CVD is a term that describes a broad range of diseases affecting heart or blood vessels, including coronary artery disease, heart attack, heart failure, high blood pressure and stroke. Atherosclerosis, probably the most common cause of CVD, is a complex disease characterised by a strong inflammatory component involving a chronic dysfunction of both endothelial and smooth muscle cells and the activity of a number of different circulating cell types, such as macrophage and lymphocytes(Reference Libby1). TNF-α is a pro-inflammatory cytokine playing a pivotal role in triggering and maintaining tissue-specific inflammatory response(Reference Hansson, Robertson and Soderberg-Naucler2). The endothelium constitutes an important target for TNF-α action, and the subsequent response of vascular endothelial cells may trigger vascular pathology in different conditions. In fact, TNF-α induces definite effects, such as cell activation, proliferation and apoptosis, by binding to a specific family of receptors (tumor necrosis factor receptor)(Reference Bradley3). The major result of receptor-mediated signalling is the transcriptional activation of specific genes. NF-κB is a key factor in TNF-α associated cell response(Reference Grivennikov, Kuprash and Liu4). Its activation, transfer to the nucleus and binding to a specific sequence in the promoter of different genes involved in inflammation are finely modulated by a wide range of variables, leading to the final control of transcription. Moreover, the majority of genes, such as those encoding for cell adhesion molecules, chemotactic factors and prothrombotic molecules, typically contains multiple binding sequences that recognise different transcription factors. This multiple regulation makes the transcriptional response to TNF-α the result of a complex interplay of a number of factors including activator protein-1 (AP-1) and cAMP response element-binding proteins, a group of related transcription factors including cAMP response element binding, cAMP response element modulator, activating transcription factor-2 and c-jun(Reference Ono, Ichiki and Ohtsubo5, Reference Medcalf6).
Epidemiological investigations have indicated that a light-to-moderate consumption of red wine (RW), corresponding to about a glass of wine per day, is associated with a reduced risk for CVD(Reference Gronbaek, Becker and Johansen7). This beneficial effect has been attributed to either a generic antioxidant activity mainly exerted by its polyphenolic constituents(Reference Aviram and Fuhrman8) or to a more specific anti-inflammatory action(Reference Wannamethee, Lowe and Shaper9) as suggested by the reduction of serum inflammatory biomarkers observed in association with a moderate consumption of RW(Reference Estruch, Sacanella and Badia10, Reference Sacanella, Vazquez-Agell and Mena11). Overall, RW components, and in particular polyphenols and alcohol, have been reported to act as anti-inflammatory agents through the modulation of different signal transduction pathway(Reference Rahman, Biswas and Kirkham12). In particular, one of the most accredited mechanisms proposed on the basis of experiments on cultured cells is the inhibition of two key factors of inflammatory pathway, NF-κB and AP-1, and the correlated decrease or suppression of gene transcription(Reference Murase, Kume and Hase13–Reference Kobuchi, Roy and Sen15). However, most of the in vitro studies dealing with the molecular mechanisms associated with polyphenols have utilised the molecules under investigation in the form present in the ingested food, not considering the profound consequences of gastrointestinal absorption and metabolism. Food metabolites circulating in body fluids are possibly characterised by a different chemical structure and biological activity in comparison with those that were originally present in food. No study is so far available on the effect of a complex food matrix such as RW, which has taken into account the metabolisation process of its components, on inflammatory molecular mechanisms in cultured endothelial cells, in conditions close to physiological environment. According to this consideration, the aim of the present study was to investigate the effect of metabolised polyphenols on NF-κB, AP-1 and cAMP response element-binding proteins pathways. To deal with this issue, we utilised a novel experimental approach based on the culturing of human umbilical vein endothelial cells (HUVEC) in the presence of serum collected from healthy volunteers, after RW supplementation(Reference Canali, Ambra and Stelitano16). This experimental design allows the assessment of the effect of the complex metabolic transformations occurring during digestion and intestinal absorption processes and the possible synergism between the different components of RW. Thus, the effect of serum collected after RW consumption and therefore containing RW metabolites on TNF-α-dependent activation of transcription factors (NF-κB, AP-1 and cAMP response element-binding proteins) and downstream gene expression in cultured HUVEC was examined.
Experimental methods
Experimental model
Five healthy men aged 35–40 years were recruited and asked to drink an acute dose of RW (5 ml RW/kg body weight) in fasting conditions. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of the National Institute for Food and Nutrition Research. Verbal informed consent was obtained from all the subjects. Verbal consent was witnessed and formally recorded. Teroldego Rotaliano wine was selected among others due to its high content in polyphenols(Reference Mattivi, Zulian and Nicolini17). Phenolic compounds content of this wine moiety has been reported previously(Reference Canali, Ambra and Stelitano16). Blood was withdrawn from each subject just before and 40 min after RW supplementation. Serum was isolated by centrifugation at 1800 g for 10 min. The time point for post-drinking blood withdrawal was chosen on the basis of previous studies indicating that this is the average peak time for polyphenols and alcohol absorption(Reference Goldberg, Yan and Soleas18, Reference Abu-Amsha Caccetta, Croft and Beilin19). Fasting control serum (CS) and serum enriched of RW metabolites (RWS) were stored at − 80°C in several aliquots and utilised for different independent experiments. HUVEC were cultured in the absence of bovine serum in culture media supplemented with 20 % (v/v) with CS and RWS. After 16 h of cell incubation with either CS or RWS, 5 ng/ml TNF-α was added to HUVEC medium for different times as indicated in the result section. RWS contained 0·077 (sd 0·01) % (w/v) of alcohol and 5·6 (sd 2·07) μg/ml of total phenolic compounds. Therefore, after appropriate dilution (20 %), endothelial cells were exposed to about 0·015 % (w/v) and 1·09 μg/ml of alcohol and phenolic compounds, respectively. In each independent experiment, cells were either incubated with RWS or with CS as control of the effects of RWS metabolites.
Phenolic compounds and ethanol analysis
Total phenolic compounds in human serum were estimated after deproteinisation by Folin–Ciocalteau method according to Serafini et al. (Reference Serafini, Maiani and Ferro-Luzzi20). Results are expressed as μg of (+)-catechin equivalent per ml. Ethanol level was measured after serum isolation using alcohol dehydrogenase enzymatic assay (Sigma, St Louis, MO, USA).
Endothelial cell culture
HUVEC were obtained from umbilical cords kindly provided by the nursery of ‘Annunziatella’ hospital of Rome, as described previously(Reference Jaffe, Nachman and Becker21). HUVEC were grown on gelatin-coated tissue culture plates in 199 medium (Sigma) containing 20 % bovine serum (Sigma), HEPES (20 mm), heparin (50 U/ml (0·1 mg/ml); Sigma), l-glutamine (1 %; Sigma) and penicillin/streptomycin (1 %; Sigma) under 5 % CO2 at 37°C. Cells were utilised for experiments at a density corresponding to 90–100 % apparent confluence and within passages 3–4. When cells reached the indicated density, bovine serum was substituted with human serum as described above. Passages were performed according to standardised protocols and by diluting the cell population 1:3. Cultures were made from at least three different preparations from different umbilical vein cords pooled together.
Transcription factor nuclear translocation
Nuclear extracts were prepared from confluent HUVEC grown in T25 flasks. Briefly, cells were lysed for 20 min in a hypotonic buffer in ice (10 mm HEPES, pH 7·8, 1 mm MgCl2, 10 mm KCl, 0·1 mm EDTA, 0·5 mm ethylene glycol tetraacetic acid and 5 % glycerol), containing a cocktail of protease inhibitors. Nuclei were treated with 0·625 % Nonidet P-40 for 5 min and pelleted by centrifugation at 20 000 g for 30 s. Nuclear proteins were obtained by incubating with a hypertonic buffer (50 mm HEPES, pH 7·8, 400 mm NaCl, 1 mm MgCl2, 0·1 mm EDTA, 1 mm ethylene glycol tetraacetic acid and 10 % glycerol) also containing a cocktail of protease inhibitors. After treatment, nuclei were centrifuged at 20 000 g for 5 min, and the supernatant retained for use in the DNA-binding assay. Annealed complementary oligonucleotides (10 pmol) were labelled in 1X kinase buffer with 5 U T4 polynucleotide kinase (USB, Cleveland, OH, USA) and 0·3700 MBq (10 μCi) [γ32P]-ATP (Perkin Elmer, Boston, MA, USA) at 37°C for 30 min. After kinase inactivation at 65°C for 5 min, probes were purified using Micro Biospin30 columns (Biorad, Hercules, CA, USA). Binding reactions were performed incubating 5 μg nuclear proteins for 20 min at room temperature with 2 μg poly(deoxyinosinic-deoxycytidylic acid)–poly(deoxyinosinic-deoxycytidylic acid) (Sigma) and 50 000 cpm (Cherenkov counting) of labelled oligonucleotides. DNA–protein complexes were resolved in 6 % polyacrylamide (acrylamide–bisacrylamide, 29:1) gels and then autoradiographed. The resulting films were subjected to densitometric scanning using SCION IMAGE program and normalised against loading control. A 100-fold excess of unlabelled oligonucleotides were added as cold competitors as binding specificity control. In case of supershift, 1 μl of 1 μg/μl c-jun phosphorylated (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was added to the binding reaction mix before adding the labelled probe. Oligonucleotide consensus sequences are (from 5' to 3'): NF-κB, AGT TGA GGG GAC TTT CCC AGG C; AP-1, CGC TTG ATG AGT CAG CCG GAA. Moreover, the specific cAMP responsive element (CRE) consensus was ATT CAA TGA CAT CAC GGC TGT T for tissue plasminogen activator (t-PA) and TTC AGAGTG ACC TCA TCC TCC for plasminogen activator inhibitor-2 (PAI-2) genes(Reference Costa, Shen and Medcalf22).
Real-time PCR
At the end of the incubations, RNA was extracted using TRI®reagent (Sigma) and quantified by spectrophotometry. Gene expression at level of mRNA was assessed by real-time quantitative PCR utilising an ABI Prism® 7900 HT Instrument (Applied Biosystem, Foster City, CA, USA) coupled with the SYBR Green JumpStart™ Taq Ready Mix kit (Sigma).
Fluorescence data were collected and processed by an SDS 2.2 software and expressed as threshold cycle (C t). The C t values for each target and reference genes were obtained and their difference was calculated (ΔC t). Quantitative differences in the cDNA target among samples were determined using the mathematical model of Pfaffl(Reference Pfaffl23). Primer efficiencies for the test genes were comparable with those for the glyceraldehyde-3-phosphate dehydrogenase (considered as reference/housekeeping gene) (Table 1). The last step in quantification was the conversion of C t to absolute values. Results are expressed as fold of increase or decrease as compared with the control.
Table 1 Sequences for primers utilised to assess gene expression by RT-PCR

RT, real time; VCAM, vascular cell adhesion molecule; ICAM, intercellular adhesion molecule; MCP-1, monocytes chemoattractant protein; t-PA, tissue plasminogen activator; PAI-2, plasminogen activator inhibitor-2; G3DPH, glyceraldehyde-3-phosphate dehydrogenase.
U937 adhesion on endothelial cells
U937 cells (a human leukaemic monocyte lymphoma cell line) were labelled with 5 μm fluorescent dye calcein acetoxymethyl ester (Molecular Probes, Eugene, OR, USA) in serum-free Roswell Park Memorial Institute medium for 30 min at 37°C. Then cells were washed twice with Roswell Park Memorial Institute. Adhesion assays were performed in ninety-six-well tissue plates by adding pre-labelled U937 cell suspension (1 × 105 per well) to confluent CS- or RWS-treated (20 % serum for 16 h and 5 ng/ml TNF-α for 4 and 6 h at 37°C) endothelial cells for 60 min at 37°C. Non-adherent cells were removed by washing with Roswell Park Memorial Institute, and 200 μl Triton X-100 (0·1 %) was used to lyse adherent cells. Finally, the fluorescence was measured using a fluorescent plate reader (excitation: 495 nm; emission: 535 nm). Controls included the measurement of total fluorescence of labelled U937 and the control for autofluorescence of unlabelled cells. Data are expressed as percentage control (control being the rate of U937 adhesion on endothelial cells incubated with CS).
Data analysis
RWS and their respective CS were isolated from subjects and independently utilised for at least three separate experiments. Each experiment was repeated in triplicate. Values are presented as means and standard deviations of the fold of changes (or percentage) in comparison with control. Control is HUVEC incubated with CS for 16 h, unless differently specified. Multifactorial ANOVA was used to test the significance between differences taking into account the variability within the experiments and among the treatments. P value < 0·05 was considered the threshold level for significance.
Results
NF-κB and activator protein-1 nuclear translocation modulation by red wine consumption before and after TNF-α stimulation
The presence of RWS in the culture medium induced a progressive activation of both NF-κB and AP-1 nuclear translocation in endothelial cells (Fig. 1). After 16 h incubation with RWS, according to the electromobility shift assay, the amount of NF-κB and AP-1 transferred inside the nucleus was, respectively, 76 and 49 % higher in comparison with the control. NF-κB and AP-1 transfer inside the nucleus was not accompanied by an increase in transactivating activity, as indicated by our previous results that show a substantial absence of important changes in the expression levels of genes bearing NF-κB- and AP-1-binding sequences in their promoter(Reference Canali, Ambra and Stelitano16).
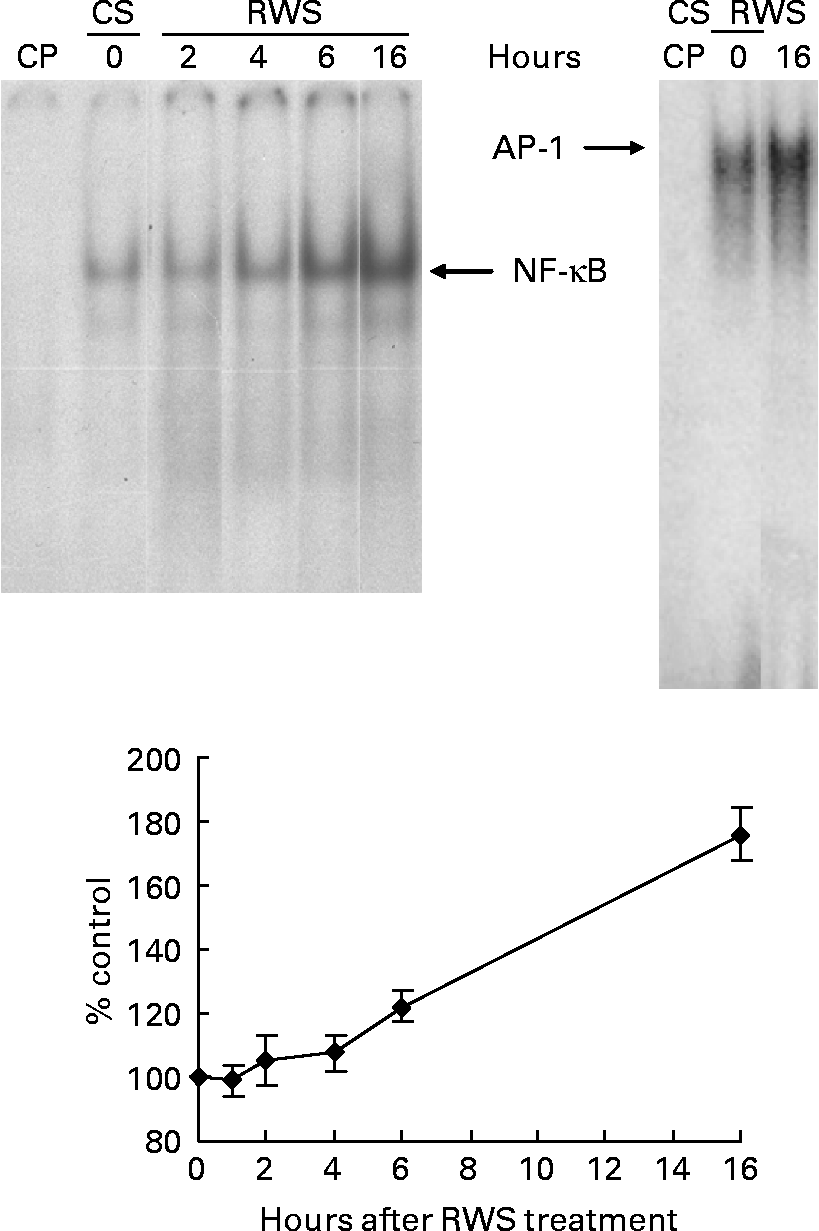
Fig. 1 Human serum obtained after red wine consumption (RWS) incubation activates NF-κB and activator protein-1 (AP-1) nuclear translocation. A representative electromobility shift assay (one out of at least three separate experiments) showing nuclear protein interactions with consensus NF-κB and AP-1 probes is shown. Human umbilical vein endothelial cells were incubated for 2, 4, 6 and 16 h with 20 % of RWS (or control serum (CS)) in 199 medium in the absence of bovine serum. Protein interactions with AP-1 oligonucleotide were carried out only at 16 h of RWS incubation. The films obtained from the experiments were analysed using SCION IMAGE program. Results, from at least three separate experiments, are indicated as means and standard deviations of the relative densitometric intensity and expressed as percentage of CS-treated cells at each time of the analysis. The lane ‘CP’ was loaded with an excess of cold (unlabelled) probe.
At 16 h from the beginning of the incubation with either CS or RWS, HUVEC were stimulated with 5 ng/ml of TNF-α. The nuclear translocation of NF-κB and AP-1 was assessed at 30, 60 and 120 min after the administration of the pro-inflammatory cytokine. As shown in panels (a) and (b) of Fig. 2, TNF-α induced a fast increase of the nuclear transfer of both NFκB and AP-1 in cells incubated with CS. On the other hand, the increase of transcription factor nuclear translocation associated to TNF-α in RWS pre-incubated cells was much less evident than in CS pre-incubated cells. These data indicate that a delay in TNF-α-dependent NF-κB and AP-1 activation is associated to RWS.

Fig. 2 Human serum obtained after red wine consumption (RWS) incubation delays NF-κB and activator protein-1 (AP-1) nuclear translocation induced by TNF-α. A representative electromobility shift assay (one out of at least three separate experiments) showing nuclear protein interactions with consensus NF-κB (a) and AP-1 (b) probes is shown. After 16 h of pre-incubation with 20 % of control serum (CS) or RWS in 199 medium, human umbilical vein endothelial cells were stimulated with 5 ng/ml TNF-α for 30, 60 and 120 min. The films obtained from the experiments were analysed using SCION IMAGE program. The lane CP was loaded with an excess of cold (unlabelled) probe. Results, from at least three separate experiments, are indicated as means and standard deviations of the relative densitometric intensity and expressed as percentage of control. Control is considered as, respectively, CS and RWS baseline cells before TNF-α stimulation.
Gene expression induced by TNF-α in human umbilical vein endothelial cells is modulated by red wine consumption
In order to study the transactivation effect in response to TNF-α in the presence of RWS, we assessed the kinetic expression of the mRNA encoding for marker genes presenting both NF-κB- and AP-1-binding sequences in their promoter. Vascular cell adhesion molecule (VCAM), intercellular adhesion molecule (ICAM) and monocytes chemoattractant protein-1 (MCP-1) were chosen as markers of the activation of cell adhesion response, and t-PA, PAI-2 as molecules involved in the activation of fibrinolysis process. HUVEC were stimulated for 20, 40, 90 and 240 min from TNF-α treatment.
The incubation with either CS or RWS was associated with a similar trend of activation for all the genes considered, with the exception of t-PA (Figs. 3 and 4). VCAM and ICAM mRNA expression peaked at 90 min from TNF-α treatment in the presence of both CS and RWS (Fig. 3). At 90 min from TNF-α administration, cells incubated in the presence of RWS expressed significantly lower levels of VCAM and ICAM mRNA (20 and 40 %, respectively) in comparison with CS pre-incubated cells and treated with TNF-α. At 240 min from TNF-α treatment, mRNA levels of both VCAM and ICAM dropped, still remaining significantly higher than the baseline, irrespective to the presence of either RWS or CS in the culture media.

Fig. 3 Effect of human serum obtained after red wine consumption (RWS) incubation on TNF-α-dependent adhesion molecule expression. After 16 h of incubation with 20 % of control serum (CS) or RWS in 199 medium, human umbilical vein endothelial cells were stimulated with 5 ng/ml TNF-α for 20, 40, 90 and 240 min. At the end of the incubation times, RNA was isolated and gene expression assessed by real-time PCR. Values are presented as mean values and standard deviations of the fold of changes of the gene expression in comparison with control. * P < 0·05; † P < 0·01 compared with CS pre-incubated cells and stimulated with TNF-α.
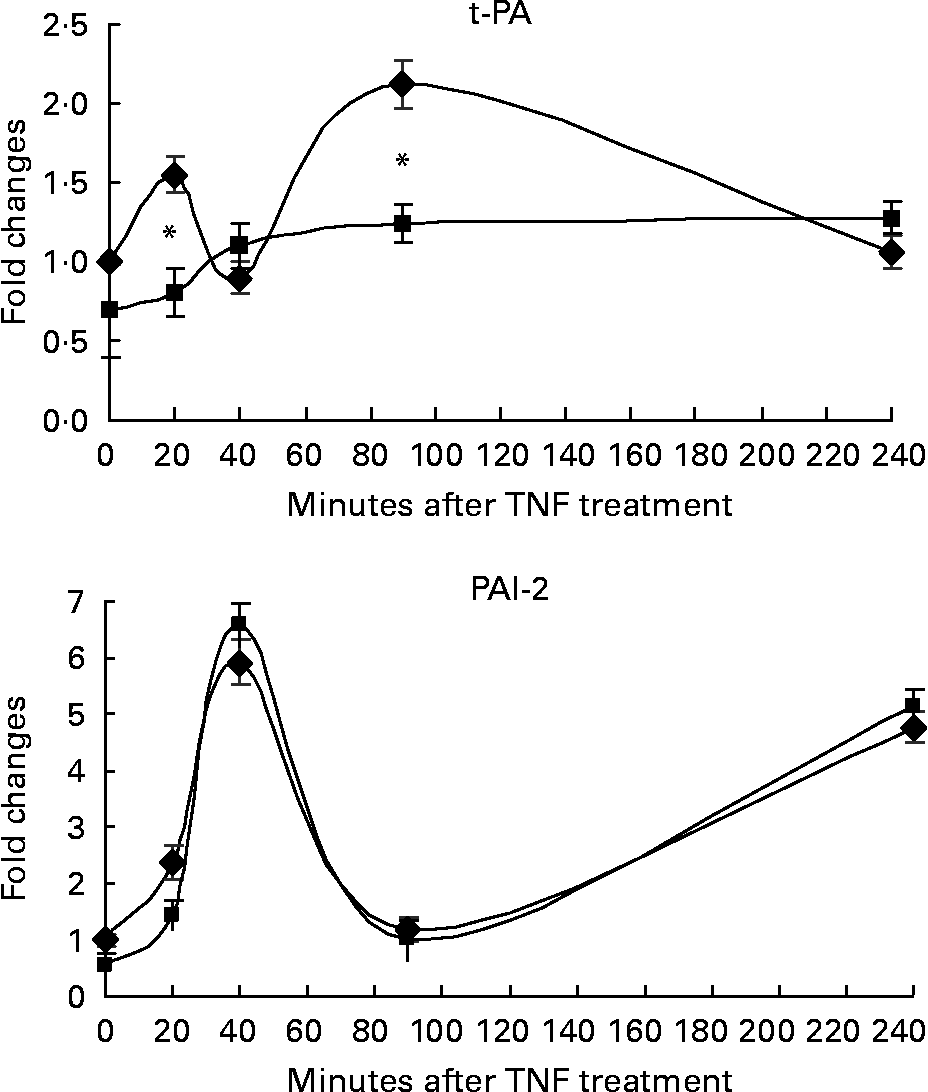
Fig. 4 Effect of human serum obtained after red wine consumption (RWS) incubation on TNF-α-dependent fibrinolysis molecule expression. After 16 h of incubation with 20 % of control serum (CS) or RWS in 199 medium, HUVEC were stimulated with 5 ng/ml TNF-α for 20, 40, 90 and 240 min. At the end of the incubation times, RNA was isolated and gene expression assessed by real-time PCR. Values are presented as mean values and standard deviations of the fold of changes of the gene expression in comparison with control. * P < 0·01 compared with CS pre-incubated cells and stimulated with TNF-α.
MCP-1 mRNA expression induced by TNF-α showed two peaks at 40 and 240 min, respectively, in the presence of both CS and RWS. The expression of MCP-1 mRNA was significantly lower in cells incubated with RWS than in control cells during the first 90 min from treatment, but rose up to a level significantly higher than control at 4 h from TNF-α treatment (Fig. 3).
Fig. 4 shows the effect of RWS on TNF-α-dependent fibrinolysis molecules expression. TNF-α induced the expression of t-PA and PAI-2 in cells incubated with CS. However, the incubation in the presence of RWS in the culture media significantly inhibited t-PA expression induced by TNF-α, while did not affect PAI-2 expression in comparison to the control.
Monocytes adhesion on endothelial cells is affected by red wine consumption
According to our observation indicating that the presence of RWS in the culture media affected the expression of genes involved in adhesion, we sought to study the interaction and adhesiveness of circulating cells to the endothelial cell surface.
Fig. 5 shows that the presence of RWS in the culture medium was associated with a slight but significant decrease of leucocyte adhesion in comparison with CS, both in baseline conditions and after 4 h from the administration of TNF-α. On the other hand, no difference in adhesion rate associated with RWS and CS incubation was observed at 6 h from TNF-α stimulation.

Fig. 5 Human serum obtained after red wine consumption (RWS) incubation decreases TNF-α-induced adhesion of U937 to human umbilical vein endothelial cells (HUVEC). After 16 h of incubation with 20 % of CS or RWS in 199 medium, HUVEC were stimulated with 5 ng/ml TNF-α for 4 and 6 h. Adhesion assays were performed by adding U937 cell suspension (1 × 105 per well), pre-labelled with calcein acetoxymethyl ester, to endothelial cells for 60 min at 37°C. At the end of the incubation, non-adherent cells were removed and the fluorescence of cell lysate was measured using a fluorescent plate reader. Data are expressed as percentage control (control is the rate of U937 adhesion to endothelial cells incubated with CS). * P < 0·05 compared with CS-incubated cells in the presence or absence of TNF-α.
Our data indicate that the presence of RW metabolites in the culture medium induces a decrease in the adhesion between endothelial cells and monocytes, which is recovered at longer incubation times.
c-Jun binding to cAMP responsive element sites is affected by red wine consumption
As shown in Fig. 4, the presence of RWS in the culture media quenched the activation of TNF-α-dependent t-PA expression, but did not affect PAI-2 expression. PAI-2 and t-PA expression is highly regulated and requires multiple transcription signals. An important role in their expression is played by CRE-binding site within both t-PA and PAI-2 genepromoters(Reference Medcalf, Ruegg and Schleuning24, Reference Cousin, Medcalf and Bergonzelli25). c-Jun is an abundant tissue-type plasminogen activator CRE (t-PACRE) and PAI-2 CRE-binding protein, and has been suggested as a possible candidate regulatory molecule for t-PA and PAI-2 gene expression(Reference Costa, Shen and Medcalf22).
In order to determine the role of RWS in c-jun modulation of t-PA and PAI-2 mRNA expression induced by TNF-α, the nuclear extracts obtained after 16 h of CS or RWS incubation (time 0) and at 30 min from TNF-α treatment were submitted to electrophoresis in the presence of both labelled gene-specific CRE sequences and with phosphorylated c-jun antibody. Fig. 6 shows that in cells incubated in the presence of CS, c-jun binding to t-PACRE sites increased after 30 min from TNF-α treatment. c-Jun binding to t-PACRE site, before TNF-α stimulation (time 0), was higher in the presence of RWS than in the presence of CS in culture media. Moreover, no increase in c-jun binding to t-PACRE was observed in cells incubated in the presence of RWS, after 30 min from TNF-α stimulation in comparison with time 0.

Fig. 6 Human serum obtained after red wine consumption (RWS) affects c-jun binding on t-PA and PAI-2 CRE sites. A representative electromobility shift assay showing nuclear protein interactions with the t-PA- and PAI-2-specific cAMP responsive element probes is shown. After 16 h of incubation with 20 % of control serum (CS) or RWS, human umbilical vein endothelial cells were stimulated with 5 ng/ml TNF-α for 30 min. Arrows indicate the protein band supershifts with phosphorylated c-jun antibody.
A significant supershift of PAI-2 CRE band indicated that TNF-α also induced a strong increase in c-jun binding to PAI-2 CRE sites. However, no differences associated with the presence of either RWS or CS in the culture medium were observed indicating that the presence of RW metabolites in HUVEC culture medium did not significantly affect c-jun binding to PAI-2 CRE.
Discussion
The present study provides a novel indication that RW metabolites (polyphenols, alcohol and others not identified) induce a pre-adaptation to eventual inflammatory stimuli. This pre-adaptation is characterised by a progressive increase of nuclear translocation of NF-κB and AP-1 in HUVEC. However, the binding activity of transcription factors, as detected by electromobility shift assay methodology, could not strictly reflect the specific activity on individual promoters(Reference Saccani, Pantano and Natoli26). In our experimental conditions, RWS evidently promoted cytoplasmic activation and nuclear transfer of both AP-1 and NF-κB. However, the absence of a parallel increase in transcriptional activity suggests that RW metabolites contained in RWS were not sufficient per se to induce the specific conditions leading to the transcription of the considered target genes.
Several studies have demonstrated the protective effect of RW constituents on the activation of adhesion of leukocytes on endothelial cells stimulated with an inflammatory stimulus(Reference Badia, Sacanella and Fernandez-Solà27, Reference Carluccio, Siculella and Ancora28), and the inhibition of NF-κB nuclear translocation has been suggested to play an important role within this mechanism(Reference Carluccio, Siculella and Ancora28, Reference Lee, Na and Namkoong29). Monocytes–endothelial cell adhesion is an early event in atheromatous plaque formation, and therefore the reduction in this process has been proposed to be one of the mechanisms underlying the beneficial effects of RW on atherosclerosis(Reference Endemann and Schiffrin30). Our observations indicate that the presence of RW metabolites in the culture medium is associated with a reduction of the adhesion of the monocytes to endothelial cells both in baseline conditions and at 4 h from TNF-α treatment. This effect is associated to a decrease in VCAM, ICAM and MCP-1 expression during the first 90 min of TNF-α treatment in comparison with control cells stimulated with TNF-α. However, after 6 h from TNF-α stimulation, no difference was observed in monocyte adhesion in RWS and CS pre-incubated cells. This effect is possibly associated to the lack of difference of VCAM and ICAM mRNA levels both in CS and RWS pre-incubated cells after 4 h from TNF-α stimulation and to the increase in MCP-1 mRNA induced by RWS. The present results suggest that the key event correlated to the biological effect observed is the pre-activation, i.e. nuclear import, of NF-κB and AP-1 induced by RW metabolites. Unexpectedly, such pre-activation state subsequently determines a delay of transcription factors activation when endothelial cells are stimulated with TNF-α and induces a decrease in the inflammatory response. The mechanism underlying this delay could involve a sort of adaptative cellular setting and surely warrants further research.
Moreover, we have also considered the role of RW metabolites in some molecular aspects of the control of blood flow, haemostasis and fibrinolysis by the endothelium in vascular dysfunctions. Available literature is somehow conflicting when addressing the effects of pro-inflammatory cytokines on the expression of t-PA. In agreement with previous reports(Reference Gingras, Nyalendo and Di Tomasso31, Reference Ulfhammer, Larsson and Karlsson32), our observations indicate an early stimulatory effect of TNF-α on t-PA gene expression. Moreover, the present results strongly confirmed that t-PA and PAI-2 mRNA are inversely regulated, in agreement with the observations previously reported by Costa et al. (Reference Costa, Shen and Medcalf22) in HT-1080 cells.
t-PA and PAI-2 promoters are very complex regulatory units: a down-regulation of t-PA expression has been demonstrated to be mediated by NF-κB and p38 mitogen-activated protein kinase, whereas c-Jun N-terminal kinase activity has been reported to act by stimulating gene expression(Reference Ulfhammer, Larsson and Karlsson32). Moreover, CRE elements have been shown to play important regulatory roles in t-PA and PAI-2 gene promoters(Reference Medcalf, Ruegg and Schleuning24, Reference Cousin, Medcalf and Bergonzelli25), with activating transcription factor-2, cAMP response element binding, cAMP response element modulator and c-jun representing the most important proteins interacting with CRE sequence(Reference Costa, Shen and Maurer33). Our data indicate that the up-regulation of t-PA and PAI-2 gene expression induced by TNF-α in endothelial cells pre-incubated with CS is associated to an increase in the binding of phosphorylated c-jun on specific t-PA and PAI-2 CRE sites, indicating its involvement in the activation of mRNA transcription.
Published studies have frequently reported that individual polyphenols enhance the fibrinolytic activity by increasing t-PA gene transcription in HUVEC in vitro (Reference Abou-Agag, Aikens and Tabengwa34). Similarly, low ethanol has been reported to transcriptionally up-regulate t-PA gene expression and to down-regulate PAI-1 expression(Reference Booyse, Aikens and Grenett35). As observed for NF-κB and AP-1 nuclear translocation activation, RWS incubation was also associated to an increase in c-jun binding to t-PACRE, as indicated by the higher intensity of the supershifted phosphorylated c-jun electrophoretic band in comparison with control. Moreover, the presence of RWS in the culture media quenches TNF-α-dependent activation of t-PA gene expression, in comparison with control cells stimulated with TNF-α. This effect was associated with the maintenance of c-jun binding to t-PACRE site at the level of RWS-unstimulated cells. Overall, these observations suggest that NF-κB, activated by RWS and present in high levels inside the nucleus, is a main candidate for a negative modulation of t-PA gene transactivation(Reference Ulfhammer, Larsson and Karlsson32). Finally, the incubation in the presence of RWS does not affect c-jun binding to PAI-2 CRE elements induced by TNF-α treatment in HUVEC. Similarly, the correlated kinetic of gene expression is not affected by the presence of RWS in the culture medium.
Overall, the present results suggest that the metabolites contained in RWS (including alcohol) are not associated to the inhibition of the activation of transcription factors, as proposed in the literature. Rather, the effect of RW components on endothelial function seems to be due to a cellular adaptative response that ultimately leads to a reduction of the inflammatory response.
Acknowledgements
The research was supported by the Italian Ministry of Agriculture and Forest Policy (‘Food Quality’ Project) and by a special research project by the ministry of Education, University and Research of Italy (FISR ‘Safe-eat’). No competing financial/commercial interests exist. R. C. (Raffaella Canali) and R. C. conceived and designed the experiments. R. C., R. C. and R. A. performed the experiments. R. C., R. C. and F. V. analysed the data. R. C., R. C. and F. V. wrote the manuscript.









