In March 2020, the WHO declared the Corona Virus Disease 19 (COVID-19) to be a pandemic(1). Infections with Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) can be asymptomatic (40 % of the cases) or cause a mild illness (40 %), but in about 15 % of the cases, severe disease develops, characterised by clinical signs of pneumonia (fever, cough and dyspnoea) plus one of the following: respiratory rate > 30 breaths/min; severe respiratory distress or SpO2 < 90 % on room air as defined by the WHO. Patients with acute respiratory distress syndrome (ARDS), sepsis or septic shock are categorised as critically ill which is the case in about 5 % of the cases(2). Amongst the co-morbidities, resulting in severe COVID-19 progression, inappropriate nutrition is increasingly attracting attention(Reference Bencivenga, Rengo and Varricchi3). According to the WHO, 1·9 billion adults are overweight or obese, while 462 million are underweight(4), underlining the relevance of taking inappropriate nutrition into account when discussing prevention and treatment of COVID-19. It is important to mention that Zn deficiency is frequently observed in undernutrition as well as in obesity, although the underlying mechanisms are different(Reference Rios-Lugo, Madrigal-Arellano and Gaytán-Hernández5).
In a previous article, we drew attention to the strong overlap of risk groups for severe progression of COVID-19 with the groups where Zn deficiency is frequently diagnosed(Reference Wessels, Rolles and Rink6). The effects of Zn supplementation were described and discussed(Reference Wessels, Rolles and Rink6,Reference Skalny, Rink and Ajsuvakova7) . In this article, we would like to discuss how pre-existing Zn deficiency might increase the susceptibility to COVID-19 infections as well as pre-dispose individuals for severe progression of disease as summarised in Fig. 1. Despite the many improvements in Zn research, we still lack a valid biomarker to reliably assess the Zn status of an individual(Reference Sandstead and Freeland-Graves8,Reference Lowe, Fekete and Decsi9) . Serum or plasma Zn levels are often used but are not completely reliable. Thus, serum levels below 642·5 μg/l are taken as an indication of Zn deficiency, but only partially reflect intracellular concentrations and the Zn status of an individual. Therefore, clear clinical signs of Zn deficiency can be observed even if serum Zn levels are above this critical value or in the normal range. Circadian variations of serum Zn levels were observed, and serum Zn also depends upon recent food intake and the degree of hydration/dehydration of an individual(Reference Heller, Sun and Hackler10,Reference Guillard, Piriou and Gombert11) . Early effects of Zn deficiency are often general and include functional changes that can be associated with various diseases. For this reason, mild Zn deficiency can be ‘hidden’(Reference Sandstead and Freeland-Graves8). Functional deficiencies in Zn-dependent immunological processes have been shown in human subjects and mice without any significantly different serum or plasma Zn levels compared with controls(Reference King, Frentzel and Mann12–Reference Beck, Prasad and Kaplan14). Currently, Zn deficiency is mostly defined by using a combination of clinical symptoms, calculating Zn and phytate intake from food and measuring immunological changes(Reference Trame, Wessels and Haase15,Reference Prasad16) . For growing infants (<2 years) and children (<5 years), the ‘height-for-age ratio’ should be determined as an additional parameter(Reference King, Brown and Gibson17). Moreover, it has been suggested that serum and plasma Zn values need to be adjusted for situations where inflammation is present(Reference McDonald, Suchdev and Krebs18,Reference Likoswe, Phiri and Broadley19) . For these reasons, Zn deficiency is often investigated using animal models of severe and induced Zn deficiency and well-defined low-Zn diets. Alternatively, Zn deficiency can reliably be modelled in cell cultures with either Zn-depleted media or by using Zn-specific chelators. Whether the latter rather models severe or mild Zn deficiency in the context of the whole organism is hard to predict. In this review, we describe data derived from clearly Zn-deficient humans and mice. Individuals with subclinical Zn deficiency might be less severely affected, but the effects are probably still not negligible(Reference Sandstead20). The consequences of Zn deficiency are manifold(Reference Prasad16,Reference Prasad21–Reference Hambidge and Krebs24) , and only effects that are relevant regarding the susceptibility and progression of infectious diseases such as COVID-19 (Fig. 1) are included here. In regard to innate immunity, the article will focus on the effects of Zn on the integrity of the epithelial cell barriers, on neutrophil and macrophage maturation and functions, and in regard to adaptive immunity, we focus on lymphocyte maturation and differentiation, and cytokine and antibody production. Known effects of Zn deficiency on the vascular system and the association of those effects with diseases affecting the heart, kidney, central nervous system and intestine are described in relation to COVID-19.

Fig. 1 Summary of complications that can be expected in patients with pre-existing zinc deficiency, when challenged by Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2). A patient with no co-morbidities and a balanced zinc homoeostasis will most likely develop no or mild symptoms or complications if infected with SARS-CoV-2 because immune cell numbers and functions are balanced, as are the other parameters listed in the Figure. However, zinc deficiency alone will result in the alterations indicated in the Figure. Preconditions resulting from zinc deficiency may result in the development of severe symptoms, critical illness and even death if the patient becomes infected with SARS-CoV-2. ARDS, acute respiratory distress syndrome; CNS, central nervous system; IFN, interferon; MMP, matrix metalloproteinase; TH, T helper cell; Treg, regulatory T cell; ZA, zinc adequate; ZD: zinc deficient.
In addition to nutritional causes (undernutrition, malnutrition, veganism, geophagy, a phytate-rich diet, low-Zn parenteral nutrition), conditioned Zn deficiency has been observed in association with many diseases and inflammatory reactions(Reference McDonald, Suchdev and Krebs18,Reference Wessels and Rink25) . Attention was drawn to Zn deficiency in the 1960s due to a traditional soil-eating diet (geophagy) in a group in Iran leading to a severe Zn deficiency associated with dwarfism. The group revealed that a severely disturbed immune response, was more susceptible to infection, suffered from lethargy, and none survived beyond the age of 25 years(Reference Prasad16). Untreated severe Zn deficiency, such as that seen during acrodermatitis enteropathica, has a high mortality rate often because of the inefficient clearance of infections(Reference Barnes and Moynahan26). The subjects with acrodermatitis enteropathica, and the above-described group in Iran, suffered from severe Zn deficiency. However, studies in mice and human subjects have shown that detrimental effects are seen not only in severe Zn deficiency but that also a slight to moderate Zn deficiency can result in alterations of haematopoiesis and defects in the functions of immune cells(Reference King, Frentzel and Mann12–Reference Beck, Prasad and Kaplan14,Reference Kahmann, Uciechowski and Warmuth27) , which thus increases the susceptibility to infection. It is important to recall that the immune system is affected negatively by Zn deficiency before any other symptoms become obvious and before serum Zn levels drop below 642·5 μg/l(Reference Roohani, Hurrell and Kelishadi28). Besides being essential for a robustly functioning immune system, Zn is also important for DNA synthesis, cell proliferation, cell differentiation, apoptosis, protein structure, protein–protein interactions and signal transduction as a second messenger for all kinds of cells. In the nervous system, Zn serves as an individual neurotransmitter that is secreted into the synaptic cleft(Reference Beyersmann and Haase29–Reference Tóth33). Zn deficiency can manifest itself in a variety of ways; amongst others, there are increased frequencies of pneumonia and diarrhoea, an altered sense of smell and taste, cytopenia, poor wound healing, hair thinning, eczema, reduced fertility, increased fatigue, sicca syndrome and nail dysplasia(Reference Ackland and Michalczyk22). Zn deficiency is a significant public health problem, and high numbers of deaths worldwide, especially in children, are associated with severe Zn deficiency(Reference Sandstead and Freeland-Graves8,Reference Hambidge and Krebs24,Reference Caulfield, Onis and Blössner34,Reference Fischer Walker, Ezzati and Black35) .
The magnitude of the effects of a pre-existing Zn deficiency, and the significance of mild compared with severe Zn deficiency, remains to be clearly defined and clarified in relation to COVID-19. A series of studies have been registered to analyse retrospectively the serum Zn levels of patients (online Supplementary Table S1), and the first published data in this regard are starting to appear. Data from further registered studies, investigating prophylactic Zn supplementation to decrease the susceptibility for infections and severe disease, especially in medical and military personnel, are also underway (online Supplementary Table S1). In the absence of experimental data, we extrapolate the information from the existing literature, in anticipation of the data from clinical studies, which should soon be available (online Supplementary Table S1).
Zinc deficiency alters haematopoiesis and disturbs the balance of innate and adaptive immune cells largely to the detriment of cells from the lymphoid lineage
Severe infections with SARS-CoV-2 can cause major hematopoietic changes. Most prominently, a decrease in lymphocytes has been noted, especially affecting the T cells. In COVID-19 patients with severe symptoms, the reduction in number and the functional exhaustion of CD4+ as well as CD8+ T cells, as detected by elevated expression of Tim-3 and PD-1, is frequently described and observed early during disease(Reference Diao, Wang and Tan36,Reference Liu, Zhang and He37) . The recovery of T cell numbers in severely ill patients was paralleled with the improvement of the symptoms and with positive prognosis and survival(Reference Liu, Li and Liu38).
Available data on the effects of SARS-CoV-2 on CD4+ compared with CD8+ T cells are somewhat controversial. While, in one study, no significant difference in the CD4+:CD8+ ratio but increased expression of CD8+ was found(Reference Ganji, Farahani and Khansarinejad39), other studies have reported a decrease particularly of CD8+ T cells, or a significantly elevated CD4:CD8 ratio in COVID-19 patients(Reference Liu, Zhang and He37,Reference Liu, Li and Liu38,Reference Jiang, Guo and Luo40) . As high levels of either perforin or granulysin, or both, were detected in CD8+ T cells(Reference Xu, Shi and Wang41), it can be assumed that CD8+ cells are overreacting initially and are subject to exhaustion and apoptosis at later stages. However, this hypothesis remains to be addressed. In contrast, B cell numbers and serum levels of Ig (IgA, IgG and IgM) have been reported to be rather weakly affected during COVID-19(Reference Liu, Li and Liu38).
Haematopoiesis is severely disturbed during both severe and mild Zn deficiency, which was found in human and animal studies as illustrated in Fig. 2. Especially, a loss in pre-B cells and immature B cells, as well as early developmental T cells, including CD4/CD8 double positive and pre-T cells, was described for humans and rodents with Zn deficiency as diagnosed by low plasma Zn levels. These effects can be corrected by Zn supplementation, as shown in subjects over 65 years of age suffering from mild serum Zn deficiency and in obese subjects with decreased serum Zn levels(Reference Rios-Lugo, Madrigal-Arellano and Gaytán-Hernández5,Reference Prasad16,Reference Kahmann, Uciechowski and Warmuth27,Reference Prasad, Rabbani and Abbasii42–Reference Hönscheid, Rink and Haase44) . Several mechanisms were described to underlie this decrease in cell numbers. Most importantly, thymus atrophy and decreased serum concentration of thymulin, which is necessary especially during maturation of T cells(Reference King, Osati-Ashtiani and Fraker45,Reference Mocchegiani, Giacconi and Costarelli46) , and lower levels of growth factors such as IL-2 (T cells) were reported in individuals with decreased serum Zn levels, and a disruption of IL-2 signalling was found when analysing cell cultures, where cellular Zn was depleted using a Zn chelator(Reference Kaltenberg, Plum and Ober-Blöbaum47). During dietary Zn deprivation in humans and rodents, a decreased ratio of type 1:type 2 T-helper cells, with reduced production of T-helper type 1 cytokines like interferon γ, is observed due to increased apoptosis(Reference Prasad16,Reference Truong-Tran, Carter and Ruffin30,Reference Hönscheid, Rink and Haase44) . Assuming that a Zn-deficient individual has fewer B cells compared with a person with a balanced Zn homoeostasis, a decreased generation of pathogen-specific antibodies can be expected. This might suggest that individuals, especially with a pre-existing severe Zn deficiency, would not be able to generate a sufficiently strong antibody response against SARS-CoV-2(Reference Anzilotti, Swan and Boisson48,Reference Fraker and King49) .

Fig. 2 Alterations in haematopoiesis are reported during zinc deficiency as well as in Corona Virus Disease 19 (COVID-19). During zinc deficiency, indicated by the red arrow, differentiation of myeloid cells, including polymorphonuclear neutrophils (PMN) and monocytes (Mo), is prioritised over development of adaptive immune cells, this especially impacts T cells (T). Amongst others, the prioritisation of myeloid cells may be explained by changes in growth factor expression: granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte-colony-stimulating factor (G-CSF) were described to be highly expressed, while levels of IL-2 are decreased during zinc deficiency. Furthermore, the T helper cell (TH)1:TH2 ratio is imbalanced during zinc deficiency, Th17 cell numbers are increased, while regulatory T cell (Treg) numbers were described as decreased as well as their functions. Most of those haematopoietic disturbances found during zinc deficiency are generally described for COVID-19 patients, as detailed in the text. B, B cell; BCP, B-cell progenitor; E, erythrocyte; EPO, erythropoietin; GM, granulocyte-macrophage progenitor; HSC, hematopoietic stem cell; MEP, megakaryocyte–erythroid progenitor; NK, natural killer cell; Pl, platelets; SCF, stem cell factor; TC: cytotoxic T cell; TNK, T and NK cell progenitor; TPO, Thrombopoietin.
In COVID-19 patients, especially those with severe symptoms, TH17 cell numbers were elevated, which is in line with the hyperinflammatory status of the immune system(Reference Xu, Shi and Wang41). Recent studies underline that differentiation into the main CD4+ cell subtypes is disturbed when Zn supply is low. In vivo data from patients with allergic asthma reveal that impaired Treg-mediated suppression can be correlated with decreased serum Zn levels(Reference Nurmatov, Nwaru and Devereux50). Data from in vitro differentiation experiments, using Zn-deficient compared with Zn-adequate culture medium, further strengthen the hypothesis, that development of proinflammatory TH17 cells is supported as a consequence of Zn deficiency(Reference Kulik, Maywald and Kloubert51).
In contrast to the consistently observed lymphopenia in COVID-19 patients, the numbers of myeloid cells in the blood and in lung tissue were strongly elevated. Neutrophilia was associated with the progression of severe disease to ARDS and with increased mortality therefrom, similarly to that described for bacteria-induced lung injury(Reference Huang, Wang and Li52–Reference Liu, Liu and Xiang55). As blood analyses of non-survivors revealed severe lymphopenia combined with significantly elevated numbers of neutrophils, the neutrophil:lymphocyte ratio was suggested as a prognostic marker for COVID-19 patients(Reference Liu, Li and Liu38,Reference Liu, Liu and Xiang55) . Similar to the neutrophil:lymphocyte ratio shift in COVID-19 patients, the balance between adaptive and innate immune cells is shifted towards the latter during Zn deficiency. Investigation of severely Zn-deficient rodents, that were fed on a low-Zn diet, showed high numbers of neutrophils and their specific products in the bone marrow and blood compared with Zn-adequate animals(Reference Fraker and King49,Reference Fraker, King and Laakko56,Reference Sakakibara, Sato and Kawashima57) . Our own unpublished results suggest that maturation of myeloid precursors into granulocytes in Zn-deficient human cell cultures is also increased compared with cells that differentiate in Zn-adequate cell cultures, while Zn supplementation attenuates the development into mature neutrophils(Reference Tillmann, Rink and Wessels58). As an underlying mechanism, the increased response to growth factors, for example, to the granulocyte-macrophage colony-stimulating factor and granulocyte-colony-stimulating factor, can be named, as was determined in cell culture experiments where Zn was added to the culture medium(Reference Wessels, Pupke and Trotha54,Reference Aster, Barth and Rink59) .
Thrombocytopenia, which we will come back to later, and a decline in Hb are also common in COVID-19 patients(Reference Liu, Zhang and He37). Lower Hb and erythrocyte counts were found in severe COVID-19 cases compared with moderate cases. Furthermore, higher ferritin was found in severe COVID-19 cases, and a significant difference in the mean ferritin levels was found between survivors and non-survivors(Reference Taneri, Gómez-Ochoa and Llanaj60). Additional research is necessary to prove the suggested important role of anaemia and a disturbed Fe in severe cases of COVID-19. This might uncover new treatment options.
Alterations in bone marrow metabolism were related to decreased serum Zn concentrations in humans(Reference Prasad21,Reference Mir, Hossein-nezhad and Bahrami61) . This finding together with the observation that the osmotic fragility of erythrocyte membranes is elevated in animals with dietary Zn deficiency, as are levels of lipid peroxidation in mitochondrial and microsomal membranes, suggests that there might be some interconnection between Zn deficiency and anaemia(Reference Vallee and Falchuk62). Indeed, serum hypozincaemia is commonly observed in anaemic subjects(Reference Mir, Hossein-nezhad and Bahrami61,Reference Abdelhaleim, Abdo Soliman and Amer63–Reference Shweta, Prantesh and Shashvat66) . However, importantly, a causal association between Zn deficiency and anaemia has so far not been established clearly and is discussed controversially. For example, serum Zn concentration was correlated with serum ferritin concentration in patients undergoing peritoneal dialysis(Reference Kaneko, Morino and Minato67). Morover, lower serum ferritin was significantly correlated with smaller sizes of Zn pools in premenopausal women, although without anaemia(Reference Yokoi, Sandstead and Egger68). Regarding the effects of adjuvant Zn therapy for improving anaemia in haemodialysis patients, Hb levels were found to increase significantly in Zn-supplemented patients compared with patients not supplemented with Zn. The authors suggest a ‘zinc deficiency anemia’, which needs further evaluation(Reference Fukushima, Horike and Fujiki64). In this regard, it should be pointed out that nutritional deficits often include several elements concomitantly, as shown for Zn and Fe, Se and others(Reference Heller, Sun and Hackler10,Reference Abdelhaleim, Abdo Soliman and Amer63,Reference Houghton, Parnell and Thomson65,Reference Gombart, Pierre and Maggini69) . Since the association of anaemia with an increased risk and severe progression of COVID-19 has not been clearly established, this will not be discussed further in this article. However, as anaemia is generally related to poor outcomes of infectious diseases(Reference Viana70), possible nutritional deficits in COVID-19 risk groups should be addressed and not only Zn but also Fe, Se and other elements might need to be supplemented if applicable.
Comparing the disturbance of haematopoiesis observed in individuals with low serum Zn levels or with COVID-19, various congruencies become apparent. As lymphopenia, neutrophilia and a decline in Hb are associated with progression to severe COVID-19, it can be hypothesised that a pre-existing severe Zn deficiency will predispose patients to stronger progression of infections with SARS-CoV-2 and that even a mild Zn deficiency should be corrected to prevent more severe progression of the viral infection. A pre-existing elevated neutrophil:lymphocyte ratio, even one of low magnitude, as during Zn deficiency, might be detrimental in the case of severe and aggressive infections such as COVID-19. At first sight, elevated numbers of innate immune cells as a first line of defence might appear beneficial. However, they are easily overrun during viral infections as a specific response, and the release of anti-viral factors and antibodies, especially by adaptive immune cells, is of major importance here. The elevated numbers of hyperactivated innate immune cells can even lead to high levels of inflammatory factors and oxidative stress causing destruction of host tissue. In SARS-CoV-infected mice, the recruitment of high numbers of monocytes and macrophages to the lungs was observed, secreting high numbers of proinflammatory cytokines and chemokines, which are associated with vascular leakage, underlining the detrimental effect of highly reactive immune cells(Reference Channappanavar, Fehr and Vijay71). Similar scenarios are suggested for SARS-CoV-2 in humans(Reference Henderson, Canna and Schulert72–Reference Chen, Di and Guo74).
The next chapters will show that the alterations in immune cell counts are not the only indication of an association between Zn deficiency and COVID-19.
Pre-existing zinc deficiency could prime for the cytokine release syndrome
A frequent complication among patients with severe COVID-19 is the cytokine release syndrome, which spreads throughout the body from the focal infected area and may lead to death because of subsequent ARDS or multiple organ dysfunction syndrome and other complications(Reference Ye, Wang and Mao75,Reference Chousterman, Swirski and Weber76) . It was reported that among the COVID-19 patients, the classic serum proinflammatory cytokines like TNF-α, IL-2, IL-6, IL-7, IL-8, IL-10, granulocyte-colony-stimulating factor and C-reactive protein are elevated(Reference Henderson, Canna and Schulert72,Reference Conti, Ronconi and Caraffa73,Reference Chousterman, Swirski and Weber76) . IL-6 in particular, which is produced by lung resident macrophages and circulating immune cells(Reference Guan, Ni and Hu77–Reference Vinciguerra, Romiti and Fattouch79), has been associated with severe COVID-19 and increased mortality(Reference Aziz, Fatima and Assaly80). Moreover, D-dimers, ferritin, lactate dehydrogenase, aspartate aminotransferase, alanine aminotransferase and soluble CD25 (IL-2 receptor) are increased, while fibrinogen is decreased(Reference Henderson, Canna and Schulert72).
Although there are planned and ongoing trials to counter the cytokine storm using approved antibodies such as tocilizumab (anti-IL-6 receptor), anakinra (IL-1 receptor antagonist, IL-1RA) and anti-TNF antibodies used to treat other hyperinflammatory conditions, and in spite of some benefits, so far their efficacy was not proven in large-scale, randomised controlled trials(Reference Henderson, Canna and Schulert72,Reference Di Giambenedetto, Ciccullo and Borghetti81–Reference Feldmann, Maini and Woody83) , and therefore, therapeutic options are still limited.
In recent years, in vivo and in vitro data supporting the hypothesis that a pre-existing Zn deficiency augments the activation-induced inflammatory response, and results characterizing the possible underlying mechanisms, are constantly accumulating. Serum hypozincaemia was correlated with increased serum levels of, amongst others, IL-1β, IL-6, TNFα, IL-8, granulocyte-colony-stimulating factor, IL-10, IL-1RA, IL-17, C-reactive protein and calprotectin; thus, a whole battery of proinflammatory mediators is increased, especially during severe Zn deficiency, and particularly in combination with the inflammatory response to a pathogen(Reference Wessels, Pupke and Trotha54,Reference Reda, Abbas and Mohammed84–Reference Wessels, Haase and Engelhardt88) . In the case of IL-1β and TNFα, Zn chelation was shown to induce epigenetic changes in the promoters of both genes in cell culture experiments. More specifically, the accessibility of regions in the DNA close to the transcriptional start site was significantly increased so that after inflammatory activation of the cells, by, for example, lipopolysaccharide, gene expression was augmented(Reference Wessels, Haase and Engelhardt88). Moreover, activation of NFκB a central player in the signalling pathways involved in the generation of inflammatory factors is increased when Zn is limiting, as found in mice with diet-induced serum hypozincaemia(Reference Bao, Liu and Lee87). Cell culture experiments using Zn-depleted medium revealed increased expression of calprotectin in myeloid precursors and mature monocytic cells(Reference Lienau, Rink and Wessels89)
Activated T cells express lower IL-2 and interferon γ mRNA levels, as was shown in vitro and observed in individuals with low serum Zn(Reference Prasad, Meftah and Abdallah90). IL-2 is essential for natural killer cell and cytotoxic T lymphocyte activity. Interferon γ is essential for killing viruses, parasites and bacteria. Thus, the decreased efficiency of the immune response in Zn-deficient subjects is easily explained(Reference Beck, Prasad and Kaplan14,Reference Prasad, Meftah and Abdallah90,Reference Tapazoglou, Prasad and Hill91) . Defects in T cell function as a consequence of Zn deficiency can also be explained by the accumulation of deoxyguanosine, which results from decreased Zn-dependent nucleoside phosphorylase activity in human lymphocytes, derived from human Zn-deficient volunteers before and after Zn supplementation(Reference Meftah and Prasad92). Serum Zn deficiency strongly affects Th1 cells, while Th2 cells are largely unaffected, and production of IL-4, IL-6 and IL-10 (Th2 cytokines) remains rather stable. However, production of interferon γ and IL-2 (Th1 cytokine) is decreased(Reference Prasad93).
Although Treg cell numbers might be constant, or even elevated, during in vitro differentiation under Zn deficiency, it was suggested that their function is disturbed(Reference Kulik, Maywald and Kloubert51). In vivo data are so far scarce, but some studies in mice suggest decreased transforming growth factor β (Treg cytokine) levels during Zn deficiency, pointing to a malfunctioning of Treg cells and thus imbalance of the immune response(Reference Finamore, Roselli and Merendino94). As Treg cells are important master regulators within the immune system, essential for tolerance and balance and differentiation of the remaining CD4+ T cell subtypes, a disturbed immune response can be expected.
Treatment of cell cultures with a Zn chelator disturbed the cytotoxic activity of natural killer cells(Reference Rolles, Maywald and Rink95,Reference Wessels, Maywald and Rink96) . Similar effects were reported for rats fed on a Zn-deficient diet(Reference Öztürk, Erbas and Imir97). This effect might decrease the killing of host cells which become infected by the virus.
A number of effects described above are due to the requirement of Zn for intracellular signal transduction and the consequent disruption of a multitude of signalling pathways when Zn supply is limited. Zn’s effect on phosphatases and kinases is central here, as is its ability to induce changes in membrane fluidity and thus receptor expression and dimerization as found in vitro and in vivo (Reference Kaltenberg, Plum and Ober-Blöbaum47,Reference Aster, Barth and Rink59,Reference Sadighi, Roshan and Moradi98) . Finally, epigenetic changes occur during Zn deficiency as described above as found in various models of Zn deficiency(Reference Wessels99,Reference Rosenkranz, Metz and Maywald100) .
In the case of IL-6, another connection to Zn deficiency has been described. A SNP was found in the IL-6 gene at position −174. It is associated with a disturbed age-related Zn deficiency, and it seems to be relevant during the regulation of Zn-related genes such as metallothioneins. The frequency of this polymorphism increases with age and offers an additional explanation for the high risk of Zn deficiency described for the elderly(Reference Mocchegiani, Giacconi and Costarelli46,Reference Wessels and Cousins101) . Interestingly, the IL-6–174 SNP was also associated with an increased risk for severe progression of and mortality from COVID-19, as was suggested previously for sepsis, but never proven up to now(Reference Kirtipal and Bharadwaj102–Reference Mayor-Ibarguren, Busca-Arenzana and Robles-Marhuenda104). Individuals with this SNP could be actively supplemented with Zn, not only to help prevent severe COVID-19 but also to enable a balanced immune response in general(Reference Mocchegiani, Giacconi and Costarelli46,Reference Mariani, Neri and Cattini105) .
Glucocorticoids were suggested as a means to attenuate the cytokine storm and proposed as a treatment option during the hyperinflammatory phase of COVID-19(Reference Henderson, Canna and Schulert72). On the other hand, chronically increased glucocorticoids may augment lymphopenia(Reference Grant, Rotstein and Liu106,Reference Olnes, Kotliarov and Biancotto107) . Serum Zn deficiency was associated with chronically elevated levels of glucocorticoids, especially corticosteroids. However, data are not clear in this regard yet, and studies have been published not recommending the use of glucocorticoids during COVID-19 treatment, or at least recommend caution. Criticism of glucocorticoid use is largely based on data on SARS from 2003, where improper use of systemic corticosteroids increased the risk of osteonecrosis of the femoral head, which is, however, a classical side effect of glucocorticoid therapy and not related to the virus(Reference Chen, Tang and Tan108–Reference Isidori, Arnaldi and Boscaro111). At first sight, this is one of the only consequences of Zn deficiency that might be viewed as an advantage in terms of COVID-19. In this regard, it should also be mentioned that the chronically increased glucocorticoid levels were suggested to be associated with the increased apoptosis of lymphocytes and probably also of cells of the thymus, thus explaining thymic atrophy in mice and humans with decreased serum Zn levels(Reference Garvy, King and Telford112–Reference Taub and Longo114). However, those suggestions require experimental verification.
The cytokine storm is central to the progression from mild or severe disease to complications and critical illness associated with COVID-19 and should be prevented by any possible means. The hyper-inflammation is largely involved in damaging various organs, including the lung, heart, liver, kidney and probably also the intestine and the brain. Interestingly, the central nervous system, the gastrointestinal tract, lungs, liver, the epidermal, reproductive and skeletal system are clinically affected by severe Zn deficiency which causes elevation of inflammatory markers(Reference Prasad16,Reference Vallee and Falchuk62) . As the treatment of the cytokine storm is complex and the individual patient response to certain treatments is almost impossible to predict, the best option is to prevent the cytokine storm. Thus, groups that are at risk of Zn deficiency should be supplemented routinely. Of course, individuals with severe pre-existing Zn deficiency will benefit the most; however, adjusting mild Zn deficiencies is also of importance especially in individuals from COVID-19 risk groups such as the elderly, diabetic patients and individuals with heart and vascular co-morbidities.
Additional roles of Zn in the regulation of immune cell function, but perhaps not obviously relevant for what is known of SARS-CoV-2 infection, have been reviewed extensively elsewhere(Reference Wessels and Rink25,Reference Beyersmann and Haase29,Reference Hönscheid, Rink and Haase44,Reference Kulik, Maywald and Kloubert51,Reference Bao, Liu and Lee87,Reference Wessels, Maywald and Rink96,Reference Wessels99,Reference Haase and Schomburg115,Reference Gammoh and Rink116) .
Zinc deficiency and vascular complications: possible association with complications affecting multiple organs
Cardiovascular complications are frequently reported during COVID-19, especially in patients with pre-existing pathologies of the heart and vascular system, such as atherosclerosis(Reference Vinciguerra, Romiti and Fattouch79,Reference Gavriilaki and Brodsky117) . Venous, arterial and microvascular thromboses are increased in patients with COVID-19. Moreover, COVID-19-associated hypercoagulopathy closely resembles the pathophysiology and phenotype of complement-mediated thrombotic microangiopathy(Reference Gavriilaki and Brodsky117). An increase of proinflammatory cytokines, increased complement activation, endothelial dysfunction and immunothrombosis are considered to be key mechanisms of hypercoagulopathy. For instance, venous thromboembolisms, also driven by a hyperinflammatory milieu, were described in 20–31 % of severe COVID-19 cases(Reference Liu, Zhang and He37,Reference Middeldorp, Coppens and van Haaps118–Reference Levi, Thachil and Iba121) . Moreover, an increased number of especially polymorphonuclear neutrophils (PMN) together with high amounts of neutrophil extracellular traps were observed in the thrombi of COVID-19 patients(Reference Nicolai, Leunig and Brambs122). Arterial embolism, including acute pulmonary embolism, ischaemic stroke and acute myocardial injury, was also increased in patients with severe SARS-CoV-2 infection(Reference Kashi, Jacquin and Dakhil123–Reference Bavishi, Bonow and Trivedi126). Subsequent thrombocytopenia was associated with poor prognosis for COVID-19 patients. Concerning the endothelial dysfunction, it was proposed that direct endothelial damage can lead to an increased thrombogenic effect in the microcirculation(Reference Ziegler, Allon and Nyquist127). An impaired microcirculation can cause complications in various organs including the lung, the kidneys, the heart, the brain, the liver or the pancreas.
As already indicated, an increased activation and tissue recruitment of PMN in Zn-deficient individuals are likely(Reference Wessels, Pupke and Trotha54,Reference Knoell, Julian and Bao128,Reference Knoell, Smith and Sapkota129) . Thus, pre-existing Zn deficiency may be indirectly associated with thrombus formation. The association of pre-existing Zn deficiency with hyperinflammation was already described in this article and can also be related to an increased risk for thromboembolism. In addition, Zn is essential for various aspects of physiological coagulation and might impact thrombogenesis as well as fibrinolysis(Reference Vu, Fredenburgh and Weitz130,Reference Taylor and Pugh131) . However, Zn’s effects seem to depend on the microenvironment and might be locally restricted and temporary(Reference Vu, Fredenburgh and Weitz130). For example, Zn can be secreted by activated platelets resulting in locally increased Zn concentrations in the vicinity of a thrombus, while the systemic Zn homoeostasis remains probably rather stable. The direct effects of pre-existing Zn deficiency on coagulation are not entirely clear. Studies in Zn-deficient humans, rodents and guinea pigs revealed clotting abnormalities, impaired platelet function as well as an increased and prolonged bleeding tendency(Reference Emery, Browning and O’Dell132,Reference Gordon and O’Dell133) . Those Zn-deficiency-induced defects were reversible by Zn supplementation(Reference Marx, Krugliak and Shaklai134). In a recent in vitro study, Zn deficiency inhibited the agonist-activated production of reactive oxygen species (ROS) by platelets and decreased glutathione levels and glutathione peroxidase activity, which might result in altered thrombus formation(Reference Lopes-Pires, Ahmed and Vara135). Due to the lack of detailed and consitent data, a clear conclusion on the effects of Zn deficiency regarding fibrinolysis and coagulation cannot be drawn. However, as with many topics discussed in this review, a well-balanced Zn homoeostasis seems to be key to a physiological balance also in the example of coagulation and fibrinolysis.
The risk of developing an acute coronary syndrome during SARS-CoV-2 infection is especially increased in patients with atherosclerotic vascular disease(Reference Bonow, Fonarow and O’Gara136). The development and subsequent rupture of vulnerable plaques can result in heart attack or stroke, and subsequent heart failure and death(Reference Lopez, Keen and Lanoue137,Reference Libby138) . During the development of atherosclerosis, up-regulation of adhesion molecules on endothelial cells is one of the central events, largely involving the transcription factor NFκB. An increased activation and DNA binding of selected transcription factors during Zn deficiency were established in vitro (Reference Connell, Young and Toborek139,Reference Hennig, Meerarani and Toborek140) . In addition, the role of Zn in NFκB-related signalling has been described in various studies(Reference Bao, Liu and Lee87,Reference Wessels, Maywald and Rink96) . The association of severe pre-existing serum Zn deficiency in mice with an increased risk of atherosclerosis was additionally explained by the Zn-dependent alteration of endothelial surface markers, changes of the plasma lipid composition and the promotion of the proinflammatory milieu(Reference Bao, Prasad and Beck85,Reference Wessels, Maywald and Rink96,Reference Reiterer, MacDonald and Browning141) . Results from various in vivo and in vitro studies as summarised by Choi et al. indicate that Zn supplementation may reduce the risk of atherosclerosis and protect against myocardial infarction as well as ischaemia/reperfusion injury(Reference Choi, Liu and Pan23). The vasculitis described in COVID-19 patients resembles the reaction to infections with Varicella zoster virus, where the viral replication in the cerebral arterial wall directly triggered local inflammation(Reference Gilden, Cohrs and Mahalingam142). Zn supplementation in cell culture experiments was shown to decrease viral replication(Reference te Velthuis, van den Worm and Sims143), and Zn supplementation might thus attenuate virus-induced vasculitis. Recently, a molecular modelling study predicted an interaction of Zn with RNA-dependent, RNA-polymerase and 3C-like proteinase enzymes of SARS-CoV-2, which awaits experimental verification.
Associations of diseases such as arterial hypertension, atherosclerosis, congestive heart failure and CHD are described in both Zn deficiency and COVID-19(Reference Bao, Prasad and Beck85,Reference Tomat, de los Ángeles Costa and Arranz144–Reference Little, Bhattacharya and Moreyra150) , but a causal link between Zn deficiency and the observations in COVID-19 remains to be established.
Pre-existing zinc deficiency is associated with severe progression of respiratory diseases
SARS-CoV-2 enters the human body predominantly via the respiratory tract. In healthy individuals, viral entry is hampered by the mucous-coated membrane of the alveoli as well as the immune cells and their anti-viral products protecting the lungs(Reference Carrillo, Rodríguez and Coronado151). When SARS-CoV-2 has crossed the epithelial barrier, it can elicit extensive alveolar injury and pulmonary fibrosis, which are irreversible pathological changes. The progression of mild COVID-19 to pneumonia, acute lung injury and subsequently to ARDS is the leading cause of mortality, affecting 5–10 % of the COVID-19 patients worldwide(Reference Ruan, Yang and Wang152,Reference Chen, Zhou and Dong153) .
As illustrated in Fig. 3, the expression of tight-junction proteins is decreased under Zn-deficient conditions. This as well as reduced expression of adherens junction proteins reduces the integrity of the endothelial barrier and might facilitate viral entry, as shown in a variety of studies investigating human and rodent tissue in vivo, ex vivo and in vitro (Reference Wessels, Pupke and Trotha54,Reference Reiterer, MacDonald and Browning141,Reference Roscioli, Jersmann and Lester154–Reference Bao and Knoell157) . Experiments using an ex vivo model of differentiated human airway epithelium showed that exposure to Zn-depleted medium significantly augmented the down-regulation of the tight junction proteins such as Zonula Occludens-1 and Claudin-1 that was induced by cigarette smoke extract(Reference Roscioli, Jersmann and Lester154). Another study, which investigated primary human upper airway and type I/II alveolar epithelial cells that were grown in Zn-depleted compared with Zn-adequate medium, revealed that Zn deprivation augmented activation-induced proteolysis of E-cadherin and β-catenin, both adherens junction proteins(Reference Bao and Knoell157). Since intracellular Zn levels of endothelial cells largely depend on the protein-bound Zn pool in the blood serum, the cells are deprived of Zn during serum hypozincaemia. Low endothelial Zn disturbs cellular metabolism and is associated with oxidative stress. Increased serum levels of oxidised LDL and high amounts of inflammatory cytokines derived from activated monocytes are frequently observed in individuals with serum Zn deficiency, and together with the high oxidative stress, this leads to increased apoptosis of epithelial cells. Consequently, mild pre-existing Zn deficiency combined with inflammation-induced serum hypozincaemia may exacerbate epithelial barrier permeability of the lung in COVID-19 patients.

Fig. 3 Pulmonary effects observed in Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) infected patients with pre-existing zinc deficiency as compared with patients with a balanced zinc homoeostasis. Pre-existing zinc deficiency (left) was suggested to increase the number, recruitment and inflammatory potential of especially PMN to the insides of the bronchi. Lymphocyte numbers are generally decreased, most prominently affecting T helper cell (TH) cells. The zinc deficiency-related decrease in tight junction expression and the increase in endothelial cell apoptosis have several consequences. Thus, infiltration of the lung by host cells, as well as the leakage of pathogens such as SARS-CoV-2 and secondary pathogens such as Streptococcus pneumoniae into the vascular system, is frequently observed during zinc deficiency. Detailed explanations can be found in the text. For comparison, the characteristics of zinc-adequate individual are indicated on the right. Ab, antibody; B, B cell; E, erythrocyte; G-CSF, granulocyte colony-stimulating factor; GC, glucocorticoid; GM-CSF, granulocyte-macrophage CSF; MMP, matrix metalloproteinase; Mo, monocyte; Mϕ, macrophage; NET, neutrophil extracellular trap; NK, natural killer cell; Pl, platelet; PMN, polymorphonuclear neutrophil; ROS, reactive oxygen species; Tc, cytotoxic T cell; TJ, tight junction; ZA, zinc adequate; ZD, zinc deficient.
Previous investigations on SARS-CoV-1 infections revealed that phagocytic cells largely contributed to the antibody-mediated elimination of the virus(Reference Yasui, Kohara and Kitabatake158). Amongst the phagocytes, resident macrophages are constantly patrolling the lung, while high numbers of PMN are recruited during infections, abundant ones. PMN are highly reactive cells, equipped with their complete anti-microbial weaponry when they leave the bone marrow. Upon activation, they release their granular content which includes highly reactive mediators such as ROS, reactive nitrogen species, antimicrobial peptides, matrix metalloproteases that degrade extracellular matrix and more(Reference Wessels, Jansen and Rink159,Reference Cowland and Borregaard160) . Those factors are primarily secreted to destroy invading pathogens. However, if secreted in excessively high amounts, they can destroy the host tissue as well(Reference Grommes and Soehnlein161), as was suggested to explain tissue injury in SARS-CoV-2 infections.
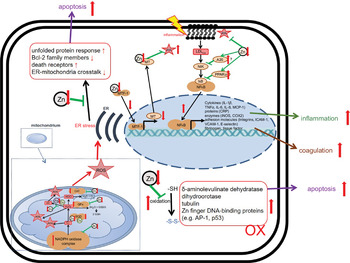
With respect to PMN activity, the effects of Zn deficiency are not clearly defined. While some studies describe attenuated motility of PMN in moderately Zn-deficient individuals(Reference Briggs, Pedersen and Mahajan162,Reference Hasan, Rink and Haase163) , the numbers of PMN found in the infected tissues of animals with pre-existing Zn deficiency are higher compared with animals with adequate Zn supply(Reference Knoell, Julian and Bao128,Reference Knoell, Smith and Sapkota129) . Whether the defect in chemotaxis is compensated by the elevated numbers of PMN observed in Zn-deficient rodents, remains to be investigated(Reference Fraker and King49). The formation of ROS and neutrophil extracellular traps by PMN was reported to be decreased in Zn-deficient cells in culture(Reference Hasan, Rink and Haase164). Surprisingly, Zn supplementation of mice in vivo, or of human neutrophils in cell culture, also decreased activation-induced neutrophil extracellular trap formation(Reference Wessels, Pupke and Trotha54). In this context, we would like to mention that it was shown for various cell types, mostly in cell culture models, that Zn deficiency alters redox metabolism and results in oxidative stress(Reference Kloubert and Rink165–Reference O’Dell169). There are several suggestions for the mechanisms responsible for the elevated ROS levels in Zn-deficient conditions, as summarised in Fig. 4. First, Zn deficiency was related to the decreased activity of enzymes which are central to ROS metabolism, such as the Cu/Zn superoxide dismutase in vitro. Here, the inactivation of enzymes due to the lack of Zn in their catalytic centre was described(Reference Lee168,Reference Ramirez, Gomez-Mejiba and Corbett170,Reference Taylor, Bettger and Bray171) . Second, expression of metallothioneins, not only the major intracellular Zn binding proteins but also an important free radical scavenger, is decreased during Zn deficiency, which was shown using various models of Zn deficiency and was recently summarised(Reference Lee168). As a third mechanism, Zn is necessary to protect the free sulfhydryl groups in proteins from oxidation. A lack of Zn might also alter the formation of intramolecular disulphide bonds, causing steric hindrance and conformational changes, which can be associated with increased activity or the loss of function of molecules involved in balancing the redox state of the cells, determined in cell culture experiments and suggested by in vivo examination of Zn-deficient animals(Reference Bray and Bettger172). In Zn-adequate conditions, Zn competes with other redox-active metal ions with similar coordination chemistry such as Cu or Fe for protein binding. The lack of Zn as competitor is a fourth suggested mechanism explaining the increased oxidative stress when Zn is limited. This was investigated for the oxidation of myoglobin and the activity of superoxide dismutase(Reference Ramirez, Gomez-Mejiba and Corbett170,Reference Hegetschweiler, Saltman and Dalvit173) . Zn also competes with Fe and Cu for binding to the NADPH oxidase and usually inhibits NADPH oxidase activity. Increased NADPH oxidase activity was reported for neuronal cells cultured in the Zn-depleted medium(Reference Aimo, Cherr and Oteiza174). In this context, Zn can bind NADPH, but not NADH, and thus inhibits NADPH-dependent enzymes in vitro (Reference Ludwig, Misiorowski and Chvapil175,Reference Verstraeten, Nogueira and Schreier176) . Moreover, Zn interferes with the Fenton reaction in vitro suppressing lipid peroxidation(Reference Friedrich, Mendes and Silva177,Reference Zago, Verstraeten and Oteiza178) . As a fifth point, Zn deprivation was associated with dysfunctions of mitochondria and the endoplasmic reticulum. Finally, Zn’s effect on gene expression might affect redox metabolism. Zn was shown to be involved in the up-regulation of several transcription factors, and some antioxidant molecules such as glutathione and detoxifying enzymes such as glutathione S-transferase and haemeoxygenase-1 mostly investigated using Zn-deficient cell cultures(Reference Verstraeten, Nogueira and Schreier176,Reference Jarosz, Olbert and Wyszogrodzka179) . The nuclear factor erythroid 2-related factor 2 can be induced by Zn, as was investigated in rats fed on a low-Zn, Zn-adequate or high-Zn diet(Reference Verstraeten, Nogueira and Schreier176,Reference Jarosz, Olbert and Wyszogrodzka179,Reference Wang, Nie and Lu180) . Whether Zn deficiency has the opposite effect to Zn supplementation remains to be explored, but in summary, the multiple mechanisms described above can explain the overall increase in ROS during Zn deficiency, which was consistently found in various models of Zn deficiency. We thus hypothesise that in combination with the infection-induced inflammation observed in COVID-19 patients, pre-existing Zn deficiency might augment the formation of ROS and reactive nitrogen species causing severe tissue damage. On the other hand, the anti-oxidative properties of Zn are widely described and accepted(Reference Choi, Liu and Pan23,Reference Hennig, Meerarani and Toborek140,Reference Abdulhamid, Beck and Millard181,Reference Cao, Duan and Zhang182) , suggesting benefits of Zn supplementation for COVID-19 patients.

Fig. 4 Effects of zinc deficiency on stress-induced changes in redox metabolism. Green arrows indicate zinc-dependent cellular functions. Red arrows illustrate the effects of zinc deficiency. A detailed description of the mechanisms underlying disturbed redox metabolism during zinc deficiency can be found in the text. AP-1, Activator protein 1; Bcl-2, B-cell lymphoma 2; CAT, catalase; COX, Cyclo-oxygenase; CRP, C-reactive protein; ER, endoplasmic reticulum; GPx, glutathione peroxidase; ICAM, intercellular adhesion molecule-1; iNOS, inducible nitric oxide synthase; MT, metallothionein; MTF, metal-responsive transcription factor-1; Ox, oxidated; MCP, monocyte chemoattractant protein; NIK, NFκB-Inducing Kinase; ROS, reactive oxygen species; SOD, superoxide dismutase; VCAM, vascular cell adhesion molecule.
COVID-19 often shows systemic effects in the patient’s tissues and organs, often resulting in multi-organ failure and high death rates(Reference Bencivenga, Rengo and Varricchi3,Reference Chen, Di and Guo74,Reference Cavalli, Luca and Campochiaro82,Reference Petrilli, Jones and Yang183) . Furthermore, ‘septic shock’ is another cause of mortality from SARS-CoV-2 and is currently observed in 4–8 % of COVID-19 patients(Reference Huang, Wang and Li52,Reference Wang, Hu and Hu53,Reference Rodriguez-Morales, Cardona-Ospina and Gutiérrez-Ocampo184) . When reading all the articles on COVID-19 discussing the symptoms in individuals undergoing mild compared with severe viral disease, one cannot help but notice the parallels to mild bacterial infections compared with bacterial sepsis and its progression to ARDS(Reference Liu, Zhang and He37,Reference Grommes and Soehnlein161,Reference Matthay, Zemans and Zimmerman185) . Regarding bacterial sepsis, various studies in animals and humans describe an association of disease progression and mortality in relation to the Zn status, which could be extrapolated to COVID-19. It was shown that pre-existing Zn deficiency was a prerequisite for the progression from mild inflammation to pneumonia and severe sepsis in mice. Severity of disease was monitored by analysing the serum levels of proinflammatory cytokines (i.e. assessment of the cytokine storm) and damage to the lungs, the liver and the kidney. Also, serum Zn concentrations were inversely correlated with sepsis severity. Thus, serum Zn was suggested as a prognostic marker for mortality in septic mice, pigs, adult humans and infants. In critically ill children, complications of sepsis, the necessity for mechanical ventilation and resulting mortality rates were correlated with low serum Zn levels.(Reference Wessels and Cousins101,Reference Knoell, Julian and Bao128,Reference Knoell, Smith and Sapkota129,Reference Hoeger, Simon and Beeker186–Reference Alker and Haase193) . Moreover, Boudreault et al. revealed that pre-existing Zn deficiency primes the lungs for severe complications derived from mechanical ventilation, including the progression from acute lung injury to ARDS(Reference Boudreault, Pinilla-Vera and Englert194). In cystic fibrosis, Zn deficiency, caused by a splice switch in the Zn Importer ZIP2, caused hypersecretion of the glycoprotein mucin in airway epithelial cells, significantly increasing disease severity(Reference Kamei, Fujikawa and Nohara195). Pre-existing serum Zn deficiency was implicated to be responsible for the high incidence of pneumonia in elderly, hospitalised patients(Reference Barnett, Hamer and Meydani192,Reference Bhat, Rather and Dhobi196,Reference Meydani, Barnett and Dallal197) . Enhanced infection and virulence of Streptococcus pneumoniae in Zn-deficient mice were reported. In addition to disrupted epithelial barriers and inadequate immune response, the enhanced virulence was explained by the sensitivity of S. pneumonia to Zn intoxication, reduced during Zn deficiency(Reference Eijkelkamp, Morey and Neville189). Direct effects of Zn deficiency on viral replication have not been addressed to date. Finally, the correlation between Zn deficiency and infection severity may be due to reverse causality, that is, the negative effects that inflammation has on serum Zn concentration. We thus suggest that when the serum Zn levels fall below a certain threshold, the inflammatory response will be self-perpetuating. Again, most tissue damage and detrimental consequences can be expected for patients with pre-existing severe Zn deficiency, but in view of the manifold effects of already mild deficiency, normalising the Zn status offers an easy and cost-efficient approach to reduce disease symptoms.
The hypothesis that Zn deficiency is a risk factor for severe COVID-19 progression and the development of pneumonia and ARDS is supported by successful supplementation studies using Zn to prevent or attenuate respiratory diseases, as we summarised previously(Reference Wessels, Rolles and Rink6). Moreover, first data indicating the congruency of low-Zn status of COVID-19 patients as well as the inverse correlation between serum Zn levels and COVID-19 severity were recently published(Reference Heller, Sun and Hackler10,Reference Vogel-González, Talló-Parra and Herrera-Fernández198) . However, the low serum Zn levels might, once more, be the result of the severe inflammatory response elicited by the virus(Reference McDonald, Suchdev and Krebs18). Clear data on possible pre-existing serum Zn defiicencies are still lacking.
Disrupted epithelial barrier integrity during zinc deficiency: opening the way for Severe Acute Respiratory Syndrome-Coronavirus-2 and co-infections
Evidence is accumulating that, in addition to attacking the lungs and the respiratory tract, SARS-CoV-2 frequently damages other organs (heart, vessels, nerves/brain, kidneys and skin). Disruption of tissue barriers is an integral part of the pathophysiology of infectious diseases, as it facilitates distribution of the pathogen within the body(Reference Carrillo, Rodríguez and Coronado151). The effects of Zn deficiency, described above regarding the lung endothelial barrier, were similarly described for other endothelial layers, including those of kidney, liver, intestine and brain.
It should also be mentioned that the expression of ACE2, lately also called ‘SARS-CoV-2 receptor’, is not limited to the lungs, that is, the goblet and ciliated epithelial cells of the upper airways, alveolar (Type II) epithelial cells and cells of the pulmonary vasculature. ACE2 is also expressed on migratory angiogenic cells, and vascular smooth muscle cells, cardiofibroblasts, cardiomyocytes, pericytes and epicardial adipose cells of the heart; glomerular endothelial cells, podocytes and proximal tubule epithelial cells of the kidneys; cholangiocytes and hepatocytes of the liver; pigmented epithelial cells, rod and cone photoreceptor cells and Müller glial cells of the retina; enterocytes of the intestines and on cells from circumventricular organs of the central nervous system. Binding of SARS-CoV-2 was claimed to result in the loss of ACE2 from the cell surface due to receptor endocytosis and proteolytic cleavage(Reference Gheblawi, Wang and Viveiros199). On the other hand, ADAM17-mediated ACE2 shedding facilitates SARS-CoV-1 entry and induces tissue damage by TNF-α production, which remains to be shown for SARS-CoV-2(Reference Haga, Yamamoto and Nakai-Murakami200). However, disturbed ACE2 expression levels on the cell surface and increased viral entry result from both scenarios. Amongst the normal physiological functions of the ACE2 system are protection against heart failure, myocardial infarction and hypertension. This can explain heart-related COVID-19 complications. Furthermore, defects in the ACE2 system were associated with lung disease, diabetes mellitus and gut dysfunction(Reference Gheblawi, Wang and Viveiros199). ACE2 is a Zn-metalloenzyme, and its normal function is therefore Zn-dependent. Thus, a likely explanation for the association of pre-existing Zn deficiency with COVID-19 complications is the decreased ACE2 activity reported for animals fed on a low-Zn diet(Reference Reeves and O’Dell201,Reference Apgar and Everett202) . ACE activity was even suggested as a biomarker for moderate Zn deficiency in patients with idiopathic taste impairment(Reference Takeda, Takaoka and Ueda203). A Zn deficiency-related, mildly restricted ACE2 activity might not result in clinical symptoms. However, if ACE2 activity is further impaired by the virus, it might fall below a certain threshold and cause vascular complications, heart problems, gut disturbances and so on. Conversely, one study reported increased ACE2 activity in the lung tissue of Zn-deficient rats(Reference Reeves and O’Dell204). Thus, further clarification is needed before conclusions can be drawn regarding the relation to COVID-19. As Zn is a structural element of ACE2, the receptor’s conformation and subsequent affinity for the virus might be altered in patients with pre-existing Zn deficiency, which remains to be tested(Reference Reeves and O’Dell201). Furthermore, Zn deficiency might impair ACE2 expression, as was reported for other Zn-containing metalloenzymes(Reference Cao, Duan and Zhang182). Zn supplementation led to decreased Sirtuin-1 activity as found in cell culture. Interestingly, Zn removal from the closely related Plasmodium falciparum Sirtuin-2 deacetylase, resulted in structural collapse and malfunction of the enzyme. Since Sirtuin-1 is involved in regulating ACE2 transcription, this might result in disturbed ACE2 expression in patients with a Zn imbalance(Reference Rosenkranz, Metz and Maywald100,Reference Min, Landry and Sternglanz205,Reference Chakrabarty and Balaram206) .
In summary, pre-existing Zn deficiency might alter ACE2 expression, structure and/or activity in a tissue-specific manner, which could affect viral entry and pre-dispose to virus-induced tissue damage, but more and detailed studies are necessary to verify those speculation.
Acute kidney injury is another complication that can cause high mortality in COVID-19 patients(Reference Chen, Shao and Hsu207). The total incidence of acute kidney injury in COVID-19 patients is estimated to be about 4–9 %, while in a retrospective study, it was demonstrated that the percentage of patients with complications can reach 37–78 %(Reference Chen, Shao and Hsu207,Reference Argenziano, Bruce and Slater208) . In addition to increased epithelial barrier permeability and the infection with the virus via ACE2, Zn deficiency was associated with renal insufficiency(Reference Bao, Prasad and Beck85). Although described for rats, severe Zn deficiency that was observed in parallel decreased the glomerular filtration rates and renal blood flow, while renal vascular resistance increased(Reference Kurihara, Yanagisawa and Sato209). The resulting renal insufficiency might be a pre-requisite for acute kidney injury and kidney failure during COVID-19. This hypothesis is supported by the finding that the role of Zn in renal function seems to be more crucial in diseased animals than in healthy ones. Tubulointerstitial nephropathy and glomerular haemodynamics were, for example, aggravated in rats with pre-existing Zn deficiency that were suffering from unilateral ureteral obstruction. Zn deficiency further increased the disease-related high expression of endothelin-1 in the glomeruli of the obstructed kidneys(Reference Yanagisawa, Moridaira and Wada210,Reference Yanagisawa, Nodera and Wada211) . Since during kidney diseases and dialysis, Zn loss is increased, Zn deficiency is self-perpetuating and a vicious circle develops causing more severe disease(Reference Cabral, Diniz and Arruda212).
Diarrhoea was reported as a consequence of COVID-19 in a high number of cases(Reference Guan, Ni and Hu77). The association of Zn deficiency with intestinal alterations and a leaky gut are well described in clinical investigations and Zn supplementation studies, and there are excellent reviews focusing on the underlying mechanism(Reference Skrovanek213–Reference Maares and Haase215).
Infection routes of COVID-19 may not include the intestinal tract. However, the leaky gut increases the risk of secondary infections, and intestinal morbidities as commensals are able to enter the human body(Reference Maguire and Maguire216,Reference Anders, Andersen and Stecher217) , especially if the immune system is otherwise occupied by the response to SARS-CoV-2. During renal diseases, nutrients not only Zn but also other elements important for an effective immune response can be lost from the body together with fluids. Consequently, dehydration and deficiency of various minerals can be expected(Reference Skrovanek213,Reference Sturniolo, D’Inca and Parisi218) .
Finally, it is not without reason that Zn supplementation, especially of children in developing countries, is recommended by the WHO to prevent and treat diarrhoea, underlining the relevance of Zn for preserving a healthy gut, as a basic step towards improving the overall health status of individuals(219).
Pre-existing Zn deficiency decreases wound healing and tissue regeneration
Long-term consequences of COVID-19 including the damage to multiple organs are becoming more and more apparent. This is of course partly due to the severe damage caused by the virus but also due to slow and inefficient recovery and healing. Again, there are striking parallels between COVID-19 symptoms and impaired healing observed in Zn deficiency(Reference Kogan, Sood and Garnick220,Reference Khorasani, Hosseinimehr and Kaghazi221) , as found during ex vivo investigation of differentiated human airway epithelium and described by several research groups(Reference Little, Bhattacharya and Moreyra150,Reference Roscioli, Jersmann and Lester154) .
In Zn-deficient rats, intestinal cell proliferation and the quality of intestinal wound healing after intestinal surgery were decreased compared with Zn-adequate controls. This was explained by higher expression of matrix metalloproteinases 2, 9 and 13 and decreased expression of Ki67 (proliferation marker). In addition, the collagen type I:III ratio was reduced in the Zn-deficient animals(Reference Binnebösel, Grommes and Koenen222). Whereas collagen type III dominates the early phase of wound healing, collagen type I rather represents late phase wound healing.
When the influx of Zn into the liver after partial hepatectomy was inhibited in a knock-out mutant of the Zn importer Zip14 in mice, proliferation of hepatocytes was significantly decreased(Reference Aydemir, Sitren and Cousins223). Pre-existing Zn deficiency had similar effects regarding regeneration of heart and lungs. Moreover, Zn supplementation improved the recovery from ischaemia as for example shown in rats where Zn was added during re-perfusion or to the diet(Reference Roscioli, Jersmann and Lester154,Reference Karagulova, Yue and Moreyra224,Reference Turan and Tuncay225) .
A Zn-adequate nutrition may thus also be relevant during recovery from COVID-19.
Zinc deficiency as pre-requisite to virus-induced neuronal damage and loss in smell and taste
In healthy and Zn-adequate individuals, the brain is usually separated from most of the immune cells by the blood–brain barrier. If the blood–brain barrier is damaged, for example, due to high levels of matrix metalloproteinase-9 or other matrix-degrading factors, the brain can easily be infiltrated by immune cells as well as by pathogens, causing neuronal damage(Reference Choi, Jung and Suh226). Thus, entry of a virus into the brain and subsequent damage of the neuronal system culminating in disturbances of their sensory function might be expected during severe Zn deficiency.
Neurological complications of COVID-19 include meningitis and encephalitis, followed by delirium and coma, acute disseminated encephalomyelitis, myelitis, Guillain-Barré syndrome and cerebrovascular complications (stroke, transient ischaemic attack, central nervous system vasculitis)(Reference Ellul, Benjamin and Singh227). However, in comparison with patients with respiratory complications, the proportion of patients with neurological manifestations of COVID-19 might be rather small. Since a high percentage of the world’s population is likely to be infected with the virus, the total number of patients with neuronal complication might be expected to be high. Moreover, lifelong disabilities can result from encephalitis and stroke. Psychosis and paralysis are also discussed as COVID-19-related(Reference Ellul, Benjamin and Singh227,Reference Paterson, Brown and Benjamin228) . Subsequent health, social, care and economic costs to society will be high(Reference Ellul, Benjamin and Singh227,Reference Paterson, Brown and Benjamin228) . Although the exact mechanisms underlying the neurological disturbances in COVID-19 patients are so far not clearly defined, a combination of direct viral invasion with secondary effects of the over-responding immune system is likely.
Serum Zn deficiency has been related to neuronal conditions such as autism, depression, psychosis, Alzheimer’s disease, stroke and schizophrenia. Disturbed neurogenesis and elevated apoptosis of neuronal cells, which can result in defects in learning and memory, were described during Zn deficiency, as was shown in animals fed on a Zn-deficient diet. Retrospective studies on stroke patients also suggest a clinical significance for serum Zn deficiency(Reference Andrews229–Reference Pochwat, Domin and Rafało-Ulińska235). The increase in neuronal apoptosis might involve mitochondrial p53 as well as p53-dependent caspase-mediated mechanisms as shown in vitro (Reference Seth, Corniola and Gower-Winter236). Moreover, a deficiency in synaptic Zn, achieved by Zn chelation, elevated the susceptibility to epileptic seizures in rodents(Reference Cole, Robbins and Wenzel237,Reference Blasco-Ibáñez, Poza-Aznar and Crespo238) . Also, Zn deficiency reduces the amount of Zn available for signal transmission and processing of information, considering that Zn functions as a neurotransmitter, as reviewed in detail elsewhere(Reference Weiss, Sensi and Koh239). Zn is usually packaged into synaptic vesicles of a large sub-population of excitatory neurons for the purpose of neurotransmission. In addition, Zn functions as an important neuromodulator in the olfactory bulbs in rodents(Reference Horning and Trombley240,Reference Sekler, Moran and Hershfinkel241) . Restricting the release of Zn by knocking out the Zn exporter ZnT3 inhibited cell proliferation and neuronal differentiation in the adult hippocampus in mice(Reference Choi, Hong and Jeong242). Surprisingly, Zn in the brain remains unaltered or might even be elevated and involved in Alzheimer-related plaque formation in Zn-deficient animals and humans(Reference Chowanadisai, Kelleher and Lönnerdal243–Reference Datki, Galik-Olah and Janosi-Mozes245). Thus, the relevance of direct effects of Zn deficiency to explain neuronal damage and defects in brain function awaits further data to assist verification. However, ROS, reactive nitrogen species and matrix metalloproteinase-9, which can cross the blood–brain barrier, are elevated during Zn deficiency and affect blood–brain barrier integrity, thus explaining the neuronal damage that has been found in vitro and in vivo (Reference Kumar246,Reference Gilgun-Sherki, Melamed and Offen247) .
Although not in itself life threatening, descriptions of disturbed sense of smell or taste, or both, in COVID-19 patients have accumulated(Reference Beltran-Corbellini, Chico-Garcia and Martinez-Poles248–Reference Russell, Moss and Rigg251). An association between Zn deficiency and the (partial) loss of smell, taste or both has been described in several studies(Reference Russell, Cox and Solomons252,Reference Lyckholm, Heddinger and Parker253) . However, underlying mechanisms are so far not clear. Thus, a connection between Zn deficiency and the disturbances in taste and smell in COVID-19 patients must be carefully analysed in future studies. Extrapolating from the literature, however, still suggests some logical associations.
The elderly: a risk group not only for Zn deficiency
The above-discussed consequences of Zn deficiency are relevant for all age groups. However, in a large number of subjects older than 65 years, co-morbidities may exist. Thus, the association of the age-related decline of serum Zn with the high susceptibility of the elderly for severe COVID-19 is hard to estimate. Instead, we would like to point out that although this article’s focus is Zn, a deficiency in other nutritional elements could also worsen COVID-19 prognosis(Reference Bencivenga, Rengo and Varricchi3,Reference Calder, Carr and Gombart254,Reference Handu, Moloney and Rozga255) . Especially, the elderly suffer not only from Zn deficiency but often from inadequate nutrition. Thus, their nutritional status should generally be checked regularly. It was estimated that the prevalence of inappropriate nutrition risk in Europe is 8·5 % in the community setting, 17·5 % in residential care and 28 % in hospitalisation for individuals ≥65 years(Reference Leij-Halfwerk, Verwijs and van Houdt256). The evidence of the relationship between inappropriate nutrition, immunosenescence and the higher morbidity and mortality from COVID-19 in elderly patients was recently discussed(Reference Bencivenga, Rengo and Varricchi3,Reference Calder, Carr and Gombart254) . Those articles may be consulted in regard to options especially for supporting the aged population in addition to Zn supplementation. The articles provide an elaboration on the impact of malnutrition on the immune system specifically of older subjects including cell-mediated immunity, cytokine production and phagocytic function(Reference Bencivenga, Rengo and Varricchi3,Reference Wessels, Rolles and Rink6,Reference Skalny, Rink and Ajsuvakova7,Reference Calder, Carr and Gombart254) .
However, we believe that Zn supplementation of groups at risk of Zn deficiency and especially in case of the elderly can significantly reduce the severity of infectious diseases such as COVID-19, especially when combined with a generally optimised and nutritious diet, and physical exercise(Reference Wessels, Rolles and Rink6,Reference Skalny, Rink and Ajsuvakova7,Reference Calder, Carr and Gombart254) .
Next step: clinical trials
Based on the available literature, this article suggests a multitude of mechanisms as to how pre-existing Zn deficiency poses a risk of higher susceptibility to SARS-CoV-2 infections and a more severe progression of disease. To test this hypothesis, clinical studies are necessary and some are already registered(257) (online Supplementary Table S1). Moreover, first clinical data support the hypothesis that serum Zn levels are decreased in COVID-19 patients and that disease severity and mortality might be inversely correlated with serum Zn concentration(Reference Heller, Sun and Hackler10,Reference Jothimani, Kailasam and Danielraj258) . A decreased serum Zn level might perhaps be expected in COVID-19 patients due to the strong inflammatory response. Indeed, in serum samples from thirty-five patients with COVID-19, Zn levels were below those from healthy controls(Reference Heller, Sun and Hackler10). Furthermore, in a study of pregnant women, COVID-19 was associated with lower serum Zn levels and serum Zn was negatively correlated with inflammatory markers(Reference Anuk, Polat and Akdas259). Thus, subjects starting out with an inherent Zn deficiency might be expected to be less well prepared for a COVID-19-induced decrease in serum Zn. In this regard, serum samples from non-survivors of COVID-19, taken at various time points, showed that the majority were below the threshold categorised as Zn-deficient. This was also noted for half of the surviving patients. The same study also found a Se deficiency in the majority of patients. The levels of Selenoprotein P and Zn in relation to the age of the subject were identified as reliable prognostic indicators for COVID-19 survival(Reference Heller, Sun and Hackler10). Analysis and correction of Se and Zn status were recommended. Another study also found that a significant number of COVID-19 patients were Zn deficient. Here, Zn-deficient patients revealed more complications, a prolonged hospital stay and higher mortality(Reference Jothimani, Kailasam and Danielraj258). Low Zn levels in COVID-19 patients at clinical admission were associated with poor disease outcomes(Reference Vogel-González, Talló-Parra and Herrera-Fernández198). Finally, in Sakai City Medical Center (Osaka, Japan), most severely ill patients with COVID-19 showed Zn deficiency. Regarding those patients, critical illness could be predicted by serum Zn values. The authors thus suggest serum Zn levels as a predictive factor for a critical illness of COVID-19(Reference Yasui, Yasui and Suzuki260). Additional studies on correlating serum Zn levels with disease severity are ongoing(261).
The data we present strongly suggest that individuals with severe pre-existing Zn deficiency should be included in potential risk groups for COVID-19. We also suggest that prophylactic Zn supplementation, addressing mild pre-existing Zn deficiency, would be more promising than using Zn for the treatment of active disease. In several registered studies, Zn supplementation of groups with high risk of close contact with SARS-CoV-2, including medical or military personnel, is being investigated. Finally, the use of Zn supplementation alone or in combination with other treatment strategies is being tested in clinical studies. First data on the benefits of Zn supplementation as monitored by improved disease status in four confirmed cases of COVID-19 which were supplemented with up to 200 mg of elemental Zn per d were recently published(Reference Finzi262). However, only a minimal effect of Zn on the survival of Zn treated (100 mg elemental Zn per day) v. untreated COVID-19 patients was found by others(Reference Yao, Paguio and Dee263). Supplementation studies using Zn together with the ionophore chloroquine have so far produced contradictory results(Reference Frontera, Rahimian and Yaghi264–Reference Carlucci, Ahuja and Petrilli268).
Combining the Zn-related data from descriptive, preventive and treatment studies will be necessary to increase our knowledge of the importance of Zn homoeostasis during COVID-19 infections and for developing optimal Zn-based supplementation strategies.
Conclusion
Zn is not without reason called an ‘essential’ trace element. Although its single actions on the various cells of the human body might be small and the symptoms of mild to moderate Zn deficiency are rather subtle, the pre-existing lack of Zn in combination with a pathogen such as SARS-CoV-2 can be detrimental and life threatening. Unfortunately, the current data for COVID-19 patients do not allow to distinguish, whether the low serum Zn levels repeatedly found are elicited by virus-induced inflammation, or are reflecting a pre-existing Zn deficiency which cause a more severe disease. Irrespective of this ambiguity, it is quite obvious that groups at risk of Zn deficiency may also be at risk of severe progression of COVID-19, in which the literature on the effects of Zn deficiency, summarised in this article, emphasises. Still, this hypothesis needs to be tested experimentally in clinical studies, some of which are currently in progress. At present, the hypothesis is only supported by data derived largely from animal and cell culture models of Zn deficiency.
This article underlines the various ways as to how a vicious circle of pre-existing, low-grade Zn deficiency and mild pathogen-induced symptoms, followed by increased loss of Zn from the body and the switch to more severe symptoms and serious complications, can be generated. Especially since Zn and its deficiency can have a wide variety of individually very different effects, the consequences of pre-existing Zn deficiency in combination with a pathogen like SARS-CoV-2 that causes so many different symptoms and complications by itself are almost impossible to predict. However, as a conclusion, it can be assumed that Zn deficiency represents a risk for severe progression of SARS-CoV-2-induced disease and a high mortality therefrom. As Zn supplementation is cost-effective and can be regarded as safe, it is highly recommended to supplement individuals who are at risk of Zn deficiency. Finally, we would like to add that more attention should be paid to monitoring nutritional status, since minerals and trace elements are inevitably associated with an efficient immune response. Collaborations between the wide range of clinical and research expertise from the nutritional field along with those involved in intensive care treatment, forming a COVID-19 Nutrition Network is desirable.
Acknowledgements
We thank Wenlei Liu for great support in designing the Figures and the Table. L. Rink is a member of Zinc-Net.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
I. W., B. R. and L. R. drafted the original manuscript and figures. A. J. S. edited language and style and contributed substantially to the revised manuscript. All authors supported writing the manuscript and designing figures and table. The manuscript, figures and the table were critically proofread by all authors.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114521000738