Whole grains (WG) consist of the bran, the starchy endosperm and the germ of the grain. Whole-grain (WG) products are rich in dietary fibre, certain vitamins, minerals and phytochemicals, whereas refined grains have much lower contents of micronutrients and dietary fibre(Reference Slavin, Jacobs and Marquart1).
Prospective studies have found that intakes of WG products are associated with a lower risk of developing certain types of chronic diseases including type 2 diabetes(Reference de Munter, Hu and Spiegelman2), CVD(Reference Jacobs, Meyer and Kushi3, Reference Jensen, Koh-Banerjee and Hu4), obesity(Reference Newby, Maras and Bakun5, Reference Liu, Willett and Manson6) and some types of cancer(Reference Kasum, Nicodemus and Harnack7–Reference Chatenoud, Tavani and La10).
In several studies, WG consumers have been characterised as having healthier dietary habits as well as a healthier lifestyle in general, including a higher socio-economic status(Reference Jacobs, Meyer and Kushi3, Reference Egeberg, Frederiksen and Olsen11–Reference Thane, Jones and Stephen14). Owing to the suggested healthier lifestyles of WG consumers, the results of studies on WG and disease prevention might be confounded by other healthy dietary habits and lifestyle factors. Studying the possible health benefits of WG products requires better knowledge on the associations with other factors. Much of the research on the health effects of WG has been conducted in the USA and UK, where the average intake is much less compared with the intake in Scandinavia(Reference Cleveland, Moshfegh and Albertson12). In the Scandinavian countries, WG is part of the traditional diet and therefore the population consumes much more WG(Reference Ross, Becker and Chen15), with a large qualitative and quantitative variability in the WG consumption across Scandinavia. According to food disappearance data, the consumption of WG products in, for instance, Norway is four times the reported intake for the USA(Reference Jacobs, Meyer and Solvoll16). Consequently, the Scandinavian countries are a good setting for research on WG.
In nutritional epidemiology, most studies addressing associations of the intake of WG with other dietary and lifestyle factors assess WG intake using an FFQ, with WG typically being assessed in intakes of ‘WG products’ (grams of WG product) rather than in ‘absolute amounts’ (grams of calculated WG). Most FFQ do not always give a detailed picture of the types of WG products consumed, because they are usually not developed with the specific aim of assessing WG products. Studies with a more detailed and precise description of WG intake are therefore lacking. The open-ended dietary method 24 h dietary recalls (24HDR) provide more information on the types of food consumed(Reference Slimani, Ferrari and Ocke17) and therefore serve as a better basis for a detailed description of the intakes of WG products.
The objective of the present study was to identify factors associated with the intake of WG in Norway, Sweden and Denmark, with the aim of identifying potential confounders of importance in studies on the health effects of WG. It is hypothesised that the intake of WG is associated with other healthy factors.
Methods
Study population and design
The HELGA cohort is a Scandinavian cohort consisting of three prospective cohorts combined: the Norwegian Women and Cancer Study (NOWAC); the Northern Sweden Health and Disease Study (NSHDS); and the Danish Diet, Cancer and Health Study (DCH). All three cohorts are part of the European Prospective Investigation into Cancer and Nutrition (EPIC). Participants were recruited from the general population, but NOWAC only included women. At baseline (1992–1998), the participants filled in a questionnaire regarding their habitual food intakes and lifestyle. The three cohorts are described in detail elsewhere(Reference Lund, Dumeaux and Braaten18–Reference Tjonneland, Olsen and Boll21).
In 1995–2000, a calibration study was completed on a random sample from each of the cohorts as part of the EPIC study(Reference Slimani, Kaaks and Ferrari22). The present study is based on the calibration subsample: 8716 of the 120 017 participants of three cohorts combined. The subsample was randomly selected and weighted according to the cumulative number of expected cancer cases over 10 years of follow-up per gender and 5-year age stratum. A single 24HDR interview questionnaire was created using a standardised software program, EPIC SOFT, that served as a common structure and interview interface for the 24HDR interviews. Although EPIC SOFT has common standard features to describe, quantify and probe the reported food similarly across countries, food and recipe lists are country-specific to ensure that local and traditional foods and dishes are included(Reference Slimani, Ferrari and Ocke17).
Method
In Sweden, the 24HDR interviews were completed face-to-face at the centre. In Denmark, the interviews were essentially completed at the centre. In Norway, telephonic interviews were used(Reference Brustad, Skeie and Braaten23). In Sweden and Denmark the interviews were completed in 1995–1998, whereas in Norway they were completed in 1999–2000(Reference Slimani, Kaaks and Ferrari22). Participants were recruited for the 24HDR study ‘by surprise’ when possible. The participants in Norway and Sweden were recruited by letter and the Danish participants were recruited at the centre(Reference Slimani, Kaaks and Ferrari22). The participants were asked to recall all food items consumed during the last 24 h. First, the participant was asked to remember in generic terms all food items consumed within the last 24 h. Second, the participant was asked to describe and quantify the foods in detail. The reason for this two-phased interview is that it minimises recall errors(Reference Slimani, Ferrari and Ocke17).
Source of data
All dietary variables including all WG variables were from the 24HDR collection of data, whereas lifestyle factors were from the baseline collection of data. The variables from baseline include: BMI, age, alcohol, alcohol from beer, alcohol from wine, highest school level completed and smoking status. A comparison of WG intake from the different data sources was made using the calculated intakes from FFQ and 24HDR, respectively.
Dietary information
The definition of WG in the present study is in accordance with the definition given by the American Association of Cereal Chemists: ‘Whole grains shall consist of the intact, ground, cracked or flaked caryopsis, whose principal anatomical components – the starchy endosperm, germ and bran – are present in the same relative proportions as they exist in the intact caryopsis’(24). Grain types included in the definition used in the present study consist of the following (all part of the Poaceae family): wheat, rye, oats, barley, rice, millet, corn/maize (dried), triticale and sorghum/durra. All products of the following categories were included as WG products (no limit was set on the minimum content as long as they contained WG): breakfast cereals, WG bread, WG crisp bread, WG rice, WG flour and starch, other grains (100 % cereal), salty biscuits, aperitif biscuits and crackers, cream dessert and puddings (milk based), cakes, sweet pies, pastries and puddings (non-milk based), dry cakes and biscuits.
The information on WG intake collected using the 24HDR was very detailed because a set of descriptors was used to describe the food products during the interview(Reference Slimani, Ferrari and Ocke17). The descriptors varied by food group, but could include information on whether the product was homemade or commercial, as well as information on its brand name, physical state, type of fat used, processing and cooking method(s). In order to quantify the intake of WG in grams, it was necessary to obtain information on the content of the WG products registered in the 24HDR. Different approaches were used for doing this in different countries. The purchase and market data on the contents of WG from a Danish report on WG were used to calculate the contents of WG from the 24HDR in Denmark(25). In Norway and Sweden it was necessary to retrieve the data. This was done by contacting manufacturers, using recipe books and analysing information on the wrapping of WG products. The WG contents were expressed as the WG contents of the unprepared ingredients divided by the weight of the prepared product. In the calculations, it was necessary to aggregate WG products because of lack of information on their contents. This was done according to the methods used in other databases used for nutritional assessment such as the EPIC Nutrient Database(Reference Slimani, Deharveng and Unwin26).
Lifestyle factors, anthropometric measurements and socio-economic factors
In the baseline questionnaires participants provided information on socio-economic status, anthropometry and certain lifestyle factors. Weight and height measurements were recorded at the study centres in Sweden and Denmark, whereas they were self-reported in Norway. Information on physical activity differed among the countries, and was therefore not comparable. All three countries had information on current smoking status (current, past and never) and the highest school level completed (primary school completed or lower, technical/professional school, secondary school, higher education).
Exclusion criteria
Of the 120 016 participants included in the HELGA cohort, 24HDR data were available for 8716. Of the 8716 participants, fourteen were excluded because of missing values on highest school level completed or missing value on alcohol intake. Thus, 8702 participants were included in the analysis (1797 Norwegian women, 1617 Swedish women, 1372 Swedish men, 1994 Danish women and 1922 Danish men).
Statistics
For baseline data on lifestyle, socio-economic and dietary characteristics, the continuous variables are all presented as median intakes with their corresponding percentiles (5th and 95th) and categorical variables are presented as percentages.
Multivariable linear regression analysis was used to explore the relationship between a set of independent variables and WG intake as the dependent variable. The dependent variable was tested for normal distribution and was normalised by log-transformation because of skewness of the data distribution. The intake of WG used as the dependent variable was the calculated total amount of WG per day. Results are presented as estimates (Δ) reported as the percentage change in WG intake per defined unit of change in the independent variable with corresponding 95 % CI and P values. The values are back-transformed from log values. The independent variables investigated in the model included: age at baseline, BMI, alcohol, alcohol from beer, alcohol from wine, highest school level (primary school completed or lower, technical/professional school, secondary school, higher education), smoking status (never smoker, former smoker, smoker, unknown), potatoes, vegetables, fruits, nuts (spread) and seeds, milk, yoghurt, cheese, white bread, red meat, poultry, processed meat, fish and shellfish, eggs, sugar and confectionery, cakes and biscuits, coffee, tea, vegetable oils, butter and margarine. The dependent variable was the intake of WG in ‘absolute amounts’ (g WG/d) calculated by assessing the total WG content of products of the following food categories: breakfast cereals, WG bread, WG crisp bread, WG rice, WG flour and starch, other grains (100 % cereal), salty biscuits, aperitif biscuits and crackers, cream desserts and puddings (milk-based), cakes, sweet pies, pastries and puddings (non-milk based), dry cakes and biscuits.
All continuous independent variables were tested for linearity using linear splines(Reference Greenland27). The assumption of linearity was made by visual inspection of the plots and F tests. No significant departures from linearity were found.
All models were weighted for sampling differences on weekdays (Monday–Friday) compared with weekends (Saturday and Sunday) and for seasons (summer, autumn, winter and spring). Two models are presented, one adjusted for age and country only and one additionally including all variables in a mutual adjustment. Tests for interactions between the three countries have been conducted to identify differences among the associations found for the countries.
The SAS statistical software package version 9·1 (SAS Institute, Cary, NC, USA) on a Windows platform was used for all statistical analyses. The GLM procedure was used for the multiple linear regression analyses and the tests for interactions and the UNIVARIATE and FREQ procedures were used for the descriptive analyses.
Results
Baseline characteristics
Table 1 shows the lifestyle, socio-economic and dietary baseline characteristics of the participants, including the WG intakes in ‘absolute amounts’ (g WG/d). The median age at recruitment was 52 years for women and 57 years for men. The median BMI was 24 and 26 kg/m2 for women and men, respectively. The median intakes of WG in ‘absolute amounts’ were 36 g/d for women and 43 g/d for men. In all, 6–7 % of non-users of WG were identified for men and women from the 24HDR, whereas no non-users were defined in the FFQ (data not shown). The validity of the 24HDR v. FFQ is beyond the scope of the present study, but a few initial calculations have been performed, which showed that 68 % of the study population had an intake of WG in the same (±1) quintile when assessed by 24HDR and FFQ (data not shown).
Table 1 Lifestyle, socio-economic and dietary baseline characteristics of participants included in the 24HDR of the HELGA cohort

24HDR, 24 h dietary recall; P5–P95, 5th–95th percentile; WG, whole grains.
Factors associated with the intake of whole grains (g/d)
Table 2 (women) and Table 3 (men) show the regression estimates (Δ) indicating the percentage change in WG intake per defined unit of change in dietary, lifestyle or socio-economic variable with the corresponding 95 % CI and P values.
Table 2 Percentage change in grams of WG intake (Δ) and 95 % CI by dietary, lifestyle and socio-economic variables among 5408 women included in the 24HDR of the HELGA cohort
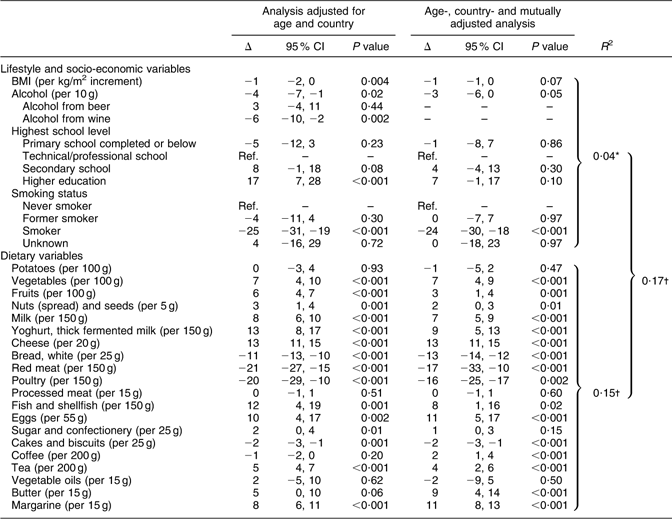
WG, whole grains; 24HDR, 24 h dietary recall; Ref., reference (Δ = 0).
Δ, Estimates reported as percentage change in WG intake per defined unit of change in lifestyle/socio-economic/dietary variable. All models are weighted according to recall day and season. The percentage change estimates were derived from regression with log-transformed dependent variable. The results shown are back-transformed from log values.
*Variation in intake of WG explained by socio-economic and lifestyle variables.
†Variation in intake of WG explained by dietary variables.
‡Variation in intake of WG explained by socio-economic, lifestyle and dietary variables.
Table 3 Percentage change in grams of WG intake (Δ) and 95 % CI by dietary, lifestyle and socio-economic variables among 3294 men included in the 24HDR of the HELGA cohort
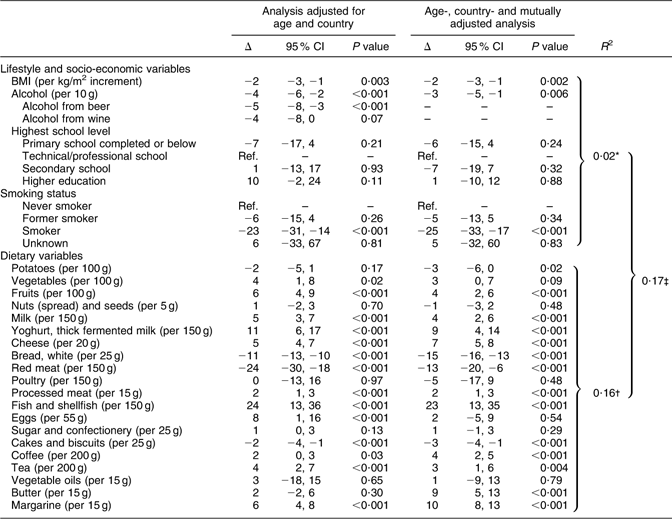
WG, whole grains; 24HDR, 24 h dietary recall; Ref., reference (Δ = 0).
Δ, Estimates reported as percentage change in WG intake per defined unit of change in lifestyle/socio-economic/dietary variable. All models are weighted according to recall day and season. The percentage change estimates were derived from regression with log-transformed dependent variable. The results shown are back-transformed from log values.
*Variation in intake of WG explained by socio-economic and lifestyle variables.
†Variation in intake of WG explained by dietary variables.
‡Variation in intake of WG explained by socio-economic, lifestyle and dietary variables.
For lifestyle and socio-economic factors, being a smoker was inversely associated with WG intake for both men and women. The highest school level completed was only directly associated with WG intake among women, but the association was no longer significant after adjusting the model mutually for other assessed variables. Alcohol was found to be inversely associated with WG intake for both men and women, but alcohol from beer was inversely associated only for men and the same was the case for alcohol from wine for women. BMI was inversely associated with intake of WG for both men and women, meaning that a higher BMI would predict a lower intake of WG. However, in the mutually adjusted model for women, only a tendency of an inverse association between BMI and the intake of WG was found. Tests for interaction (data not shown) by country revealed some differences for women in the lifestyle and socio-economic factors analysed, whereas none were identified for men. Alcohol was inversely associated with intake of WG in Danish women, whereas no significant association was found among Swedish and Norwegian women. Further, alcohol from wine was inversely associated with the intake of WG in Swedish and Danish women.
For dietary variables, the intake of WG was directly associated with intakes of vegetables (not statistically significant in the adjusted model for men), fruits, dairy products (cheese, milk and yoghurt), fish and shellfish, coffee, tea and margarine and inversely associated with intakes of white bread, red meat and cakes and biscuits. In addition, in women, the intake of nuts was further found to be directly associated with the intake of WG and poultry was found to be inversely associated. For men, processed meat was found to be directly associated with WG intake, whereas potatoes were found to be inversely associated (only in the adjusted model). Tests for interaction among the countries also revealed country-dependent differences in the dietary variables (data not shown). For men, potatoes were not significantly associated with WG intake in Swedes, whereas an inverse association was found among Danes. Furthermore, yoghurt was directly associated with the intake of WG in Swedish men, but not among Danish men, and red meat was inversely associated among both Swedish and Danish men, but even stronger so for Swedish men. Finally, processed meat, fish and shellfish, and eggs were associated with WG intakes in Danish men, but not in Swedish men. For women, the test for interaction identified only two dietary variables that significantly differed among the countries. One of them was processed meat, which, even though no statistically significant association was found when stratified by country, was differently associated with the intake of WG. Furthermore, the association found in women for vegetable oil was driven by Danish women only. In addition to the age-adjusted and mutually adjusted model, a model adjusting for energy was also made (data not shown). The energy adjustment did not affect the result.
For women, 15 % of the variation in the WG intake could be explained by dietary variables and 16 % of the variation could be explained for men. Lifestyle and socio-economic variables explained 4 % and 2 % of the variation in WG intake for both women and men, respectively. Altogether, 17 % of the variation in WG intake was explained by dietary, lifestyle and socio-economic variables for both men and women.
Discussion
In the present study, the associations between lifestyle, socio-economic and dietary factors and the intake of WG were investigated among 8702 men and women from three population-based cohorts by assessing their WG intakes from a 24HDR.
The intake of WG was directly associated with other foods generally associated with a healthy lifestyle, including fruits, vegetables, fish and shellfish and tea. Furthermore, red meat and white bread were found to be inversely associated with the intake of WG. For lifestyle and socio-economic variables, BMI and smoking were inversely associated with WG intake, whereas highest school level was directly associated (in the age- and country-adjusted model for women). This suggests that a healthy diet and lifestyle are associated with a higher WG intake. Dietary variables explained most of the variation in the WG intake (15–16 %) compared with lifestyle and socio-economic variables, which explained 2–4 % of the variation.
One of the most consistent and strongest factors inversely associated with WG intake was smoking. This is in accordance with other studies, all of which have found that being a smoker was inversely associated with WG intake(Reference Jacobs, Meyer and Kushi3, Reference Egeberg, Frederiksen and Olsen11, Reference Lang, Thane and Bolton-Smith13, Reference Thane, Jones and Stephen14, Reference Jacobs, Meyer and Solvoll16, Reference Harland and Garton28, Reference Burgess-Champoux, Larson and Neumark-Sztainer29). Alcohol was also inversely associated with the intake of WG, although not alcohol from beer for women. The findings of the present study are congruent with findings by Egeberg et al.(Reference Egeberg, Frederiksen and Olsen11), who also found that alcohol intake was inversely associated with the intake of WG. The work of Egeberg et al.(Reference Egeberg, Frederiksen and Olsen11) was on the same cohort of Danes as included in the present study, but based on WG intake from an FFQ, whereas the present study was based on a 24HDR. Length of education was directly associated with WG intake in one of the two models for women, which is in accordance with findings from a Finnish and Danish study population(Reference Egeberg, Frederiksen and Olsen11, Reference Prattala, Helasoja and Mykkanen30). In the Finnish study, however, it was additionally found that the intake of rye bread, in contrast to the intake of WG, was directly associated with having less education and living in rural areas. This suggests that the consumption patterns and associations might differ according to culture. BMI was found to be inversely associated with the intake of WG, meaning that people with a higher BMI had lower WG intakes. This has also been found previously in studies on Americans and Danes(Reference Jacobs, Meyer and Kushi3, Reference Koh-Banerjee, Franz and Sampson31, Reference Bazzano, Song and Bubes32).
When considering dietary factors, red meat and white bread were most consistently inversely associated with WG intake, whereas dairy products, tea, fruits, vegetables, fish and shellfish, coffee and tea were directly associated, but less strongly compared with red meat and white bread. The findings on red meat and white bread are congruent with findings from the Iowa Women's Health Study in which a higher intake of WG was found to be associated with a lower intake of red meat and a lower intake of refined grains(Reference Jacobs, Meyer and Kushi3). Overall, factors directly associated with the intake of WG identified in the present study are all considered part of a healthy lifestyle, including fruits, vegetables and tea. Furthermore, dairy products were identified as being directly associated with WG intake; this product category can be considered as ‘neutral’ with regard to health benefits(33–Reference Tholstrup35). Processed meat, which can be considered as an unhealthy product category(33), was found to be directly associated with the intake of WG in men, probably because of the use of processed meat products on rye bread particularly in Denmark. This is not in accordance with the other findings of the study, which identified mostly ‘healthy’ food categories as being directly associated with WG intake. The identified factors inversely associated with WG intake were red meat and white bread, which, very roughly put, can be considered part of a ‘less healthy’ lifestyle, of course depending on the specific product and the quantity consumed. In general, the results of the associations of dietary factors with WG intake in the present study indicate that WG intake is associated with having an overall healthy dietary lifestyle. This finding is in accordance with the findings of other studies by Jacobs et al.(Reference Jacobs, Meyer and Solvoll16), a systematic review by Harland et al. (Reference Harland and Garton28) and a Danish report on WG(25), all of which identified a healthy behaviour and diet/healthier lifestyle/better nutritional profile as being directly associated with WG intake. In general, no major differences were identified in the associations of WG intake with other factors between the three countries included in the present study. For potatoes, vegetable oil, margarine and eggs there were different associations with WG intake between the countries, but this does not interfere with the overall conclusion that a healthy dietary lifestyle is directly associated with WG intake in all three countries. This highlights the importance of considering these factors when conducting studies on WG with disease endpoints. Furthermore, the information on the associated factors can be helpful in understanding the disparities in the populations with regard to adapting a healthy lifestyle, and can make efforts to improve the lifestyle of people with an unhealthy lifestyle more targeted.
The strengths of the present study are the population-based design and the very detailed information on WG intake provided by the 24HDR. In addition, the fact that the 24HDR has been conducted using the same standardised program (EPIC SOFT) is a considerable strength of the present study and serves as a good basis for comparing the intake of WG and the factors with which WG intake is associated among the three countries. Several limitations of the present study exist. Only one 24HDR was conducted; more than one 24HDR per person would have contributed information on the variation in intake, which would have given a more precise estimate of WG intake at the individual level. In an initial analysis of the validity of the 24HDR compared with the FFQ it was found that 68 % of participants were classified in the same (±1) quintile in the FFQ and the 24HDR, which is acceptable and in accordance with the findings of other validity studies(Reference Tjonneland, Overvad and Haraldsdottir36). However, it is important to bear in mind that the 24HDR and the FFQ reflect two different aspects. The 24HDR reflects the diet within the last 24 h and the FFQ assesses the habitual diet. This is also shown in the differences between the percentages of non-users of WG when the 24HDR is compared with the FFQ. In the 24HDR, 6–7 % of non-users were identified, whereas none were identified from the FFQ.
Only 2 % (men) to 4 % (women) of the variation in WG intake could be explained by socio-economic and lifestyle variables, whereas 15–16 % could be explained by the dietary variables assessed. This leaves at least 80 % of unexplained variation. It would probably be possible to explain a little more variation in WG intake if information on more variables of relevance had been available for all countries, such as physical activity, but it would probably not change the results much.
All three cohorts suffer from over-representation of people with a high socio-economic status(Reference Tjonneland, Olsen and Boll21, Reference Lund, Kumle and Braaten37, Reference Weinehall, Hallgren and Westman38), and the study therefore presents a more selected group of participants compared with the general population. It is possible that the 24HDR suffers even more from over-representation of people with a high socio-economic status because of an additional selection into the 24HDR substudy. In addition, the fact that three cohorts have been combined into one can be a weakness because of different inclusion criteria and methodological differences. The findings of the present study reflect the general public in Norway, Sweden and Denmark with the exception of children and young people, who are not included in the study. Furthermore, the results do not represent Norwegian men. In Norway the entire country is chosen as the area of invitation, whereas only certain areas of Sweden and Denmark are chosen as areas of invitation; however, it is expected that the chosen areas of invitation in Sweden and Denmark represent both rural and urban areas and therefore the countries as a whole(Reference Tjonneland, Olsen and Boll21, Reference Lund, Kumle and Braaten37, Reference Weinehall, Hallgren and Westman38).
In conclusion, the present study found that the intake of WG is directly associated with healthy lifestyle, socio-economic and especially dietary factors and inversely associated with ‘unhealthy’ factors. This highlights the importance of considering both lifestyle, socio-economic and dietary factors related to WG intake when studying the health effects of WG. Future high-quality prospective studies on different populations are needed to elucidate the disease preventive role of WG, so that residual confounding can be minimised. These studies need to take the mentioned potential confounders into consideration.
Acknowledgements
The present study was supported by Nordforsk (Nordic Centre of Excellence programme HELGA). The authors have no conflict of interest to declare. G.H., E.L., K.O. and A.T. contributed to the design and collection of the HELGA cohort; C.K. was responsible for planning and conducting the statistical analyses with help from J.C., and also for drafting the manuscript under supervision of A.O., L.O.D. and G.S. The classification and calculation of data on whole grains were carried out by I.J., N.S., J.H., N.F.J. and G.S. The present work was performed at the Institute of Cancer Epidemiology, Danish Cancer Society. The authors acknowledge Katja Boll (data manager) and Jytte Fogh Larsen (project coordinator) for assistance with data collection. They also thank The International Agency for Research on Cancer (WHO–IARC) especially Jerome Vignat and Genevieve Nicolas for data checking and preparation.





