The gradual introduction of solid foods, known as the ‘weaning process’ (or complementary feeding), is essential to provide for the increasing nutritional requirements during an infant's first year(Reference Calvo, Galindo and Apres1, 2). The WHO(3) and the Department of Health and Children in Ireland(4) recommend exclusive breast-feeding during the first 6 months postpartum, with the introduction of solids at 6 months. Despite this WHO(3) recommendation, global debate over the optimal weaning age continues to challenge whether 4, 6 months or a window between these two time points should be taken as the optimal age for introducing solids to an infant's diet(Reference Khakoo and Lack5, Reference Ward Platt6). While ‘demand weaning’ challenges the notion that there may not be a particular age for introducing solids to an infant's diet(Reference Ward Platt6), this concept has gathered momentum in the years following the introduction of advice by WHO in 2001(3) to delay weaning to 6 months postpartum, and after the completion of the present study (after 2006). However, in Ireland, and in line with guidance from the European Society for Paediatric Gastroenterology, Hepatology and Nutrition(Reference Agostoni, Decsi and Fewtrell7), the consensus that the earliest introduction of solids should not be done before 4 months or 17 weeks remains an unchanged recommendation currently(8).
The deleterious short- and long-term health implications of both early and delayed weaning are well documented. Early weaning onto solids has been associated with increased risk of allergy(Reference Kajosaari9, Reference Armentia, Banuelos and Arranz10), eczema(Reference Fergusson, Horwood and Shannon11, Reference Forsyth, Ogston and Clark12) and enteritis(Reference Popkin, Adair and Akin13). Weaning at ≤ 12 weeks has been shown to increase the incidence of respiratory illness in infants aged 14–26 weeks and persistent cough at 27–39 weeks(Reference Forsyth, Ogston and Clark12). There is also some evidence to suggest that early introduction to solid foods may result in increased percentage body fat in childhood(Reference Wilson, Forsyth and Greene14). Conversely, weaning infants beyond the recommended time may deleteriously affect the feeding behaviour(Reference Northstone, Emmett and Nethersole15), and may lead to nutritional deficiencies(Reference Hendricks and Badruddin16) and failure to thrive(Reference Wright and Birks17).
Considering the international recommendation to initiate weaning at 6 months(3), studies from the UK(Reference Wright, Parkinson and Drewett18, Reference Bolling, Grant and Hamlyn19), Belgium(Reference Schiess, Grote and Scaglioni20) and Sweden(Reference Hornell, Hofvander and Kylberg21) highlight that a large proportion of infants are prematurely weaned at < 4 months of age; however, few studies have examined the reasons and risk factors for early weaning.
In addition to the importance of the timing of weaning, compliance with the recommended weaning practices can prevent certain nutritional issues in healthy term infants, including fussy eating, faltering growth, constipation and Fe-deficiency anaemia(Reference Harrod-Wild22). Furthermore, as early dietary patterns and feeding behaviours formed in infancy can determine childhood feeding habits and food preferences(Reference Devine, Wolfe and Frongillo23, Reference Skinner, Carruth and Bounds24) and as they have been implicated in the development of childhood overweight and obesity(Reference Wilson, Forsyth and Greene14, Reference Ong, Emmett and Noble25), both the types of foods consumed by infants and weaning practices deserve greater attention.
Monitoring infant feeding practices, particularly since the introduction of advice by WHO(3) to delay weaning to 6 months, is essential if targeted interventions to improve services are to be provided in an effective and structured manner(8). However, the extent to which mothers in Ireland adhere to weaning recommendations is not known. Thus, the objectives of the present study were to examine the weaning practices of mothers in Ireland at 6 months postpartum, to establish the timing of weaning and to identify the factors predicting, or associated with early weaning.
Subjects and methods
This prospective, observational study involved the recruitment of pregnant women from the Coombe Women and Infants University Hospital (CWIUH) in west Dublin (Ireland), which is one of the three maternity hospitals in the Dublin region. Details of the methods and representativeness of the sample have been reported elsewhere(Reference Tarrant, Younger and Sheridan-Pereira26). Briefly, between June 2004 and October 2006, 539 pregnant women at ≥ 24 weeks gestational age who attended either public or private/semi-private antenatal clinics were invited to participate in the study. From the 491 women who agreed to participate (91 % recruitment rate) and of those who delivered a healthy, singleton, term infant at ≥ 37 weeks gestational age, 483 (98 %) women were subsequently followed up at 6 weeks and 454 (92 %) women were followed up at 6 months postpartum. Following the application of inclusion criteria at the 6-month follow-up, including mothers who were living in Ireland and those who were willing to participate in the follow-up, 401 Irish national mothers were included in the final sample. Ethical approval for the present study was obtained from the Dublin Institute of Technology and the CWIUH Research Committees, and informed written consent was given by all the mothers.
Pre-pilot tested semi-structured questionnaires were used at each of the three contact points with mothers exclusively. The first contact point involved the completion of a subject-completed questionnaire, which aimed to elicit information on maternal/paternal socio-economic data, as well as specific enquiry about the age (week) at which an infant should be introduced to solid foods. The first questionnaire also collected data on mothers’ smoking and alcohol status during pregnancy, including smokers v. non-smokers, number of cigarettes smoked/d, alcohol consumers v. non-alcohol consumers, number of alcohol units consumed/week. Obstetric details pertaining to the birth, including type of delivery, gestational age and health status of the infants at birth, were documented from the mothers’ medical notes. Characteristics of the infants (sex, birth weight, and health status after birth) were recorded from the infants’ medical notes. The 6-week and 6-month interviewer-administered surveys were conducted via telephone or face to face by a trained paediatric dietitian. The average duration of the 6-month survey interview was between 20 and 30 min, and during this time period, information was collected on timing of weaning and practices, including additions to infants’ weaning foods (multiple response options that were given included ordinary gravy, baby gravy, salted/unsalted butter, non-infant-specific sauces, sugar/honey, vegetable stock and table salt), and maternal sources of advice on infant feeding from 6 weeks to 6 months (open-ended responses which were then categorised).
Using a non-prompted open-ended question on mothers’ reasons for initiating weaning, specific and individual ‘reasons’ were collected, which were then entered singly into the database as per mothers’ responses, e.g. ‘the infant was hungry all the time and not satisfied with milk alone’, ‘complied with weaning recommendations’, ‘breast milk supply was decreasing, so had to introduce solids’ and ‘maternal perception that the infant was a big baby and needed more than milk’. Mothers could give more than one reason for initiating weaning, e.g. infant hunger and infant sleep promotion. Cross-tabulations between each of the individually entered ‘reasons for introducing solids’ and the dichotomous variable ‘weaned at ≤ 12 weeks/>12 weeks’ were then performed. Only the reasons for initiating weaning that were statistically significant were presented.
Dietary information was obtained via maternal reporting of a short dietary history of the infant's usual diet. For infants who were consuming formula milk, data were collected on the feeding frequency/24 h as well as on the volume of milk consumed per feed and in a 24 h period. As food frequency components of the dietary assessment, data pertaining to the frequency of infants’ usual snack consumption per week were recorded. Data were also obtained on the daily volumes and type of supplementary fluids consumed by the infants.
Explanatory measures
The following weaning practices were examined:
1. Age (weeks) at the introduction of solid foods. Solid foods, or solids, were defined as any food offered to the infants other than their main milk drink. The definition of exclusive breast-feeding was in accordance with the WHO(27). In the present study, 4 weeks was taken to be 1 month, and mothers’ understanding of 4 months being equivalent to 16 or 17 (calendar) weeks was clarified.
2. Meal episodes were defined by the time of the day the particular meal was consumed, and were differentiated as breakfast (consumed from the time of waking until 12.00 hours), lunch (consumed between 12.00 and 15.00 hours) and an evening meal (consumed between >15.00 and 18.00 hours). Any sweet/savoury food consumed as a course after a meal, including fruit purée and sweet varieties of commercially prepared infant-specific desserts, were also recorded, and combined with the foods consumed at the routine meal. Commercially prepared infant-specific foods refer to both ready-prepared jarred infant foods and packets of dried infant foods, preparation of which requires addition of water/milk. Home-prepared infant-modified meals refer to meals specifically prepared for infants, conducive to healthy eating guidelines and cooked without the use of added condiments, including sauces, gravy, sugar or salt. Home-prepared non-infant-modified meals were those that included unsuitable additions (salt, gravy, sauces, vegetable stock and sugar), and were served as meals to the family, as well as to the infant.
3. A ‘snack’ was defined as any sweet/savoury food offered to infants in between the routine meals. Data elicited on the usual type of snacks consumed by infants were open-ended responses, which were then categorised as infant rusks, yoghurt (infant/non-infant-specific), infant-specific baby biscuits, fruit/vegetables, chocolates, biscuits, bread and butter, crisps and ice-cream. Data on the weekly frequency of consumption of each listed snack were collected, and the infants were differentiated as those who consumed a snack 1–4 or >4 times/week.
4. Mothers were questioned on the texture of the foods consumed by the infants at 6 months, and were provided with the following categories from which they had to choose: puréed (representing the first stage of weaning), soft/smooth, mashed/minced, soft/lumpy and roughly chopped.
5. ‘Supplementary fluids’ were defined as any fluid consumed by infants other than their main milk drink. Data elicited on the type of daily supplementary fluids were open-ended responses, which were then categorised as water, water and sugar mixture, fruit juice, diluted/undiluted baby juice, full-fat cows' milk and a separate category for non-recommended fluids for infants, including carbonated drinks and non-herbal tea. In accordance with the American Academy of Pediatrics guidelines that advise a daily limit of 120–180 ml of fruit juice during infancy(28), supplementary fluids were categorised as being consumed in volumes < 180 ml or ≥ 180 ml/d.
Statistical analyses
The Statistical Package for the Social Sciences statistical software package version 17.0 (SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses. Data were summarised using numerical descriptive statistics including means with standard deviations and medians with interquartile ranges. The relationship between categorical data was analysed using cross-tabulations and χ2 statistical tests. Statistical significance was taken at P < 0·05. The relationship between the actual age of infants at weaning (week) and mothers’ antenatal reporting of when infants should be weaned (week) onto solids (two continuous variables) was explored using Spearman correlation coefficient for non-parametric data.
In accordance with the literature(Reference Bolling, Grant and Hamlyn19, Reference Giovannini, Riva and Banderali29, Reference Alder, Williams and Anderson30), putative variables that were considered risk factors for early weaning were examined in the univariate analysis, including maternal/paternal socio-demographic factors (using education level as a proxy for socio-economic status) and infant characteristics (parity, sex, gestational age at birth and birth weight). Factors related to maternal health behaviours during pregnancy were also examined, including smoking and alcohol consumption, as well as maternal postnatal employment-related factors such as timing of return to work postpartum ( ≤ 18 v. >18 weeks), employment status at 6 months postpartum (working full-time, part-time and non-working) and infant attendance to a child-minding facility during the first 6 months. Factors pertaining to infant feeding status during the first 6 months and mothers’ reported sources of advice on infant feeding were all examined.
Binary logistic regression was then used to determine the variables that independently predicted early weaning with ‘infants weaned at ≤ 12 weeks’ being the dependent variable. Multiple forward stepwise logistic regression was performed, and multivariate binary logistic regression models were developed based on a priori literature and the results obtained from the univariate modelling in the present study. Factors were retained in the model if they were significant at the P < 0·05 criterion. The importance of each variable, adjusted for the others in its group, was assessed by the OR and 95 % CI.
Results
Characteristics of the women and their infants (n 401) are detailed elsewhere(Reference Tarrant, Younger and Sheridan-Pereira26). A total of 304 (76 %) telephone and ninety-seven (24 %) face-to-face survey interviews were conducted at 6 months (mean time of follow-up: 24·73 (sd 0·43) weeks). At this stage, one infant (0·2 %) was exclusively breastfed and 400 (99·7 %) infants were established on solids. The characteristics of the women and their infants who were established on solids are described in Table 1. Altogether, 238 (59 %) mothers reported that they received professional advice on infant feeding during the first 6 months, mainly from the public health nurse (PHN) as reported by 217 (91 %) of these mothers.
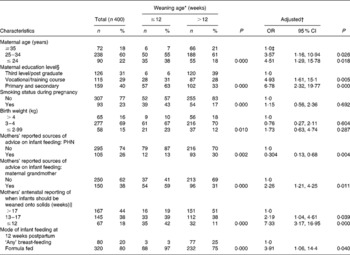
Table 1 Characteristics of the women and their infants in the ≤12 (n 91) and >12 week (n 309) weaning groups, and binary logistic regression analysis of the factors associated with weaning ≤12 weeks
(Numbers, percentages, odds ratios and 95 % confidence intervals)

PHN, public health nurse.
* Univariate analyses using cross-tabulations and χ2 statistical tests to compare the differences between women and infants in the ≤ 12 and >12 week weaning groups.
† Values are OR that were obtained from the final binary logistic regression model. The model was adjusted for maternal education level, age and smoking status during pregnancy, parity, infant birth weight and gestational age of infants at birth.
‡ 1·0 denotes the reference group.
§ Highest qualification attained.
∥ n 21 missing values.
Timing of weaning and first weaning foods
The median age of introduction to solid foods was 16 weeks (interquartile range = 14–17·7) (Fig. 1). In total, 286 infants (71·3 %) were weaned by 16 weeks (300 infants (75 %) were weaned by ≤ 17 weeks) and ninety-one infants (22·6 %) had been weaned by 12 weeks postpartum. Weaning by 6 weeks was observed in fourteen (3·5 %) infants, while only twenty-four (5·9 %) infants were weaned beyond 20 weeks.

Fig. 1 Timing of the first introduction to solids during the first 6 months (n 401). (![]() ), Total weaned by that age; (
), Total weaned by that age; (![]() ), weaned during each time period.
), weaned during each time period.
Commercially prepared puréed baby rice was reported as the most commonly used first weaning food by 239 (60 %) mothers, followed by an infant-specific, commercially prepared baby cereal reported by ninety-seven mothers (24 %). Home-prepared foods such as vegetable and fruit purées were used by twenty-three (6 %) and twelve (3 %) mothers, respectively, and gluten-containing cereals were used by three mothers ( < 1 %) as the first weaning foods. Infants consumed the first weaning food as a spoon feed on a daily basis for a mean of 11·1 (sd 9·2) d before another weaning food was introduced.
Determinants of early weaning
In the univariate analysis, mothers who weaned their infants at ≤ 12 weeks, compared with >12 weeks, were more likely to be ≤ 24 years and educated to primary and secondary level, to have smoked during pregnancy and to have formula fed their infants at 12 weeks (Table 1). The two weaning groups ( ≤ 12 v. >12 weeks) did not differ with regard to infant parity (P = 0·146), sex (P = 0·630), gestational age of the infant at birth (P = 0·718), infant attendance to a child-minding facility during the first 6 months (P = 0·821), maternal alcohol consumption during pregnancy (P = 0·308), employment status at 6 months (P = 0·605) or the timing of return to work postpartum (P = 0·067) (data not shown).
The significant factors that independently predicted weaning at ≤ 12 weeks, after adjustment, included mothers’ antenatal reporting that infants should be weaned onto solids at ≤ 12 weeks, formula feeding at 12 weeks and mothers’ reporting of the maternal grandmother as the principal source of infant feeding advice (Table 1). Reporting of the PHN as the principal source of feeding advice was associated with weaning at >12 weeks. Furthermore, a significant positive correlation between mothers’ antenatal reporting as to when infants should be weaned and actual weaning time postpartum was observed (r s 0·317; P = 0·000), indicating that later weaning during the first 6 months was associated with later antenatal prediction of weaning time.
The maternal reasons that were most significantly associated with weaning at ≤ 12 weeks (P = 0·000) included the maternal perception of infant hunger and sleep promotion (Table 2).
Table 2 Mothers’ reported reasons for introducing solids to infants’ diet during weaning at ≤12 weeks (n 91) and >12 weeks (n 309) postpartum*
(Numbers and percentages)

* ‘Reasons for introducing solids’ was an open-ended question, the specific responses to which were individually cross-tabulated with the dichotomous variable (weaned at ≤ 12 weeks/weaned at >12 weeks).
† Mothers could indicate more than one reason for introducing solid foods.
Infant dietary patterns at 6 months
Infant formula milk was consumed as the main milk drink by 386 (96 %) infants at 6 months (n 361 consumed formula milk and solids; n 25 consumed a combination of formula milk, breast milk and solids). The mean daily volume of formula milk consumed by these infants was 828 (sd 226) ml, with a mean of four (sd 0·99) formula feeds consumed in a 24 h period. Thirteen infants (3·2 %) were breastfed in addition to being fed with solids, with a mean of 5·92 (sd 1·8) breastfeeds offered to these infants in a 24 h period. One infant (0·2 %) in the total sample consumed solid foods in addition to full-fat cows' milk as the main milk drink in the absence of vitamin/mineral supplementation.
In addition to infants’ main milk drink, a daily breakfast, lunch and evening meal were consumed by 385 (96 %), 381 (95 %) and 357 (89 %) of infants, respectively, while 235 (59 %) infants consumed at least one snack per d. The type of foods consumed by infants at the routine meals (breakfast, lunch and evening meal) is detailed in Figs. 2–4. A meat and vegetable or a vegetable-based meal tended to be consumed at lunch, rather than in the evening meal. Commercially prepared infant-specific foods featured as the most commonly consumed infant foods for breakfast (63·2 % consumed sweet varieties of infant-specific cereals) and lunch (31 % consumed a meat and vegetable-based meal), while sweet varieties of infant-specific desserts (30·8 %) were the most common foods consumed in the evening meal.

Fig. 2 Type of breakfast foods consumed by infants at 6 months (n 385). * Includes maize-, wheat- and oat-based non-infant-specific cereals. CP-IS, commercially prepared infant-specific; HP, home-prepared.

Fig. 3 Type of lunch foods consumed by infants at 6 months (n 381). * For definitions of HP-IM and HP-NIM, refer to ‘explanatory measures’ section of Subjects and methods. CP-IS, commercially prepared infant-specific; HP-IM, home-prepared infant-modified; HP-NIM, HP-non-infant-modified.

Fig. 4 Type of evening meal foods consumed by infants at 6 months (n 357). * Includes sweet dessert varieties for infants, e.g. apple crumble, custard, chocolate pudding desserts; † includes both infant-specific and non-infant-specific yoghurts. CP-IS, commercially prepared infant-specific; HP, home-prepared; Veg., vegetables.
Infant rusks were the most frequently consumed weekly snack, followed by yoghurts and baby biscuits (Fig. 5). Snacks rich in refined sugar and salt, including chocolates, biscuits and crisps, were consumed >4 times/week by seven (3 %), nine (4 %) and nine (4 %) infants, respectively, while fruit/vegetables as a snack were consumed >4 times/week by twelve (5 %) infants. Few infants (n 44) were reported to consume puréed textured foods at 6 months, while soft/smooth, mashed/minced and soft/lumpy textured foods were consumed by 168 (42 %), 112 (28 %) and seventy-six (19 %) infants, respectively.
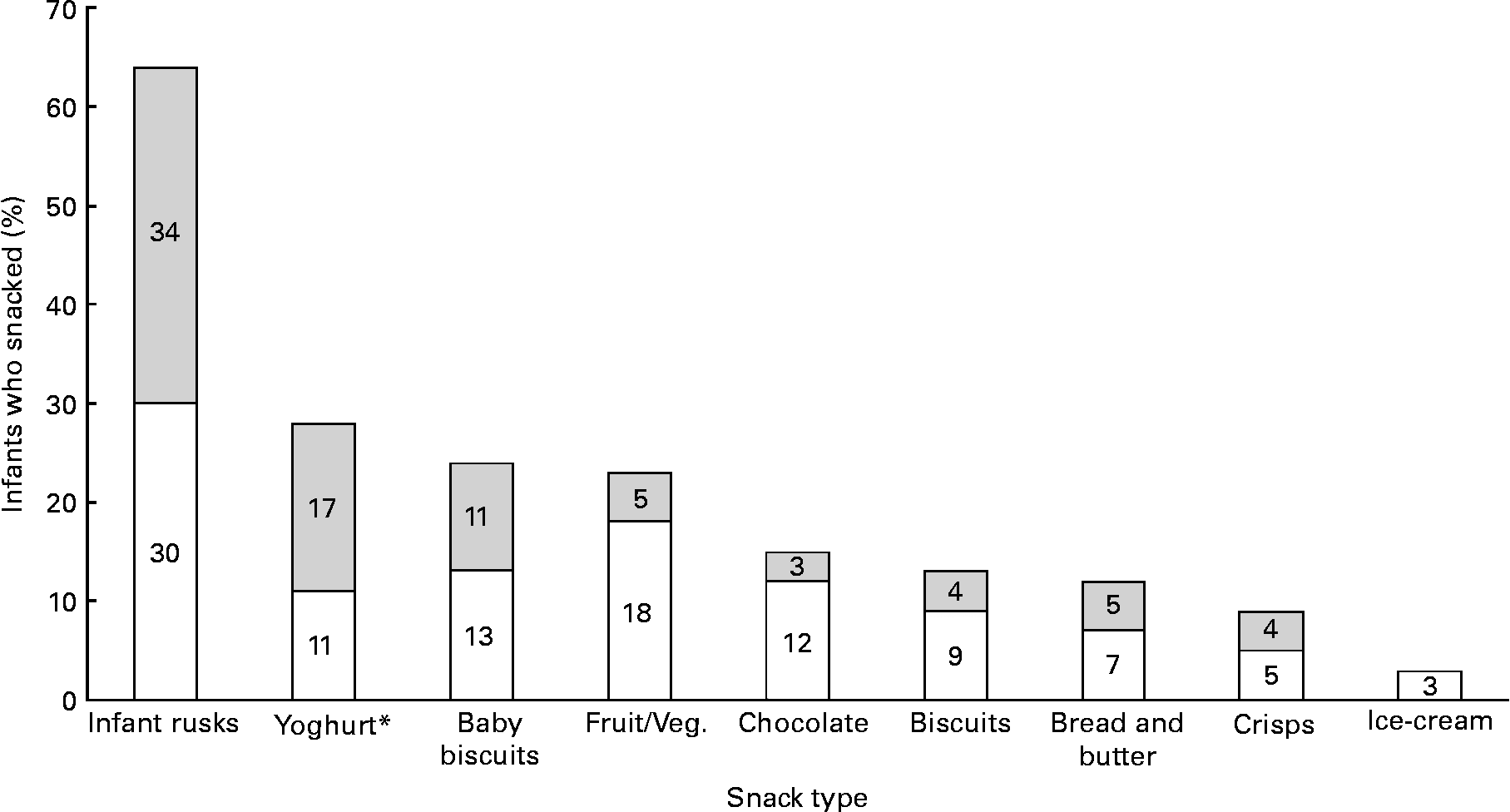
Fig. 5 Weekly snacking frequency of 6-month-old infants (n 235), with snacks consumed 1–4 (□) and >4 times/week (![]() ); mothers could indicate more than one snack type. * Includes both infant-specific and non-infant-specific yoghurts. Veg., vegetables.
); mothers could indicate more than one snack type. * Includes both infant-specific and non-infant-specific yoghurts. Veg., vegetables.
Of the 329 (82 %) infants who consumed supplementary fluids at 6 months, two (0·6 %), four (1·2 %) and twenty-three (7 %) infants consumed fruit juice, carbonated drinks/non-herbal tea and commercially prepared baby juices in volumes ≥ 180 ml/d, respectively (Fig. 6). In total, 224 (55·8 %) infants had been introduced to a lidded beaker cup, with mothers reporting cooled boiled water and infant-specific baby juice as the main fluids consumed in the beaker cup by 105 (47 %) and ninety-four (42 %) of these infants, respectively.

Fig. 6 Supplementary fluids consumed by 6-month-old infants (n 329) in volumes < 180 (□) and ≥ 180 ml/d (![]() ); mothers could indicate more than one type of supplementary fluid. * Water and sugar mixture; † tea refers to non-herbal varieties.
); mothers could indicate more than one type of supplementary fluid. * Water and sugar mixture; † tea refers to non-herbal varieties.
Maternal weaning practices
Altogether, 141 mothers (35 %) added at least one extra condiment to the infants’ weaning foods. Ordinary gravy, butter, sauces and vegetable stock were added by seventy-four (52 %), fifty-one (36 %), forty-seven (33 %) and thirty-three (23 %) of these mothers, respectively, and table salt was added by five mothers (3·5 %). Sweet additions such as sugar/honey were added by forty-one mothers (29 %).
Eighteen mothers (4·5 %) in the total sample reported adding solid foods to their infants’ bottled feeds, including baby rice (n 7) and rusks (n 11), mainly to promote sleep, as reported by fifteen of these mothers.
Mothers who weaned their infants at ≤ 12 weeks, compared with >12 weeks, were also more likely to have added non-recommended condiments (ordinary gravy, butter, sauces, vegetable stock, salt and sugar/honey) to the weaning foods (P = 0·000), to have added solids to their infants’ bottled feeds (P = 0·002) and to have offered their infants non-recommended snacks (chocolates, biscuits, crisps and ice-cream) (P = 0·000) (data not shown). In the univariate analysis, mothers who added non-recommended condiments to the weaning foods and those who offered non-recommended snacks to their infants were more likely (P = 0·000) to be single mothers, ≤ 24 years, smokers and educated to primary/secondary level, to have formula fed their infants from birth and to have reported the maternal grandmother as their principal source of advice on infant feeding.
Discussion
Significant deviations from the current weaning recommendations were demonstrated in the present study. Firstly, only one mother (0·2 %) complied with the WHO(3) recommendation to exclusively breastfeed until 6 months. A national infant feeding study(Reference McSweeney and Kevany31) carried out in 1982 (n 1195) similarly reported that < 1 % of mothers were exclusively breast-feeding at 6 months, indicating little improvement in exclusive breast-feeding rates over the past 24 years in Ireland. However, particularly low exclusive breast-feeding rates of 0·4 and 7 % at 6 months have also been reported in robust studies from the UK (n 11 490)(Reference Pontin, Emmett and Steer32) and Norway (n 2383)(Reference Lande, Anderson and Baerug33), respectively. Secondly, 75 % of infants (n 300) had been weaned onto solids before the minimum recommended weaning time of 4 months or 17 weeks(Reference Agostoni, Decsi and Fewtrell7, 8), and of even greater concern is the finding that 22·6 % of infants were prematurely weaned by 12 weeks postpartum. Although these figures indicate a marked shift towards later introduction of solids compared with previous Irish studies, which found that 68(34) and 32 %(Reference Twomey, Kiberd and Matthews35) of mothers, respectively, had weaned their infants by 12 weeks, early weaning remains a concern not just in Ireland, but internationally(Reference Schiess, Grote and Scaglioni20, Reference Ford, Schluter and Mithell36). Data obtained from the north-east of England(Reference Wright, Parkinson and Drewett18) and New Zealand(Reference Ford, Schluter and Mithell36) similarly report that 21 and 20 % of infants, respectively, are weaned onto solids by 12 weeks, while delayed weaning to 16 weeks has been demonstrated in 34·2 % of infants in Italy(Reference Giovannini, Riva and Banderali29) and 34 % of infants in Sweden(Reference Hornell, Hofvander and Kylberg21). The deleterious health consequences of early weaning have been well documented in the literature. Wright et al. (Reference Wright, Parkinson and Drewett18) showed that infants weaned onto solids before 3 v. >4 months are at greater risk of diarrhoea and are more likely to visit their general practitioner between 6 weeks and 4 months. Weaning before 15 v. >15 weeks has been shown to increase body fat and weight during childhood, as well as the probability of respiratory illness(Reference Wilson, Forsyth and Greene14).
In the present study, in addition to the early weaning, a high frequency of consumption of non-recommended snacks by infants (>4 times/week), drinking of sugar-containing supplementary fluids in volumes ≥ 180 ml/d and frequent additions of salted and sugared condiments to weaning foods was observed. In particular, sweet varieties of commercially prepared infant-specific desserts (e.g. apple crumble and chocolate pudding desserts) and jelly with ice-cream were consumed by almost one-third of the infants (31·6 %) as the usual evening meal. In comparison, fewer infants consumed less sweetened foods in the evening meal, including yoghurt and/or fruit (22·5 %) and home-prepared fruit purée (16·3 %). Similarly, among the infants who consumed snack foods, 18 % of the infants were found to consume sweet snack foods, including biscuits and chocolates >4 times/week in comparison with only 5 % of the infants who consumed fruit and vegetables >4 times/week. According to the Committee on Medical Aspects of Food Policy(2), added sugars provide energy in the infants’ diet and increase the palatability of foods; however, they should be used sparingly, and where possible, unsweetened foods should be encouraged in preference to sugar-containing varieties. As dental caries development is associated with the amount and frequency of consumption of added sugars, best practice weaning guidelines recommend that infants consume sugar-containing foods and fluids either with or after a meal(2, 8). To promote optimal short- and long-term dental health, results of the present study point towards a need to highlight this recommendation in infant feeding literature, and in advice to parents.
Although the present study did not collect quantitative data on the food portions consumed, it appears that a significant proportion of 6-month-old infants in Ireland are regularly consuming foods rich in refined sugars, energy, saturated fats and salt, which have been suggested to adversely influence both later health(Reference Dennison37, Reference Zinner, McGarvey and Lipsitt38) and child food preferences(Reference Skinner, Carruth and Bounds24). Moreover, the regular consumption of such non-recommended foods during infancy may deleteriously affect the long-term compliance with healthy eating guidelines(Reference Cooke39), and may be a behavioural determinant of later overweight and obesity risk(Reference Baughcum, Burklow and Deeks40). The present results are of further public health importance owing to the evidence that consumption of diets rich in salt during infancy cultivates a taste for salted foods(Reference Bernstein41), and a high Na intake during early life could contribute to the risk profile for higher blood pressure later in life(Reference Zinner, McGarvey and Lipsitt38).
Although cooled boiled water is the recommended supplementary drink for young infants(2, 8), a greater proportion of infants in the present study consumed baby juices (57 %) rather than water (54·4 %) as a supplementary fluid. Furthermore, thirty-three infants consumed sugar-containing supplementary fluids in volumes ≥ 180 ml/d, an amount which is in excess of the recommended 120–180 ml/d set out in guidelines by the American Academy of Pediatrics(28). Similarly, in the UK, Emmett et al. (Reference Emmett, North and Noble42) found that a greater proportion of 8-month-old infants (n 1178) consumed sugar-containing supplementary fluids, including squashes/cordials (55·8 %) and fruit juice (14·9 %), in comparison with water (19·7 %). A disproportionate intake of sugar-containing fluids during the first 2 years may result in toddler diarrhoea(Reference Hoekstra43), non-organic failure to thrive(Reference Smith and Lifshitz44) and tooth decay(Reference Holt45), and may lead to a decline in the consumption of milk and hence a decrease in the level of Ca in the diet(Reference Dennison37). Thus, our data strongly indicate that in infant feeding literature and while advising parents, greater emphasis should be placed on the appropriate use of supplementary fluids during infancy, as well as on snacking preferences and home preparation of infant foods, in line with infant feeding recommendations.
Comparable with other studies(Reference Forsyth, Ogston and Clark12, Reference Bolling, Grant and Hamlyn19, Reference Alder, Williams and Anderson30), early weaning was significantly associated with socio-economic status in the present study. In agreement with our findings, maternal education level and age have been identified by other investigators as particularly influential socio-demographic determinants not only in relation to early weaning(Reference Bolling, Grant and Hamlyn19), but also in relation to the types of supplementary fluids(Reference North, Emmett and Noble46) and foods consumed by infants(Reference Hendricks, Briefel and Novak47). Data obtained from the USA(47) report that mothers (n 2515) who had a third-level college education were more likely to offer their infants fruit, rather than sweetened beverages, desserts and sweets, while results obtained from the present study indicate that mothers who were educated in a primary/secondary school were more likely to offer their infants non-recommended snacks, including chocolates, biscuits, crisps and ice-cream. To address the high prevalence of sub-optimal weaning practices and hence decrease inequalities in health, our data highlight that younger and less educated mothers who formula feed their infants and report the maternal grandmother as their principal source of advice on infant feeding represent a highly vulnerable group who deserve greater attention with regard to infant feeding support. Mothers who weaned their infants at ≤ 12 weeks were also found to be more likely to carry out other sub-optimal weaning practices. Public health initiatives that aim to improve compliance with weaning recommendations and strategies to promote healthy infant feeding practices in Ireland should therefore target these high-risk groups.
In the present study, a significant association between mothers’ antenatal reporting of when infants should be weaned and the timing when mothers’ actually weaned was found. It is likely that mothers’ antenatal reporting is closely associated with their antenatal expectation as to when they thought they would introduce solids to their infants’ diet postpartum. Few studies have examined this antenatal and postnatal relationship; however, based on our findings, the effectiveness of public health campaigns or interventions that aim to correct mothers’ misperceptions during the antenatal period and accurately inform both mothers and grandmothers-to-be of best infant feeding practices should be studied.
In the present study, the influential role of the PHN in delaying the introduction of solids was evident. In contrast, the negative impact of the maternal grandmother on mothers’ weaning practices was also clearly demonstrated. Consistent with this finding, data obtained from Scotland report that mothers who weaned their infants at < 12 v. >12 weeks were influenced by the opinions of the infants’ maternal grandmother and friends(Reference Alder, Williams and Anderson30), similarly highlighting the importance of the social group on mothers’ infant feeding practices. Compliance with traditional feeding practices that have been inter-generationally ‘handed-down’ has been shown to be more influential on mothers’ practices than the professional advice received, and in addition, traditional feeding practices have the advantage of being readily available to mothers(Reference Daly, MacDonald and Booth48). Although it is a possibility that mothers in the present study resorted to advice from the maternal grandmother due to the lack of weaning advice offered by the PHN, it has been shown that mothers who are aware of feeding recommendations are still likely to wean prematurely based upon influences from their social network(Reference Anderson, Guthrie and Alder49). According to Forsyth et al. (Reference Forsyth, Ogston and Clark12), parents are not aware of the predicted harmful effects of early feeding with solids, and may perceive the practice to be beneficial to their infants. It has also been suggested that the potential threats to an infant's health may be stronger motivators for mothers than messages that point to the health benefits of delaying weaning to 4–6 months(Reference Horodynski, Olson and Arndt50). Although these findings raise awareness of the wide occurrence of sub-optimal weaning practices during the first 6 months among the health professionals in Ireland, effective and standardised strategies are needed to improve compliance with the weaning recommendations. The provision of detailed and standardised weaning information from the PHN no later than 12 weeks postpartum, in addition to increasing the impact of the PHN on mothers’ weaning practices, is the potential area for the development of effective interventions to improve infants’ diets.
Given the finding that 41 % of the mothers in the present study reported that they did not receive professional advice on weaning, there is a possibility that an overall deficiency in weaning information exists among these mothers. It may also have been the case that a proportion of mothers who did not comply with the weaning recommendations reported that they did not receive professional advice from the PHN or other health professionals during the first 6 months. Nonetheless, our data strongly suggest a need for the implementation of a structured national weaning policy that ensures the provision of specific weaning information to all parents, particularly, during the antenatal period.
There are a number of limitations to the present study. Firstly, this study is a prospective study that was conducted at a single centre, and therefore, it cannot be regarded as a population-based nationally representative study of weaning practices in Ireland. However, based on the literature available to date, no other prospective study done over the past decade has examined maternal weaning practices in Ireland using a large cohort of healthy term infants spanning over a 2-year period. Secondly, the accuracy of the maternal reporting of the infant's usual diet at 6 months, as well as the possibility of maternal recall bias in relation to the timing of weaning, must be considered. The short dietary history of the infant's usual diet at 6 months was reliant on mothers’ memory at the time of the interview. Completion of an infant feeding logbook by mothers during the first 6 months may have increased the accuracy of the data collected. Recall bias is also a possibility with some mothers under- or/and over-estimating the foods and fluids consumed by infants in an effort to provide more socially desirable responses(Reference Livingstone, Robson and Wallace51). As the 6-month interview may have taken up to 30 min to complete, there may have also been a possibility that mothers gave less complete responses in an effort to complete the interview more quickly. Finally, in comparison with the telephone survey interviews, increased face-to-face contact with mothers during the home-visits may have contributed to a greater rapport with the mothers, resulting in potential response bias. Nonetheless, all the interviews were performed by an experienced investigator, and every effort was made to ensure consistent interview methods throughout the study population.
In conclusion, a high prevalence of sub-optimal weaning practices among mothers in Ireland, including premature weaning at ≤ 12 weeks, was demonstrated in the present study. Initiating weaning advice to parents during the antenatal period may prove an effective measure to increase compliance with weaning guidelines. Crucially, weaning information requires greater specification with regard to healthy snack preferences, appropriate volumes of supplementary fluids and home-preparation of infant foods. In order to address the negative inter-generational influence of the maternal grandmother on weaning practices observed in the present study, intensive education, support and increased resources should be apportioned to younger and less educated mothers.
Acknowledgements
The authors thank the clinical and administrative staff of the CWIUH, Dublin 8, for their invaluable support and help in carrying out the present study. They also thank the 491 mothers who participated, and whose time and commitment to the study made its completion possible. The present study was funded by the Dublin Institute of Technology, Dublin 8, Republic of Ireland. R. C. T., J. M. K. and K. M. Y. were responsible for the study design and the interpretation of the results. R. C. T. was responsible for the recruitment and follow-up of the subjects, as well as for data collection and input, statistical analyses and preparation of the draft manuscript. J. M. K. was the project supervisor/coordinator, and was responsible for statistical analyses and manuscript revision. K. M. Y., M. S.-P. and M. J. W. were responsible for manuscript revision. The authors report no conflict of interest in relation to the present study.