MNA < 17(Reference Guigoz11, Reference Vellas, Guigoz and Garry12). In the case of cognitive impairment, self-perception questions regarding health and nutritional status were answered by the nursing staff. Patients receiving nutrient and fluid needs via tube feeding (naso-gastric or gastrostomy; n 8) were assigned the highest scores to the questions concerning the number of meals, protein and fruit/vegetable intakes, fluid consumption, appetite and mode of feeding, while those undergoing oral supplements (n 10) were considered as meeting energy and protein goals. Enteral nutrition was not counted as prescription drug use.
Geriatric Nutritional Risk Index
Nutritional risk of health complications was assessed by GNRI score through the equation of Bouillanne et al. (Reference Bouillanne, Morineau and Dupont13):
Categorisation of the patients was performed according to the following cut-offs: severe/moderate risk, < 92; low risk, 92–98; no risk, > 98(Reference Bouillanne, Morineau and Dupont13). Differently from the categorisation in four classes proposed by Bouillanne et al., in the present study we avoided distinguishing the ‘severe risk’ group (GNRI < 82) from the ‘moderate risk’ one (GNRI 82 to < 92) because these two categories have been demonstrated to present a similar increased risk (OR) of overall health complications and of those other than mortality (bedsores or infections)(Reference Bouillanne, Morineau and Dupont13). Moreover, this categorisation let us obtain a three-category tool similar to the MNA.
Follow-up and outcome definition
Patients were followed for 6 months for the occurrence of major health complications: death; infections (septicaemia, pneumonia, urinary tract infection, candida); bedsores. Sepsis was defined as fever (> 38°C) or hypothermia ( < 36°C) and ≥ one positive blood culture for pathogenic organisms. Pneumonia was diagnosed in the presence of fever (> 38°C), a clinical sign, and radiographic confirmation. Urinary tract infection required fever (> 38°C), a clinical sign, and bacteriological confirmation of ≥ 105 organisms/ml urine. Candida infection required the isolation of Candida spp. on secretion (oral, genital, cutaneous) by tampon sampling.
Statistical analyses
Data are presented as mean values and standard deviations. We evaluated the relationship between the variables and both the MNA and GNRI by Pearson's simple correlation model and we compared groups for quantitative variables by one-way ANOVA. Control for overall type I error was performed by Scheffe's post hoc comparison test.
Patients were categorised and a severity score was assigned according to nutrition state based on the MNA (MNA < 17 = 2; 17–23·5 = 1; ≥ 24 = 0) and to nutrition risk as defined by the GNRI (GNRI < 92 = 2; 92–98 = 1; > 98 = 0)(Reference Bouillanne, Morineau and Dupont13). We used the χ2 test or Fisher's exact test (used when expected values were < 5) to compare prevalence among nutritional classes and Cohen's kappa test to analyse the agreement between the assessment methods.
To evaluate the association with outcome of both these tools, we calculated gross OR and 95 % CI; for each calculation, the unexposed patients were those with a severity score = 0 (GNRI > 98 and MNA > 24, respectively). Along with this, we carried out multiple logistic regression analyses to test independent associations.
Finally, to fullfill the main purpose of the study, the sensitivity, the specificity and the predictive values (positive and negative) of different GNRI and MNA cut-offs were calculated to assess the reliability of these tools in predicting overall complications.
All statistical analyses were performed by STATISTIX 7.0 (Analytical Software, Tallahassee, FL, USA). The level of significance was established as a two-sided P value < 0·05.
Results
Baseline data analyses
A total of 241 institutionalised elderly were recruited (ninety-four males; 147 females). Mean age was 80·1 (sd 8·3) years (range 65–99 years).
The clinical and anthropometric characteristics of the GNRI and MNA categories, as well as the results of both Pearson's correlation model and ANOVA analyses, are shown in Table 1. According to the simple correlation model, the MNA score closely correlated with the GNRI (Fig. 1), most of the parameters and mildly only with sex. Similar results were found by ANOVA analysis. When considering GNRI correlates, compared with all the other variables, only muscle arm circumference and arm muscle area presented a mild association. In addition, no correlation was observed with age and sex. In the same way, after conducting ANOVA between GNRI groups, no difference was detected for age while a mild one was observed for muscle arm circumference and arm muscle area. Interestingly, when considering results from post hoc comparison of means (Scheffe's test) we observed that both malnourished and at-risk patients by the MNA presented lower values of nutritional indices than those well nourished. However, according to GNRI scoring, only high-risk subjects (GNRI < 92) showed more depleted nutritional parameters (Table 1). Moreover, whilst no association was found between nutritional risk (GNRI) and sex, females patients were more likely to be malnourished or at risk of malnutrition by the MNA.
Table 1 Statistical description and comparison of nutritional indices among Geriatric Nutritional Risk Index (GNRI) and Mini Nutritional Assessment (MNA) categories, according to Pearson's simple correlation model and one-way ANOVA
(Mean values and standard deviations)

r, Pearson's correlation coefficient; MUAC, mid-upper arm circumference; TSF, triceps skinfold; MAC, muscle arm circumference; AMA, arm muscle area.
Post hoc comparison of means was performed by Scheffe's test (* significant for overall).
Significant correlation: † P < 0·05, †† P < 0·01, ††† P < 0·001.
‡ P values were computed according to ANOVA except for the sex difference (χ2 test).

Fig. 1 Correlation between Geriatric Nutritional Risk Index (GNRI) and Mini Nutritional Assessment (MNA) according to Pearson's simple correlation model (; r 0·51; P < 0·001) and their association with 6-month outcome. (■), Complications (n 28): infections, bedsores and death; (○), no complications. A GNRI score of 92 is the cut-off (- - -) for a high risk of nutrition-related complications; a GNRI score of 98 is the cut-off for no risk of of nutrition-related complications. An MNA score of 17 is the cut-off for malnutrition; an MNA score of 24 is the cut-off for good nutritional status.
Prevalence (n and %) of subjects among GNRI and MNA categories is presented in Table 2.
Table 2 Distribution of the population and complications among nutritional classes according to the Mini Nutritional Assessment (MNA) and the Geriatric Nutritional Risk Index (GNRI)
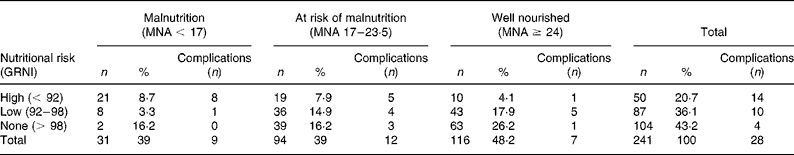
A significant difference (χ2 test; P < 0·001) in prevalence of nutrition risk and a poor agreement (linear weighted Cohen's kappa test; κ = 0·29 (95 % CI 0·19, 0·39)) was detected between the assessment methods.
Outcome data analyses
At the end of the follow-up period (6 months), major complications occurred in twenty-eight patients (11·6 %). Of these twenty-eight patients, six died (one from septic complications of bedsores). Infectious complications occurred in nineteen subjects (twelve pneumonia; two sepsis; four urinary tract infection; one candida), while eight developed bedsores. Four patients had both bedsores and infection. The coexistence of more than a complication was computed as one in overall analyses. The distribution of complicated patients among nutritional classes is presented in Table 2; their nutritional features are described in Table 3.
Table 3 Nutritional features of the population
(Mean values and standard deviations)

MUAC, mid-upper arm circumference; TSF, triceps skinfold; MAC, muscle arm circumference; AMA, arm muscle area; GNRI, Geriatric Nutritional Risk Index; MNA, Mini Nutritional Assessment.
* The coexistence of more than a complication was computed as one in overall analyses (n 28). Complications were: infections (n 19); bedsores (n 8); death (n 6).
† Statistical comparison between complicated and uncomplicated patients was performed by the two-sample t test (P values chosen according to Bartlett's test for equality of variances) except for the sex difference (χ2 test).
The risk of health complications related to GNRI and MNA categories was initially described as an OR (Table 4). For the GNRI, the overall complications OR of every risk class (GNRI < 92 and 92 ≤ GNRI ≤ 98) was significantly higher than that of unexposed subjects (GNRI > 98; OR 1), while for the MNA only malnourished patients (MNA < 17) presented a significantly increased risk. Compared with malnutrition (MNA < 17), a high nutritional risk (GNRI < 92) showed a stronger association with the risk of single complications, particularly with death. However, the risk of overall and single complications was similar (P>0·05) in both GNRI < 92 and MNA < 17 categories. Along with this, we report significant differences in overall outcomes and death OR (P < 0·05 and P < 0·002, respectively) between high-risk (GNRI < 92) and low-risk (GNRI = 92–98) patients and in death OR (P < 0·05) between malnutrition (MNA < 17) and risk of malnutrition (MNA = 17–23·5).
Table 4 Risk of overall and single complications among nutritional categories by the Mini Nutritional Assessment (MNA) and Geriatric Nutritional Risk Index (GNRI)†
(Odds ratios and 95 % confidence intervals)
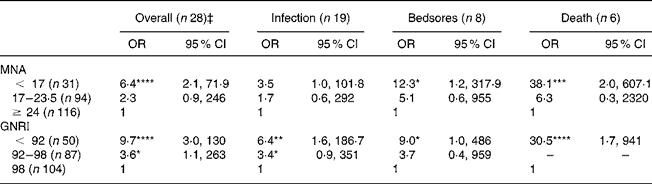
* P < 0·05, ** P < 0·01, *** P < 0·002, **** P < 0·001.
† Comparisons with the unexposed patients (OR 1; MNA ≥ 24 and GNRI > 98 respectively) were assessed by the χ2 test or Fisher's exact test (used when expected values were < 5).
‡ The coexistence of more than a complication was computed as one in overall analyses.
According to the sex- and age-adjusted univariate logistic regression model, overall complications were significantly associated with both GNRI (OR 2·93 (95 % CI 1·68, 5·10); P < 0·001) and MNA (OR 2·23 (95 % CI 1·25, 3·97); P = 0·006) severity scores. Then, all the variables were run together and only the GNRI (OR 2·54 (95 % CI 1·38, 4·68); P < 0·003) was detected as a significant independent predictor (MNA: OR 1·40 (95 % CI 0·74, 2·64); P = 0·296).
Finally, we aimed to fully investigate the ability of these tools to predict outcome by calculating the sensitivity, the specificity and the predictive values of the threshold values currently in use (Table 5). A high sensitivity and negative predictive value are required for a good screening tool, whereas a high specificity and positive predictive values are required for diagnosis. The GNRI's cut-offs ( < 92 and < 98) showed slightly higher, but not significant, sensitivity and NPV when compared with those of the MNA ( < 17 and < 24, respectively). In overall-outcome prediction, a good sensitivity (> 0·80) was detected only for a GNRI < 98. Nevertheless, the association of a GNRI > 98 with an MNA > 24 seemed to exclude best the occurrence of complications (Fig. 1).
Table 5 Analysis of different Mini Nutritional Assessment (MNA) and Geriatric Nutritional Risk Index (GNRI) cut-offs in the identification of complicated patients
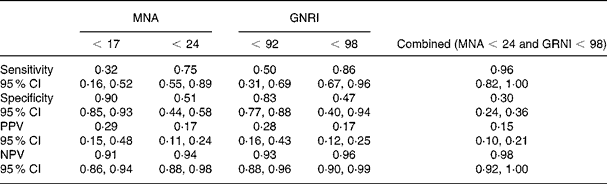
PPV, positive predictive value; NPV, negative predictive value.
Discussion
The idea for the present investigation was suggested by previously observed associations between the GNRI and other indices of nutritional status (anthropometric and biochemical)(Reference Cereda and Vanotti15–Reference Cereda, Limonta and Pusani18). In agreement with the results found in similar series(Reference Cereda and Vanotti15–Reference Cereda, Limonta and Pusani18), the GNRI confirms a good capacity, although not comparable with the MNA(Reference Guigoz11, Reference Alves de Rezende, Marquez Cunha and Alvarenga26–Reference Vellas, Guigoz and Baumgartner28), in detecting differences among frequently used nutritional parameters. In fact, the GNRI appeared strongly associated with anthropometric and biochemical variables only when low scores were recorded (GNRI < 92). However, when the MNA was considered for grading nutritional status, a more reliable discrimination of nutritional parameters was observed between classes. This fact might probably be related to the multiple items (eighteen questions) grouped in four rubrics (anthropometric assessment; general assessment; short dietary assessment; subjective assessment) on which the MNA was structured and developed as well as to the fact that some of the parameters associated are included in the method itself(Reference Guigoz11).
The present paper clearly represents an attempt to validate the GNRI as a nutritional assessment tool. This was made through the comparison with the MNA, as it is recommended by the European Society of Parenteral and Enteral Nutrition (ESPEN) for grading the nutritional state of the elderly(Reference Kondrup, Allison and Elia10). Moreover, the recent review by Guigoz(Reference Guigoz11) has summarised its sensitivity to this purpose and its reliability as a basic instrument for geriatric assessment by healthcare professionals. On the other hand, the MNA cannot be considered the ‘gold standard’ of nutritional assessment methods and this consideration puts in evidence an obvious limit of our analysis. Up to now, no consistent cross-validation of the GNRI has been produced(Reference Cereda and Pedrolli21, Reference Yamada, Furuya and Takita29).
According to the present results, and despite the significant relationship between the MNA and GNRI, these tools appeared to perform differently, showing also a poor agreement, in grading nutritional status. Moreover, at least in the present study, there is evidence that the GNRI is better than the MNA in predicting complications, although no significant differences in gross risk were found between patients malnourished (MNA < 17) and those at high nutritional risk (GNRI < 92).
It is worthy of mention that the GNRI has been described as a nutrition-related risk index and not an index of malnutrition(Reference Bouillanne, Morineau and Dupont13). In addition, the prognostic value of this tool has recently been confirmed when addressing its relationship with muscle dysfunction, another well-accepted prognostic indicator(Reference Cereda and Vanotti15, Reference Cereda and Vanotti16), and its association with total lymphocyte count in predicting short-term nutrition-related complications(Reference Cereda, Limonta and Pusani17). However, when comparing the present outcome data with those by Bouillanne et al. (Reference Bouillanne, Morineau and Dupont13), we highlight that no significant differences were detected in risk (OR) between high-/moderate-risk (GNRI < 92) and low- (92 ≤ GNRI ≤ 98) risk patients. This might probably be related to the type of patients we enrolled (institutionalised) and to the categorisation used to grade nutritional risk. Despite the high prevalence of malnutrition and risk for malnutrition in institutions, and the present results agree with previous reports(Reference Guigoz11, Reference Hudgens and Langkamp-Henken30), subjects who live in long-term care facilities are usually less complicated than patients admitted to rehabilitation sub-acute cares in which the pre-existing acute illness is frequently related to hypoalbuminaemia and weight loss(Reference Thomas, Zdrowski and Wilson3, Reference Cereda and Pedrolli6, Reference Cereda and Vanotti15, Reference Cereda, Zagami and Vanotti31). However, it is reasonable to argue that the stronger association with death is probably related to the high weight given to albumin(Reference Cereda and Pedrolli6, Reference Cereda, Zagami and Vanotti22, Reference Cereda, Zagami and Vanotti31) that, although related to hydration or inflammation(Reference Ballmer32), is usually considered an in-acute prognostic indicator(Reference Omran and Morley33, Reference Knaus, Wagner and Draper34). On the other hand, we underscored that albumin might also reflect chronic undernutrition and deconditioning, probably related to poor dietary habits(Reference Cereda and Pedrolli6, Reference Cereda and Vanotti16). Thus, the GNRI, as it is structured, seems to identify the presence of an acute stress and, at the same time, to mirror the unavailability of protein-energy stores(Reference Cereda and Pedrolli6).
Given the association with some inflammatory markers (for example, albumin and C-reactive protein), the MNA has also been reported to mirror acute stresses well(Reference Guigoz11, Reference Vellas, Guigoz and Baumgartner28) while disease-related changes in oral intake, as assessed by simple MNA questions, have been demonstrated to predict weight loss(Reference Wilson, Thomas and Rubenstein35) and poor outcome(Reference Kagansky, Berner and Koren-Morag36).
Simple, accurate and highly sensitive assessment methods are the best in clinical practice. The MNA has been consistently investigated and validated in regard to nutritional assessment and outcome prediction(Reference Kondrup, Allison and Elia10, Reference Guigoz11). Another of the advantages of using the MNA is that it does not depend on biochemical investigation, thus being inexpensive and making it theoretically suitable for every setting (community, general practitioner, home care, out-patient, hospital and institution). However, a weakness has been pointed out in the evaluation of long-term care residents(Reference Hudgens and Langkamp-Henken30). Sometimes, it is difficult to have some questions answered (for example, those concerning weight loss, cognitive impairment or objective evaluation of disabilities) without the help of caregivers (for example, family, nurses, home-care staff), and this was the case in the present experience. Moreover, other authors have also discussed limitations of the MNA in reason of its tendency to overdiagnose elderly at risk, since the consequences of a positive screening result are still uncertain(Reference Beck, Holst and Rasmussen37).
The GNRI was designed to face the frequent incapacity of the old patient to participate in the assessment procedures and, particularly, difficulty in obtaining usual weight and standing height(Reference Bouillanne, Morineau and Dupont13, Reference Cereda, Limonta and Pusani23). Requiring quick routine measurements (albumin, weight and knee height) with a low-grade participation of the patient, its use may be hypothesised in all the settings but further larger studies are required(Reference Cereda and Pedrolli6). Indeed, the present sample size is an obvious limitation of the present study.
In conclusion, after this preliminary comparison, it is difficult to assert that an index is preferable to another, particularly when considering the rich literature addressing the MNA. Further studies and comparisons with other tools (Nutritional Risk Score 2002, Malnutrition Universal Screening Tool, etc) might be considered.
Currently, performing both the assessments methods, and combining their results, would probably allow a better categorisation of the patient and the identification of those who might benefit more from early nutritional intervention. It is well accepted that the MNA detects risk of malnutrition before severe change in weight or serum proteins occurs and intervention studies have demonstrated that timely intervention can stop weight loss in elderly at risk of malnutrition or undernourished(Reference Guigoz11). However, the GNRI appears to describe the patient and assess the risk of complications on the basis of unfavourable changes in both weight and visceral proteins that have already established. Unfortunately, no intervention studies according to risk by the GNRI have been yet performed. A GNRI less than 92 was recently suggested as the clinical trigger for routine nutrition support but also other risk categories (92 ≤ GNRI ≤ 98) should be investigated in guiding effective intervention(Reference Cereda and Pedrolli6, Reference Cereda, Zagami and Vanotti31).
In the future, comparisons between tools should probably address the effectiveness of nutritional intervention, not only in institutions but also in other healthcare settings.
Acknowledgements
We grateful acknowledge Professor Alberto Battezzati and Dr Simona Bertoli for their assistance in reviewing the manuscript.
All the authors significantly contributed to the work, read and approved the final manuscript. E. C. was the main author of the manuscript, and analysed and interpreted data. E. C., C. P. and D. L. collected the data. A. V. supervised the study.
The authors certify that there are no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter or materials discussed in the paper. There are no sources of funding to declare.








