22q11.2 deletion syndrome (22q11DS) is one of the most common recurrent copy number variant disorders. It is caused by a microdeletion on the long arm of chromosome 22 and affects 1 in 2000–4000 live births.Reference Schneider, Debbané, Bassett, Chow, Fung and van den Bree1 The phenotypic expression is highly variable and characteristics of the syndrome include heart malformations, palatal abnormalities, hypocalcaemia, intellectual disability and high rates of psychiatric illness.Reference Schneider, Debbané, Bassett, Chow, Fung and van den Bree1
One of the strongest associations with psychiatric illness in 22q11DS is that with psychotic disorders.Reference Bassett, Chow, AbdelMalik, Gheorghiu, Husted and Weksberg2 Schneider et al Reference Schneider, Debbané, Bassett, Chow, Fung and van den Bree1 reported a prevalence of schizophrenia spectrum disorders of about 20–25% in young adults (aged 18–25 years) with 22q11DS and approximately 40% in patients over 25 years of age. The 22q11.2 deletion is therefore among the strongest genetic risk factors for the development of psychosis.Reference Murphy, Jones and Owen3 The increased risk for developing psychotic disorders may be partially explained by altered dopamine transmission in these patients,Reference Boot, Booij, Zinkstok, Abeling, de Haan and Baas4 posited to be related to reduced gene expression and/or enzyme activity, particularly in prefrontal areas, of catechol-O-methyltransferase (COMT), a gene in the 22q11.2 deletion region.Reference Gothelf, Law, Frisch, Chen, Zarchi and Michaelovsky5 Moreover, the COMT gene contains a common functional polymorphism, Val158Met, of which the Met variant is associated with lower enzyme activity and subsequently less dopamine breakdown.Reference Gothelf, Law, Frisch, Chen, Zarchi and Michaelovsky5
The majority of patients with idiopathic psychotic disorders use substances such as alcohol, nicotine and cannabis excessively: 95% of patients with a psychotic disorder report lifetime substance use,Reference Barnes, Mutsatsa, Hutton, Watt and Joyce6 compared with 70% in the general population.Reference Young, Corley, Stallings, Rhee, Crowley and Hewitt7 Patients with schizophrenia are 4.6 times more likely to have a comorbid substance use disorder (SUD) relative to the general population.Reference Chambers, Krystal and Self8 The underlying mechanisms of this high prevalence of SUD in schizophrenia are not well understood, but are likely to be associated with the brain dopaminergic pathway.Reference Volkow9 All substances of misuse directly or indirectly activate the mesolimbic dopamine pathway, which is associated with the reward properties of drugs and the positive symptoms of schizophrenia.Reference Volkow9
Given the dopaminergic abnormalities in both psychosis and SUD and the high prevalence of psychotic disorders and dopamine abnormalities in 22q11DS, one might hypothesize that patients with 22q11DS are at an elevated risk for substance use or SUD. We therefore investigated the prevalence of substance use and SUD in patients with 22q11DS, in non-deleted patients with psychosis and in healthy controls. We further explored the types and amounts of substances used. In addition, within the 22q11DS group we explored the relationship between substance use and psychosis, COMT Val158Met genotype, full-scale IQ and gender.
Method
This was a retrospective case study and therefore approval for this study was waived. All procedures performed were in accordance with the ethical standards of the institutional committee of the Academic Medical Center, Amsterdam. All data were processed anonymously.
Participants
In total, 833 participants were included in the study: 434 patients with 22q11DS (196 male, 238 female), 265 non-deleted patients with psychosis (219 male, 46 female) and 134 healthy controls (80 male, 54 female). The mean age was 30.91 years (s.d. 12.65) for patients with 22q11DS, 29.79 years (s.d. 8.41) for non-deleted patients with psychosis and 29.86 years (s.d. 10.22) for healthy controls. All participants underwent comprehensive psychiatric assessment. 22q11DS diagnosis was established with multiplex ligation-dependent probe amplification analysis, fluorescence in situ hybridisation with a standard 22q11.2 region probe, or other microarray method. Clinical case files from patients with 22q11DS from four sites were used. These sites included the Department of Psychiatry of the Maastricht University Medical Centre, the Netherlands (n = 95), the 22q11DS out-patient clinic of the Department of Psychiatry of the University Medical Center Utrecht, the Netherlands (n = 68), the Center for Human Genetics of the University Hospital Gasthuisberg Leuven, Belgium (n = 50) and the Dalglish Family 22q Clinic for Adults with 22q11.2 Deletion Syndrome and/or the Clinical Genetics Research Program at the Centre for Addiction and Mental Health, Canada (n = 209). Patients over 15 years of age with a confirmed diagnosis of 22q11DS were included. Non-deleted patients with psychosis and healthy controls were participants in the Genetic Risk and Outcome Psychosis (GROUP) study (Amsterdam site).Reference Korver, Quee, Boos, Simons and de Haan10 All of the GROUP participants gave written informed consent before participation after explanation of the study procedure. Inclusion criteria for non-deleted patients with psychosis were aged between 15 and 60 years and a diagnosis of a non-affective psychotic disorder (DSM-IV-TR criteria). For the healthy controls, inclusion criteria included age between 15 and 60 years, no lifetime diagnosis of a psychotic disorder and no first-degree relative with a lifetime psychotic disorder.Reference Korver, Quee, Boos, Simons and de Haan10
Psychiatric assessment
For patients with 22q11DS, psychiatric diagnoses (DSM-IV-TR) were obtained by experienced clinicians, using validated instruments (Structured Clinical Interview for DSM-IV Axis I Disorder;Reference First and Gibbon11 Schedule for Affective Disorders and Schizophrenia for School-age Children;Reference Kaufman, Birmaher, Brent, Rao, Flynn and Moreci12 MINI: Mini International Neuropsychiatric Interview;Reference Sheenan, Lecrubier, Sheenan, Amorim, Janavs and Weiler13 Mini Psychiatric Assessment Schedules for Adults with Developmental DisabilitiesReference Prosser, Moss, Costello, Simpson, Patel and Rowe14). For non-deleted patients with psychosis, diagnosis was established by a trained research assistant, psychologist, psychiatrist, nurse or PhD student, using the Comprehensive Assessment of Symptoms and History.Reference Korver, Quee, Boos, Simons and de Haan10, Reference Andreasen15
Substance use
For the 22q11DS group, lifetime substance use data were systematically gathered from the clinical files. The clinical files were based on a psychiatric consult conducted by an experienced psychiatrist, during which substance use was systematically queried. Substance use in the non-deleted psychosis and healthy controls groups was assessed by sections B, J and L of the Composite International Diagnostic Interview (CIDI).Reference Robins16 All groups were dichotomised into a substance use and non-substance use group according to the CIDI guidelines. Participants who reported to have (lifetime) used substances for at least five times or more were assigned to the substance use group whereas participants reporting fewer than five instances of substance use were included in the non-substance use group. For all groups, the mean amount of alcohol and nicotine use per week was also assessed. For illicit drug use, only frequency (weekly, monthly or less) was known. To ensure that the same quantities were used for alcohol and nicotine use across sites, it was determined that one pouch of tobacco (shag) equals 40 cigarettes; CIDI alcohol-equivalent guidelines (response card J1) were used to compute the number of glasses per week.
Intelligence
Full-scale IQ was available for a subgroup of 347 (80.0%) patients with patients. In the 22q11DS group, full-scale IQ was assessed by age-appropriate, standardised validated instruments, including the Wechsler Adult Intelligence Scale III,Reference Wechseler17 the Wechsler Intelligence Scale for Children IIIReference Wechseler18 and the abbreviated Scale of Intelligence.Reference Velthorst, Levine, Henquet, de Haan, van Os and Myin-Germeys19 Instruments varied across sites. In the non-deleted psychosis and healthy controls groups, full-scale IQ was assessed by a shortened validated version of the Wechsler Adult Intelligence Scale III.Reference Korver, Quee, Boos, Simons and de Haan10
COMT genotype
For a subgroup of 297 (70.1%) patients with 22q11DS, the COMT Val158Met genotype was determined by previously described, validated standardised methods.Reference Boot, Booij, Zinkstok, Abeling, de Haan and Baas4, Reference Bassett, Caluseriu, Weksberg, Young and Chow20–Reference van Duin, Goossens, Hernaus, da Silva Alves, Schmitz and Schruers22
Data analyses
A one-way analysis of variance and a χ 2-test were used to analyse group differences in age, full-scale IQ and gender. Prevalence of total substance use and separate alcohol, nicotine and illicit drug use was computed as the percentage in each group. In addition, we computed the prevalence of use of multiple substances and SUD per group in percentages. Prevalence rates of substance use, SUD, nicotine, alcohol, illicit drug use and multiple substance use were compared between the three groups, using χ 2-tests. To examine whether group status predicts substance use, we conducted a logistic regression (method: Enter) analyses with 22q11DS as reference category. To check for possible confounding effects of gender and full-scale IQ, we repeated this analysis and added these variables to the model. To further examine the effects of full-scale IQ on substance use and SUD, supplementary analyses were conducted to compare prevalence rates of overall substance use and SUD between patients with 22q11DS and non-deleted patients with psychosis with a full-scale IQ <80, as well as three group comparisons between participants with a full-scale IQ ≥ 80, using χ 2-tests.
The mean amount of alcohol and nicotine use per week was compared between the three groups with one-way analysis of variance. To examine the effects of gender and full-scale IQ on weekly alcohol and nicotine use, we conducted an additional analysis of covariance, including these variables as covariates. Additionally, the prevalence of drug use was computed for separate classes of drugs (cannabis, cocaine, XTC/3,4-methylenedioxymethamphetamine (MDMA), psychedelics, opiates, sedatives, stimulants and phencyclidine (PCP)). Prevalence of regular cannabis use (daily or weekly) was compared between the three groups, using χ 2-tests. This analysis was not conducted for other classes of drugs because the prevalence was very low in at least one of the groups.
Within the 22q11DS group the relationships between substance use and psychotic disorders, gender, full-scale IQ and COMT genotype were examined by means of odds ratios. In addition, the relationship between age and substance use was computed. Statistical analyses were conducted with IBM SPSS Statistics for Windows, version 23.
Results
Demographic variables
Sample characteristics are displayed in Table 1. The three groups significantly differed in gender distribution (χ 2 (2) = 95.86, P < 0.001) as well as full-scale IQ (F(2725) = 515.13, P < 0.001) (Table 1). Of the patients with 22q11DS, 281 (64.8%) were diagnosed with a psychiatric disorder, and 165 (38.1%) had a psychotic disorder.
Table 1 Sample demographics
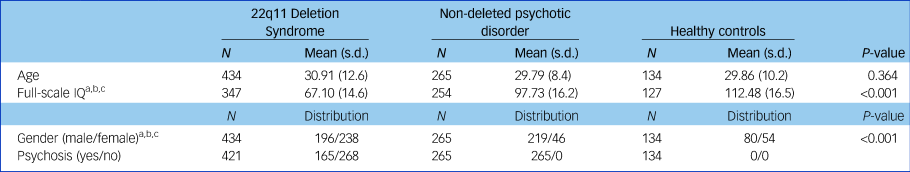
a. P < 0.05 for 22q11 deletion syndrome versus healthy controls.
b. P < 0.05 for 22q11 deletion syndrome versus non-deleted psychotic disorder.
c. P < 0.05 for non-deleted psychotic disorder versus healthy controls.
Prevalence of substance use
The prevalence of participants with any substance use was significantly lower (n = 160, 36.9%) in the 22q11DS group than in the healthy controls group (n = 112, 83.6%; χ 2 (d.f. = 2) 104.41, P < 0.001) or in the non-deleted psychosis group (n = 233, 87.9%; χ 2 (d.f. = 2) 112.40, P < 0.001) (Table 2). The prevalence of participants who met the DSM-IV-TR criteria for SUD (misuse or dependence) was also significantly lower in the 22q11DS group compared with the healthy controls and non-deleted psychosis groups, as were alcohol, nicotine, illicit drug use and multiple drug use (Table 2). Prevalence of SUD, nicotine and multi-substance use was significantly higher in non-deleted patients with psychosis compared with healthy controls. Alcohol use was significantly lower in the non-deleted psychosis group compared with healthy controls. No significant difference in prevalence of overall substance use and illicit drug use was found between healthy controls and non-deleted patients with psychosis. Supplementary comparisons of prevalence of overall substance use and SUD between a subgroup of patients with 22q11DS and non-deleted patients with psychosis with a full-scale IQ <80 yielded comparable results; both substance use and SUD were less prevalent in the 22q11DS group (P < 0.001 and P < 0.001, respectively, Supplementary Table 1 available at https://doi.org/10.1192/bjp.2018.258). Moreover, comparisons of substance use and SUD between participants with a full-scale IQ > 80, also yielded similar results (Supplementary Table 2).
Table 2 Prevalence of substance use and substance use disorders in 22q11 deletion syndrome, non-deleted psychosis and healthy controls, and group comparisons using χ 2
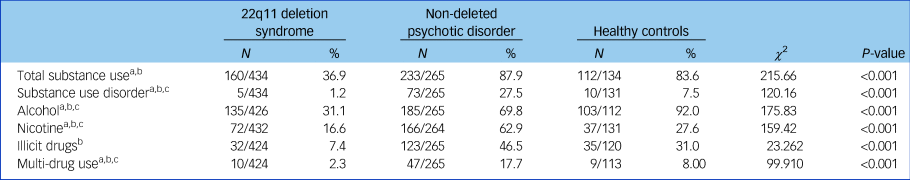
N indicates the number of participants reporting substance use relative to the total sample.
a. P < 0.05 for 22q11 deletion syndrome versus healthy controls.
b. P < 0.05 for 22q11 deletion syndrome versus non-deleted psychosis.
c. P < 0.05 for non-deleted psychosis versus healthy controls.
Logistic regression analyses showed that group status was a significant predictor of (total) substance use (P < 0.001) as well as for nicotine (P < 0.001), alcohol (P < 0.001) and illicit drugs (P < 0.001) separately (Table 3). Healthy controls were 20.6 times more likely to use substances in general, 2.0 times more likely to use nicotine, 24.7 times more likely to use alcohol and 2.0 times more likely to use illicit drugs, compared with patients with 22q11DS. Compared with patients with 22q11DS, non-deleted patients with psychosis were 11.5 times more likely to use substances in general, 8.5 times more likely to use nicotine, 5.0 times more likely to use alcohol and 3.0 times more likely to use illicit drugs. Group status explained 35% of the variance in total substance use, 24% in nicotine use, 28% in alcohol use and 5% in drug use. However, when adjusting for gender and full-scale IQ, we found that group status was no longer a significant predictor of illicit drug use (P = 0.162). After adjusting for gender and full-scale IQ, group status still predicted overall substance use as well as nicotine and alcohol use. In addition, we found that besides group status, gender was also a significant predictor of total substance use (P < 0.001), nicotine use (P = 0.003), alcohol use (P < 0.001) and illicit drug use (P = 0.003). Full-scale IQ was only a significant predictor of overall substance use (P = 0.048) and alcohol use (P < 0.001), as it did not significantly predict nicotine use (P = 0.109) or illicit drug use (P = 0.099). After adjusting for gender and full-scale IQ, healthy controls were 12.6 times more likely to use substances in general, 2.8 times more likely to use nicotine, 10.3 times more likely to use alcohol and 1.2 times more likely to use illicit drugs compared with patients with 22q11DS. Compared with the 22q11DS group, non-deleted patients with psychosis were 6.6 times more likely to use substances in general, 9.5 times more likely to use nicotine, 2.2 times more likely to use alcohol and 1.7 times more likely to use illicit drugs. The total model (including group status, gender and full-scale IQ) explained 42% of the variance in overall substance use, 27% in nicotine use, 39% in alcohol use and 9% in illicit drug use (Supplementary Table 3).
Table 3 Logistic regression analyses
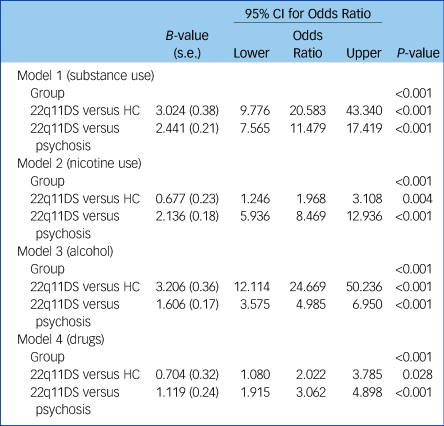
Total substance use: model 1: R 2 = 0.352 (Nagelkerke), χ 2 (1) = 0.00, P < 1.000; model 2: R 2 = 0.242 (Nagelkerke), χ 2 (1) = 0.00, P < 0.001; model 3: R 2 = 0.283 (Nagelkerke), χ 2 (1) = 0.00, P < 0.001; model 4: R 2 = 0.053 (Nagelkerke), χ 2 (1) = 0.00, P < 0.001. 22q11DS, 22q11 deletion syndrome; HC, healthy controls.
Weekly substance use
The mean weekly amount of alcohol (number of drinks) (F(4364) = 4.96, P = 0.007) and nicotine (number of cigarettes) (F(2226) = 17.52, P < 0.001) use differed significantly across all groups (Supplementary Table 4, adjusted for gender and full-scale IQ). Post hoc analyses showed that the weekly amount of alcohol use was significantly lower in the 22q11DS group compared with the non-deleted psychosis group (P = 0.004), and the trend level was significantly lower than in healthy controls (P = 0.060). There was no significant difference in weekly alcohol use between the non-deleted psychosis and healthy controls groups (P = 0.580). The amount of nicotine use was significantly lower in the 22q11DS group compared with the non-deleted psychosis group (P < 0.001), but not compared with the healthy controls group (P = 0.760). Weekly nicotine use was significantly higher in the non-deleted psychosis group compared with the healthy controls group (P < 0.001).
Frequency and characterisation of illicit drug use
Frequency of drug use (weekly, daily or less) was low in the 22q11DS group; except for cannabis, all substances were used less than weekly. The number of patients using cannabis on a regular basis (daily or weekly) was significantly lower in the 22q11DS group compared with the non-deleted psychosis and healthy controls groups (χ 2 (d.f. = 2) 48.215, P < 0.001). See Supplementary Table 4 for an overview of frequency and classes of drugs used per group. The three groups differed significantly in multiple illicit drug use (χ 2 (d.f. = 2) 60.67, P < 0.001) (Fig. 1).
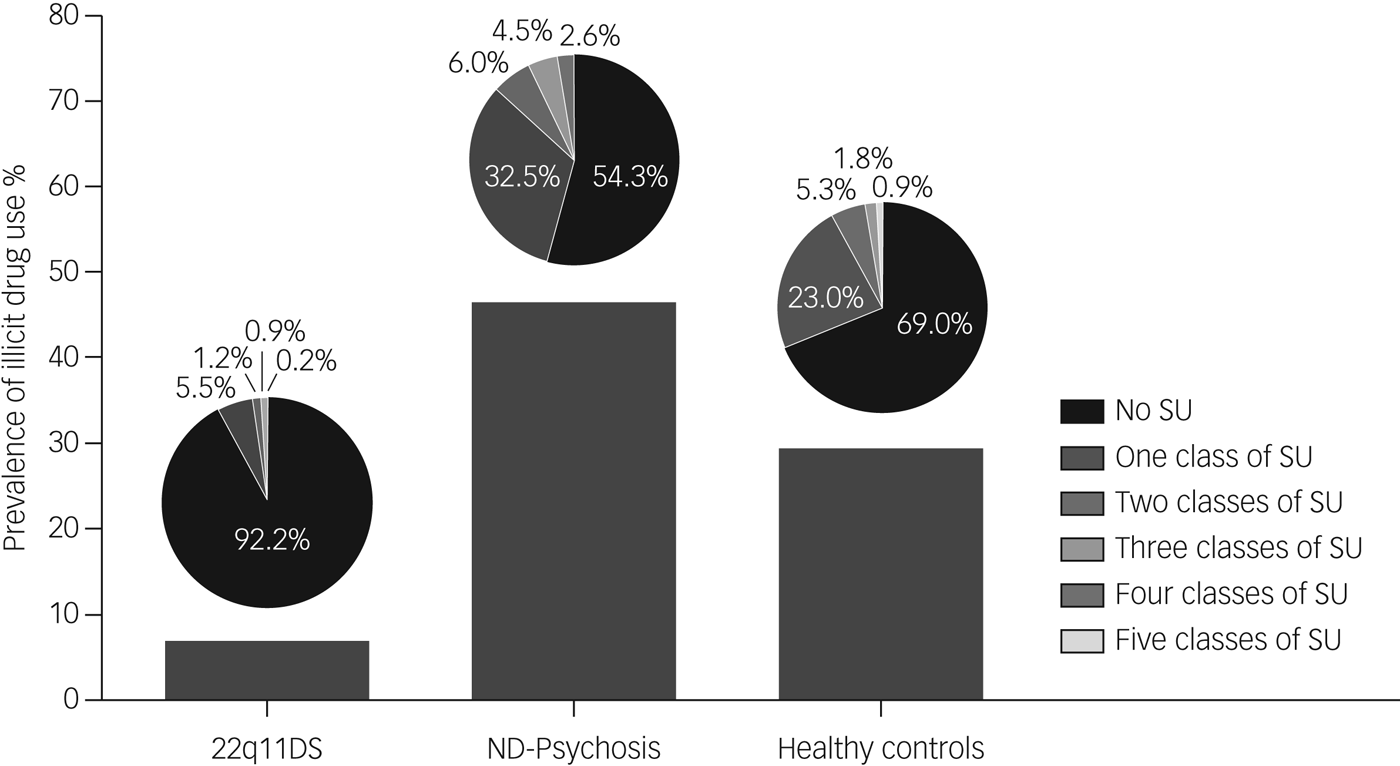
Fig. 1 Prevalence of multi-substance use per group.
Relationships between substance use and psychosis, COMT genotype, gender and full-scale IQ
Within 22q11DS, the presence of a psychotic disorder (odds ratio, 0.80; 95% CI 0.54–1.20; P = 0.28) or COMT Val158Met genotype (odds ratio, 1.09; 95% CI 0.68–1.75; P = 0.72) did not significantly increase the likelihood of substance use. Patients with a higher full-scale IQ were marginally but significantly more likely to use substances (odds ratio, 1.024; 95% CI 1.007–1.040; P = 0.004). Male patients with 22q11DS were 1.7 times more likely to use substances than female patients (odds ratio, 1.67; 95% CI 1.13–2.47; P = 0.01). Finally, a small but significant relationship was found between age and substance use (r = 0.167, n = 434, P < 0.001); prevalence of substance use increased with age.
Discussion
This is the first study to investigate the prevalence of substance use and SUD in a large sample of patients with 22q11DS compared with non-deleted patients with psychosis and healthy controls. We report a significantly lower prevalence of overall substance use and SUD in patients with 22q11DS compared with healthy controls and non-deleted patients with psychosis. Patients with 22q11DS were significantly less likely than healthy controls to use substances in general, and alcohol and nicotine. Interestingly, after adjusting for gender and full-scale IQ, patients with 22q11DS were not at decreased risk for illicit drug use. However, this could be because of the low number of participants that reported illicit drug use. These results indicate that patients with 22q11DS are at decreased risk for substance use, suggesting that the 22q11.2 deletion could be a protective factor for starting and/or continuing substance use, despite the elevated risk of psychosis.
The underlying mechanisms of this reduced lifetime prevalence of substance use in 22q11DS are currently unknown. Multiple factors are likely to be involved, including environmental, personality, maturity and neurobiological factors. To gain more insight into these mechanisms, it is important to differentiate between initiating and continuing substance use. In addition to a low prevalence of total substance use, the mean amount of alcohol and nicotine used per week was also significantly lower in 22q11DS. This could suggest that different mechanisms underlie the initiation and continuation of substance use in these patients. Decreased substance use could be related to high levels of anxiety and anxiety disorders reported in patients with 22q11DS.Reference Schneider, Debbané, Bassett, Chow, Fung and van den Bree1, Reference Angkustsiri, Leckliter, Tartaglia, Beaton, Enriquez and Simon23 High levels of anxiety are inversely related to sensation-seeking behavior,Reference Roberti24 which is in turn related to increased risk-taking behaviour and illicit substance use.Reference Wechseler17 Therefore, it could be hypothesized that patients with 22q11DS may be fearful to try new things, especially potentially harmful things such as illicit drug use. This could also explain the low use of multiple substances in patients with 22q11DS. Sensation-seeking is also related to antisocial and delinquent behaviorReference Harden, Quinn and Tucker-Drob25; in typically developing individuals and individuals with mild or borderline intellectual disability without 22q11DS, substance use and SUDs are more prevalent in those with a forensic history or displaying antisocial behavior.Reference Compton, Conway, Stinson, Colliver and Grant26, Reference Raina and Lunsky27 Although formal studies examining the prevalence of sensation-seeking or delinquency in 22q11DS are lacking, conduct disorder (often a harbinger of antisocial personality disorder) is rare in these patients.Reference Schneider, Debbané, Bassett, Chow, Fung and van den Bree1 Therefore, it is likely that levels of sensation-seeking and delinquent behaviour are low in patients with 22q11DS as well, which could also contribute to the decreased risk of substance use in these patients.
Alternatively, lower risk of substance use and SUD in 22q11DS may be related to aberrant reward processing. The reward-processing system contains multiple components, including reward anticipation (wanting, goal-directed behaviour), hedonic response (liking) and learning.Reference Berridge and Robinson28 This system is important in moderating human behaviour, including feelings of pleasure. Reward processing is mainly modulated by dopamine arising from the ventral tegmental area and projecting to the nucleus accumbens (ventral striatum) and frontal cortex.Reference Volkow9 Functional magnetic resonance imaging (fMRI) studies in SUD have shown a decreased blood-oxygen-level-dependent response in the striatum during non-drug-related reward anticipation,Reference van Hell, Vink, Ossewaarde, Jager, Kahn and Ramsey29, Reference Martz, Trucco, Cope, Hardee, Jester and Zucker30 but increased brain activation during drug-related reward anticipation (related to drug-seeking behaviour).Reference Wrase, Schlagenhauf, Kienast, Wüstenberg, Bermpohl and Kahnt31 In schizophrenia, fMRI studies repeatedly showed altered reward processing, which, consistent with SUD, has been associated with decreased striatal activity during reward anticipation.Reference Esslinger, Englisch, Inta, Rausch, Schirmbeck and Mier32 However, the hedonic response remains intact,Reference Strauss, Waltz and Gold33 thereby potentially explaining substance use continuation in non-deleted patients with psychotic illness. The reward system in 22q11DS has been little studied. The one (fMRI) study investigating the neurobiological mechanisms of reward anticipation in 22q11DS reported no differences in striatal activation between 22q11DS and healthy controls, contrary to findings in SUD and schizophrenia, suggesting different underlying mechanisms of reward processing in 22q11DS.Reference van Duin, Goossens, Hernaus, da Silva Alves, Schmitz and Schruers22 However, patients with 22q11DS had reduced activation in the medial frontal areas during monetary reward anticipation compared with healthy controls,Reference van Duin, Goossens, Hernaus, da Silva Alves, Schmitz and Schruers22 which could indicate a decreased hedonic response during anticipation of possible reward. Indeed, deficits in anticipatory and consummatory pleasure in 22q11DS compared with healthy controls have recently been reported.Reference Dubourg, Schneider, Padula and Eliez34 These results suggest that patients with 22q11DS may experience less pleasure when using substances that could help to explain low drug-seeking behaviour and continuation of substance use in 22q11DS. These potential reduced reinforcing effects of substances of misuse in 22q11DS could be related to the dopaminergic abnormalities as a result of reduced COMT activity in these patients. All substances of misuse directly or indirectly activate the dopamine systemReference Volkow9 by generating a rapid increase (phasic dopamine response) in extracellular dopamine in the striatum, which is associated with the reinforcing effects of drugs in both addicted and non-addicted individuals.Reference Volkow9 However, it must be noted that we did not find a relationship between COMT Val/Met polymorphism and substance use in this study.
Nevertheless, all of these speculations require further investigation, including more studies focusing on the dopaminergic system as well as exploration of reasons for various types of substance use.
In non-deleted individuals with mild/borderline intellectual disability, increased risk of problematic substance use and low prevalence of non-problematic substance use have been reported.Reference Chapman SL and Wu35 However, a recent study among patients with mild/moderate intellectual disability in Belgium reported non-problematic substance use to be comparable with substance use in the general population.Reference Swerts, Vandevelde, VanDerNagel, Vanderplasschen, Claes and De Maeyer36 In our study, we found that the likelihood of substance use increased with higher IQ within the 22q11DS group, although this effect was small. At present, not much is known about prevalence and risk factors for substance use in non-deleted adults with intellectual disability,Reference van Duijvenbode, VanDerNagel, Didden, Engels, Buitelaar and Kiewik37 complicating interpretation of these findings. However, one possible explanation for this relationship could be that patients with 22q11DS may be more ‘protected’ (e.g. more care by family members or institutions) than non-deleted patients with psychosis, which could contribute to low prevalence of substance use in these patients. Although this is a speculative explanation and does not rule out exposure to substance use, we cannot rule out that patients with a more severe intellectual disability are more likely to be cared for, or protected by, their families or live in a mental healthcare institution under permanent supervision. In contrast, in non-deleted individuals with intellectual disability, previous studies have reported that substance use is more likely in patients with a psychotic disorder.Reference Raina and Lunsky27 However, in our 22q11DS sample, we did not find a relationship between psychotic disorders and substance use. Similar to studies of general population samples and in non-deleted individuals with intellectual disability,Reference Chaplin, Gilvarry and Tsakanikos38 we found that male patients with 22q11DS were more likely to use substances than female patients.Reference Chaplin, Gilvarry and Tsakanikos38
Strengths and limitations
An important strength of the current study is the large sample of adult patients with 22q11DS. Because 22q11DS is a relatively rare and underrecognised disorder, most studies include small sample sizes, reducing statistical power. Another strength of this study is the inclusion of both a healthy control and a non-deleted psychosis group, enabling direct group comparisons.
When interpreting these findings, some limitations have to be taken into account. First, patients with 22q11DS were recruited through out-patient clinics for 22q11DS and genetic departments, and patients with psychosis and healthy controls were recruited for research purposes. Moreover, patients with 22q11DS were recruited from four different sites, whereas non-deleted patients with psychosis patients and healthy controls were recruited at only one site. Therefore, these samples are not true epidemiological samples and are likely to be biased to some extent by clinical ascertainment. Second, substance use data of patients with 22q11DS were based on the clinical files and/or self-reports of the patients. Therefore, for a minority of the patients not all information was complete. Third, the study had a cross-sectional design and data were assessed across different sites by multiple researchers using different instruments. Although predetermined guidelines were used, interrater differences cannot be ruled out as cross-site interreliability analyses were not performed. Finally, although we adjusted for lower IQ of patients with 22q11DS compared with healthy controls and non-deleted patients with psychosis and found that IQ only had only limited effects on prevalence of substance use, it would have been better to also include a group of non-deleted individuals with intellectual disability. Future research could address this issue.
In conclusion, we found the prevalence of substance use to be significantly lower in patients with 22q11DS compared with both non-deleted patients with psychosis and healthy controls. The results suggest that patients with 22q11DS are at decreased risk for substance use and SUD despite their increased risk of psychosis. More research on both neurobiological mechanisms and environmental factors of substance use in these patients is necessary to provide further insight into the mechanisms underlying psychosis and addiction. In addition, 22q11DS could be a valuable model to study genetic factors underlying substance use and SUD in the general population.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2018.258.
Funding
This work was supported by a grant from the Netherlands Organization for Health Research and Development (ZonMw). The GROUP project was also supported by a grant from ZonMw, within the Mental Health programme (project number: 10.000.1002). ZonMw had no further role in the study design, in the collection of the data, analyses and interpretation of the data, the writing of the report, or the decision to submit the paper for publication.







eLetters
No eLetters have been published for this article.