Management Implications
In 1998, Hadroplontus litura, the stem-mining weevil and biological control agent of Cirsium arvense (Canada thistle), was introduced into a limited area in Minnesota, with a resulting decline in C. arvense populations. The state of Minnesota is interested in expanding and augmenting its C. arvense biological control program. Before recommending H. litura for release into additional sites, we wanted to determine the host range of H. litura on Cirsium species native to the upper Midwest. Previous work documented that H. litura could complete its life cycle on Cirsium discolor (field thistle), Cirsium muticum (swamp thistle), Cirsium altissimum (tall thistle), Cirsium flodmanii (Flodman’s thistle), and Cirsium undulatum (wavy-leaved thistle) in no-choice tests in the spring. Based on the phenology of shoot emergence/initiation in the spring presented in this study, all tested Cirsium species native to the upper Midwest have the potential to be within the ecological host range of H. litura. As such, we recommend further studies on H. litura search and acceptance behavior be determined in the field, outside a screen cage, to further define the ecological host range of H. litura. Although we are not aware of published accounts of H. litura accepting these native Cirsium species as hosts in the field, it would be prudent for managers to document any observed attack.
Introduction
Native Cirsium species play an important role in landscapes across North America (Eckberg et al. Reference Eckberg, Lee-Mäder, Hopwood, Foltz and Borders2017). Of significance, native thistle flowers produce a high-sugar nectar and are a pollen source for more than 200 species of native pollinators, including a variety of butterflies, bees, and other insects (Eckberg et al. Reference Eckberg, Lee-Mäder, Hopwood, Foltz and Borders2017; Fussell and Corbet Reference Fussell and Corbet1992; Hilty Reference Hilty2015; Lye et al. Reference Lye, Kaden, Park and Goulson2010; Robertson Reference Robertson1929). Native Cirsium flowers provide a food source for birds, insect defoliators, and seed feeders (Eckberg et al. Reference Eckberg, Lee-Mäder, Hopwood, Foltz and Borders2017; Hilty Reference Hilty2015), such as the American goldfinch (Spinus tristis L.), which feeds heavily on thistle seed during its breeding season (Stokes Reference Stokes1950). The high moisture content of native Cirsium seed in the milky stage of development provides an important source of water (Gluck Reference Gluck1985).
The ubiquitous, invasive perennial Canada thistle [Cirsium arvense (L.) Scop.] is native to Europe and the Mediterranean region (Slotta et al. Reference Slotta, Foley, Chao, Hufbauer and Horvath2010) and has been introduced worldwide. Cirsium arvense may have been introduced into North America from multiple continents (Slotta et al. Reference Slotta, Rothhouse, Hovarth and Foley2006), and populations are genetically diverse (Slotta et al. Reference Slotta, Foley, Chao, Hufbauer and Horvath2010). It is considered one of the worst weeds of agricultural and natural systems (Cripps et al. Reference Cripps, Gassmann, Fowler, Bourdot, McClay and Edwards2011) In North America, C. arvense is present in 42 states and 12 Canadian provinces and has a noxious weed status in 46 states (USDA-NRCS 2022). Cirsium arvense is a herbaceous perennial plant, with aboveground shoots dying back over the winter and underground roots surviving from year to year (Moore Reference Moore1975). Plants reproduce through seed and vegetative spread via underground lateral roots to form large interconnected clonal patches (Donald Reference Donald1994; Moore Reference Moore1975). Cirsium arvense plants rapidly colonize new areas and are difficult to control in perennial and annual cropping systems (Tiley Reference Tiley2010).
In North America, Hadroplontus litura (F.) (formerly Ceutorhynchus litura) was first introduced as a biological control agent for C. arvense in 1965 (Peschken and Beecher Reference Peschken and Beecher1973). In the United States, it has since become established in Idaho, Montana, Nebraska, North Dakota, Oregon, Utah, Virginia, Washington, and Wyoming (Winston et al. Reference Winston, Hansen, Schwarzlander, Coombs, Bell Randall and Lym2009). Adult H. litura overwinter in the soil and leaf litter. In spring, the onset of adult activity is synchronized with the emergence of C. arvense shoots from the soil (Gramig et al. Reference Gramig, Burns and Prischmann-Voldseth2015; Peschken and Wilkinson Reference Peschken and Wilkinson1981; Zwolfer and Harris Reference Zwolfer and Harris1966). Adults initially feed on leaves of emerging shoots (Peschken and Beecher Reference Peschken and Beecher1973; Rees Reference Rees1990; Zwolfer and Harris Reference Zwolfer and Harris1966). Females oviposit in the midvein on the underside of leaves, and larvae progress through three instars (Zwolfer and Harris Reference Zwolfer and Harris1966). Larvae successively mine leaf midribs, stems, and crowns of C. arvense plants throughout the spring and summer (Zwolfer and Harris Reference Zwolfer and Harris1966). Third instar larvae emerge from C. arvense plants in late summer, pupate in the soil, and emerge as adults from July to October, depending on location (Peschken and Beecher Reference Peschken and Beecher1973; Rees Reference Rees1990; Zwolfer and Harris Reference Zwolfer and Harris1966). Hadroplontus litura is univoltine (produces one generation per year).
The fundamental host range of a weed biological insect is defined as the set of plant species on which the insect can complete its life cycle (Schaffner Reference Schaffner2001; Van Klinken Reference Van Klinken, Van Driesche, Heard, McClay and Reardon2000). The ecological host range is a subset of the fundamental host range and comprises plant species that biocontrol agents exploit as hosts in the field (Schaffner Reference Schaffner2001). In North America, H. litura’s primary host is C. arvense, although its host range includes the Cirsium–Silybum–Carduus complex of the Asteraceae subtribe, Carduinae (Zwolfer and Harris Reference Zwolfer and Harris1966). There are no Carduus or Silybum species native to North America, but there are at least 62 native species of Cirsium (Keil Reference Keil2006). Initial host-range testing indicated that H. litura fed on clustered thistle (Cirsium brevistylum Cronquist), wavy-leaved thistle [Cirsium undulatum (Nutt.) Spreng.], and Flodman’s thistle [Cirsium flodmanii (Rydb.) Arthur] (Zwolfer Reference Zwolfer1965; Zwolfer and Harris Reference Zwolfer and Harris1964, Reference Zwolfer and Harris1966).
Recent work by Katovich et al. (Reference Katovich, Becker, Chandler and Marek-Spartz2022) expanded the fundamental host range of H. litura to include the native Cirsium species swamp thistle (Cirsium muticum Michx.), field thistle [Cirsium discolor (Muhl. ex Willd.) Spreng.], and tall thistle [Cirsium altissimum (L.) Sprengel.]. The federally threatened Pitcher’s thistle [Cirsium pitcheri (Torr. ex Eaton) Torr. & A. Gray] (U.S. Fish and Wildlife Service 2019), was accepted for oviposition, but no adults were found in development tests, so it is not known whether H. litura can complete its life cycle on this species.
Although these native Cirsium species are within the fundamental host range of H. litura, it is unclear whether H. litura adults would accept these native Cirsium species as hosts in the field, where weevils would exhibit normal host search and acceptance behavior.
Differences in phenology between a host plant, such as C. arvense, and native non-host plants can also narrow a biocontrol agent’s host range in the field (Louda Reference Louda1998). We were unable to find reports in the literature of non-target attack by H. litura in the field. As such, the objective of our research was to further characterize the ecological host range of H. litura by comparing the phenologies of spring shoot emergence of Cirsium species native to the upper Midwest with the phenologies of C. arvense and H. litura. Our goal was to explore whether shoots of native Cirsium species could escape H. litura shoot oviposition in spring due to delayed shoot emergence relative to C. arvense. This work expands on our previous work on the fundamental host range of H. litura (Katovich et al. Reference Katovich, Becker, Chandler and Marek-Spartz2022) by further characterizing the ecological host range of H. litura on previously untested Cirsium species native to the upper Midwest.
Materials and Methods
Trial Design
A common garden was established at the University of Minnesota St Paul Field Station to compare the relative phenologies of native Cirsium species and C. arvense. We established a common garden so that all Cirsium species could be propagated at the same latitude and longitude and under the same environmental conditions (Berend et al. Reference Berend, Haynes and MacKenzie2019; Liang Reference Liang2016). Native Cirsium plants often grow at low densities at sites (Eckberg et al. Reference Eckberg, Lee-Mäder, Hopwood, Foltz and Borders2017) and do not often co-occur. Establishing a common garden was thought to be the best method to make direct comparisons of shoot emergence among species. When planting the common garden, we tried to provide optimum growing conditions for all species.
Cirsium species included in the common garden, along with seed and plant sources are listed in Table 1. Propagation methods as well as H. litura colony establishment were as described in Katovich et al. (Reference Katovich, Becker, Chandler and Marek-Spartz2022). Cirsium species included: three native biennial species—C. altissimum, C. discolor, and C. muticum; three native perennial species—C. flodmanii, C. undulatum, and C. pitcheri; and the introduced perennial C. arvense. We collaborated with the Minnesota Biological Survey to locate sources for each native Cirsium species when possible or purchased Cirsium seed or plants from local seed sources.
Table 1. Cirsium species included in the common garden (St Paul, MN). a

a All native species are present in Minnesota, except for Cirsium pitcheri, which is native and present east of Minnesota. Cirsium arvense is a nonnative invasive species.
Because H. litura adult females actively oviposit in the spring, Cirsium plants were established each summer before monitoring and overwintered so initiation of leaf growth from rosettes or emerged perennial shoots would be available in the spring when adults became active.
Cirsium species were transplanted into the common garden in a randomized complete block design with six replications each year starting in 2015, a year before data collection. Each Cirsium species was present once in each block. The soil type was a Waukegan silt loam (fine-silty over sandy, mesic Typic Hapludolls) with 6.8% organic matter and pH of 6.7. When the trial was first established in July 2015, each plot consisted of one plant spaced 1.2 m apart and watered as needed. In the fall of 2015, 2016, and 2017, Cirsium seeds were also planted in each plot in late summer so that seeds could stratify in situ over the winter to germinate and establish plants the following year. Additional propagated seedlings were transplanted each spring to replace plants that did not survive the winter; however, data were not collected on those plants until the following spring and summer.
In the summers of 2015 and 2016, the area was cultivated with a hand-driven mechanical cultivator and manually weeded for weed control within and between plots. Due to high Cirsium mortality over the winter of 2015/2016, two additional replications (eight total) were added in spring of 2016 to compensate for expected loss of plants from season to season. After continued high winter kill in the winter of 2016/2017, low-profile warm- and cool-season turfgrasses were seeded over the area in April 2017 to provide cover for Cirsium species, reduce the weed pressure, and catch snow to insulate Cirsium species during the winter, all of which reduced plant mortality.
In each spring of 2016 through 2019, dates of new leaf emergence on biennial rosettes and shoot emergence of perennials were recorded beginning as soon as the snow had melted. We rated individual Cirsium species in the common garden at 0 for no shoot emergence (perennials) or leaf initiation on rosettes (biennials) and 1 for shoot emergence or leaf initiation.
Determination of Growing Degree Days
We calculated cumulative air and soil growing degree days (GDD) to determine which had a greater influence on Cirsium shoot emergence in the spring. Phenological events were recorded by the day of the year, with Day 1 corresponding to January 1. Cumulative air and soil GDD were calculated from the Midwest Regional Climate Center online data portal using data from the on-site, University of Minnesota, St Paul reporting station (44.9902°N, 93.1824°W; elevation: 296 m). Mean percent shoot emergence as a function of cumulative soil GDD are presented for each species across years (Supplementary Figure 1a–g).
We calculated air GDD with the following equation (Midwest Regional Climate Center):
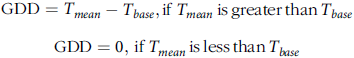 $$\matrix{ {{\rm{GDD}} = {T_{mean}} - {T_{base}},{\rm{if}}{\mkern 1mu} \, {T_{mean}}{\mkern 1mu} \, {\rm{is}} \, {\mkern 1mu} \, {\rm{greater}} \, {\mkern 1mu} {\rm{than}}{\mkern 1mu} \, {T_{base}}} \hfill \cr
{\quad \quad \quad {\rm{GDD}} = 0,{\mkern 1mu} {\rm{if}}{\mkern 1mu} \, {T_{mean}}{\mkern 1mu} \, {\rm{is}}{\mkern 1mu} \, {\rm{less}}{\mkern 1mu} \, {\rm{than}}{\mkern 1mu} \, {T_{base}}} \hfill \cr } $$
$$\matrix{ {{\rm{GDD}} = {T_{mean}} - {T_{base}},{\rm{if}}{\mkern 1mu} \, {T_{mean}}{\mkern 1mu} \, {\rm{is}} \, {\mkern 1mu} \, {\rm{greater}} \, {\mkern 1mu} {\rm{than}}{\mkern 1mu} \, {T_{base}}} \hfill \cr
{\quad \quad \quad {\rm{GDD}} = 0,{\mkern 1mu} {\rm{if}}{\mkern 1mu} \, {T_{mean}}{\mkern 1mu} \, {\rm{is}}{\mkern 1mu} \, {\rm{less}}{\mkern 1mu} \, {\rm{than}}{\mkern 1mu} \, {T_{base}}} \hfill \cr } $$
where T base = 0 C and T mean = mean temperature: (T max + T min)/2.
Cumulative GDDs required for first Cirsium emergence were estimated using a base temperature of 0 C (Donald Reference Donald2000) beginning on January 1. Cumulative GDD were also calculated beginning on April 1 for each year to compare with values reported by Donald (Reference Donald2000). Cumulative soil GDD were calculated with the following formula (Martinson et al. Reference Martinson, Durgan, Forcella, Wiersma, Spokas and Archer2007):
where T base = 0 C, T max = maximum daily soil temperature, and T min = minimum daily soil temperature.
A base temperature of 0 C (Donald Reference Donald2000) beginning on January 1 (Day 1 for all years) was used for calculations of cumulative soil GDD. To reflect the change in cover in the common garden, soil temperatures used to calculate soil GDD were taken at a 10-cm depth under bare soils in 2016 and 2017 and under sod in 2018 and 2019.
Statistical Analysis of Cirsium Common Garden Experiment
To assess whether Cirsium arvense emergence coincided with emergence of native Cirsium species, we conducted a series of survival analyses. Survival analysis, also known as time-to-event analysis, is well suited to our context, as we aim to assess differences in time to shoot emergence among Cirsium species (Klein et al. Reference Klein, van Houwelingen, Ibrahim and Scheike2013; McNair et al. Reference McNair, Sinkara and Forbish2013; Romano and Stevanato Reference Romano and Stevanato2020). The Kaplan-Meier method (Kaplan and Meier Reference Kaplan and Meier1958) provides a nonparametric means of visually assessing differences in time to emergence. Using pooled data on date of shoot emergence from 2016 through 2019, we first estimated Kaplan-Meier survival curves for each Cirsium species (Stata v. 16.1, StataCorp, 4905 Lakeway Drive, College Station, TX 77845). We then calculated the Kaplan-Meier estimate of cumulative incidence (Gooley et al. Reference Gooley, Leisenring, Crowldy and Storer1999) as follows:
We plotted the probability of shoot emergence on the y axis (Kaplan-Meier estimate of cumulative incidence) and day of the year (Day 1 = January 1) on the x axis (SigmaPlot v. 14, Inpixon Indoor Intelligence, 2479 E. Bayshore Road, Suite 195, Palo Alto, CA 94303).
To formally test whether there were significant differences in time to emergence between species, we applied the log-rank test for equality of survivor functions (Peto et al. Reference Peto, Pike, Armitage, Breslow, Cox, Howard, Mantel, McPherson, Peto and Smith1977), which compares the observed number of events per species with what would be expected if the survival curve were the same for each (i.e., H 0 = no difference in time to event between species). To assess whether there were significant pairwise differences in time to emergence between species, we conducted Holm-Sidak pairwise comparison tests and created a matrix of each of the seven Cirsium species. Each cell of the matrix shows whether the pairwise comparison between the two species was significantly different.
Finally, we extended our time-to-event analysis by estimating Cox proportional hazards models on pooled data from 2016 through 2019. We modeled time to shoot emergence for all perennial species and time to new leaf initiation for all biennial species. When we combined perennial and biennials, we modeled time of emergence, whether from shoot emergence or initiation of shoots. The Cox proportional hazards method allowed us to control for continuous-time covariates (explanatory variables) that may influence Cirsium emergence, namely cumulative air GDD and cumulative soil GDD. This method allowed us to simultaneously evaluate the importance of soil and air GDD on shoot emergence/initiation time (Cox Reference Cox1972; Templ et al. Reference Templ, Fleck and Templ2016). The global null hypothesis that air GDD and soil GDD do not affect time to emergence or leaf initiation was tested with a likelihood ratio test.
The Cox proportional hazard model creates hazard ratios associated with each model parameter, which may be interpreted as the change in risk of an event (i.e., emergence) if that parameter increases by 1 unit (e.g., 1 GDD). A hazard ratio of 1 indicates no effect of the covariate (air GDD or soil GDD), while a hazard ratio greater than 1 indicates an increased risk of emergence per 1 GDD, and a hazard ratio of less than 1 indicates a decreased risk of emergence.
Phenology of Hadroplontus litura
The phenology of H. litura was monitored in 2016, 2017, and 2018 at a C. arvense nursery on the St Paul Field Station near the common garden. Hadroplontus litura adults were added to caged, potted C. arvense plants during the preceding summer and overwintered with the pot-in-pot technique (Katovich et al. Reference Katovich, Becker, Chandler and Marek-Spartz2022; Mathers Reference Mathers2003). We used caged plants to increase the probability of collecting F1 adults, which are cryptic and difficult to find in the field (Gramig et al. Reference Gramig, Burns and Prischmann-Voldseth2015). After the first indication of adult activity in the spring, six C. arvense plants were sampled at weekly intervals. At each sampling time, two stems from each plant with adult feeding damage were dissected. Presence of eggs or first instar larvae were noted. Once first instar larvae were recorded, weekly sampling was discontinued to allow remaining larvae to continue their development. First generation adult (F1) activity was detected by observing new adult leaf feeding or finding adults crawling on screened cages. At this time, all plants were searched for adults. Accumulated air GDD were calculated from January 1 of each year to when eggs, larvae, and F1 adults were first observed on potted, caged C. arvense.
Results and Discussion
Winter and Spring Temperatures and Snow Cover
Air and soil temperatures and the amount and duration of snow cover varied considerably during the winters and springs of 2016 through 2019 (Figure 1). As we could not record shoot emergence or leaf initiation until the snowpack melted, duration of snowpack during March and April dictated when we first collected data. In March and April of 2016 and 2017, there were 0 and 5 d of snow cover, respectively (Table 2; Figure 1). As a result, we were able to begin collecting emergence data on March 14 (Day 74) in 2016 and March 20 (Day 79) in 2017 (Table 2). In 2018, we had a blizzard in St Paul, MN, on April 15 (Day 105) and the snow did not melt from the field until later in April (Figure 1). Consequently, the first observation for 2018 was delayed until April 25 (Day 115), 41 and 36 d later than 2016 and 2017, respectively (Table 2). In 2019, colder than normal winter temperatures led to lower air and soil cumulative GDD for the first data collection date (Table 2). A rapid warm-up in late March of 2019 allowed us to collect emergence data on March 25 (Day 86), but we were unable to collect data for one date in mid-April because of a 19-cm snow event (Figure 1).

Figure 1. Mean snow depth, and mean air and soil temperatures from January 1 to June 30, 2016 to 2019. Soil temperatures were taken at a 10-cm depth under bare soil in 2016 and 2017 and under sod in 2018 and 2019. St Paul Field Station, St Paul MN (44.990263°N, 93.179938°W).
Table 2. Cirsium common garden (St Paul, MN) number of days with snow cover in April and May and date of first possible data collection in spring of 2016 through 2019.
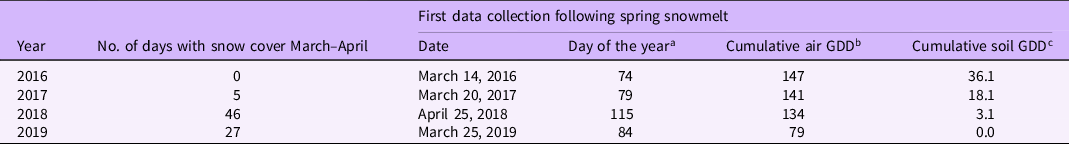
a Number of days to first data collection starting at January 1.
b Cumulative growing degree days (GDDbase 0) calculated starting on January 1 for each respective year.
c Cumulative soil GDD calculated from January 1. Soil temperatures at a 10-cm depth were used to calculate soil GDD under bare soil in 2016 and 2017 and under sod in 2018 and 2019. Soil temperatures were collected at the University of Minnesota, St Paul Field Station.
Cirsium arvense Emergence in a Common Garden
Cirsium arvense survived all plantings over all 4 yr. From 2016 through 2019, the mean date of C. arvense shoot emergence ranged from April 10 (Day 100, 372 air GDD and 64 soil GDD) in 2017, to May 6 (Day 126, 639 air GDD and 169 soil GDD) in 2019 (Figure 2; Table 3). The delay in C. arvense emergence in 2019 was most likely the result of cooler air and soil temperatures in March and April (Table 2). Donald (Reference Donald2000) created a model of C. arvense emergence using a nonlinear logistic dose–response regression model and predicted that between 1% and 80% of C. arvense shoots would emerge between 197 and 587 GDD using a base air temperature of 0 C. When we calculated air GDD, starting on April 1, there were 204, 222, 330, and 566 GDD for the average date of C. arvense emergence in 2016, 2017, 2018, and 2019 respectively. Air GDD were within the range described by Donald (Reference Donald2000) for C. arvense emergence.

Figure 2. Cirsium arvense vegetative shoot emergence in the spring in the Cirsium species common garden (St Paul MN) in 2016, 2017, 2018, and 2019. Percent maximum emergence of 1.0 is equivalent to 100% shoot emergence. Days of the year start with January 1 as Day 1.
Table 3. Hadroplontus litura and Cirsium arvense phenology 2016 through 2019. a
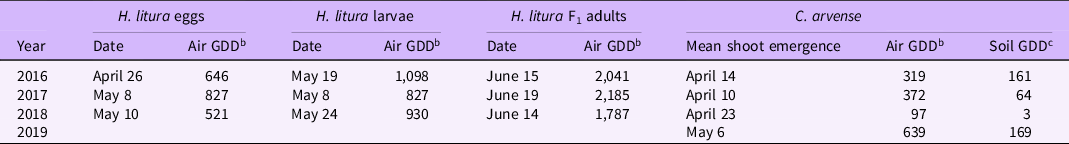
a Hadroplontus litura monitored in the field on caged Cirsium arvense plants at the University of Minnesota, St Paul, MN. Date indicates when life stage was first recorded.
b Cumulative air growing degree days (GDDbase 0) beginning on January 1 for each respective year.
c Cumulative soil GDD (GDDbase 0) calculated from January 1. Soil temperatures at a 10-cm depth were used to calculate soil GDD under bare soil in 2016 and 2017 and under sod in 2018 and 2019. Soil temperatures were collected at the University of Minnesota, St Paul Field Station.
Native Cirsium Establishment and Winter Survival
It was difficult to establish and maintain all native Cirsium species in the common garden, especially during the winters of 2016 and 2017 with bare soils and subsequent winterkill. Cirsium muticum did not readily establish in the well-drained silt loam soil at the common garden. As its common name implies, this plant grows best in moist areas near marshes and wetlands (Eckberg et al. Reference Eckberg, Lee-Mäder, Hopwood, Foltz and Borders2017). Rosettes of C. muticum suffered high rates of mortality over the course of the experiment. Spring emergence ratings were based on 43% (n = 13/30) of established plants over all 4 yr. Cirsium undulatum established each year, but only 13% of plants survived the winter over all 4 yr (emergence ratings: n = 4/30) most likely due to the St Paul location situated at the northern edge of its natural range. Cirsium pitcheri plants that survived the first winter flowered the following summer, although this plant can take 2 to 8 yr to flower in the sand dunes of its native habitat (Eckberg et al. Reference Eckberg, Lee-Mäder, Hopwood, Foltz and Borders2017; Havens et al. Reference Havens, Jolls, Marik, Vitt, McEachern and Kind2012). The native Cirsium species C. flodmanii, C. altissimum, and C. discolor established and overwintered most successfully at our site but at lower rates than C. arvense. The invasive C. arvense clearly was the most successful colonizer of the Cirsium species tested.
Emergence of Native Cirsium Species in a Common Garden
Shoots of the perennials C. flodmanii, C. undulatum, and C. pitcheri emerged before the first observation date in the spring (mid- to late March) in 2016 and 2018, before snowmelt. In 2017, only 25% of C. flodmanii shoots had emerged on the first observation date on March 20, but all shoots had emerged (maximum emergence = 1.0) by the end of March. In 2019, no C. flodmanii shoots emerged until April 8. This delayed emergence was likely due to cooler soil temperatures. When plants survived the winter, shoots of the other perennial species, C. undulatum and C. pitcheri, emerged by the first observation dates, before snowmelt during all years. However, only 4 of 30 and 6 of 30 plants (total over 4 yr of the study) survived the winter to emerge the following spring for C. undulatum and C. pitcheri, respectively.
In 2016, 2017, and 2018 all rosettes of the biennials C. discolor, C. altissimum, and C. muticum initiated growth of new leaves before snowmelt in the early spring, with two exceptions. In 2016, only 80% of C. altissimum rosettes had initiated new leaves before snowmelt. In 2019, only 43% and 83% of rosettes of C. discolor and C. altissimum had developed new leaves by the first observation date after snowmelt, respectively, most likely due to cool air and soil temperatures (Supplementary Figure 1b–d).
Because not all native Cirsium plants in each replication survived until the following spring, emergence data were collected on the surviving plants. Time to emergence (Kaplan-Meier estimate of cumulative incidence curves) were plotted for each species (Figure 3). From Figure 3, it is visually apparent that C. arvense emerged later than the native Cirsium species. Among native Cirsium species, C. undulatum emerged earlier than C. discolor, C. altissimum, or C. pitcheri (Figure 3). The log-rank test rejected the null hypothesis of equivalence among species’ survival curves at the 1% level of significance (P≤ 0.001). From this, we conclude that there were significant differences among survival curves for Cirsium species, as the number of observed events (emerging shoots/leaves) are dissimilar to the number of events expected under the null hypothesis (Supplementary Table 1). Results of the Holm-Sidak multiple comparison test showed that shoots of C. arvense emerged later than those of native Cirsium species. Cirsium undulatum emerged earlier than C. arvense, C. discolor, and C. altissimum. There were no differences in emergence time among the remaining native Cirsium species (Table 4).

Figure 3. Emergence of Cirsium arvense and native Cirsium species in 2016, 2017, 2018, and 2019 using Kaplan-Meier cumulative incidence curves. Probability of 1.0 is equivalent to 100% shoot emergence. Days of the year start with January 1 as Day 1. Common garden field trials, St Paul, MN.
Table 4. Cirsium shoot emergence in the spring in the Cirsium common garden (St Paul, MN), 2016 through 2019. a

a All pairwise comparisons (Holm-Sidak method). Comparisons should be made across rows. A plus sign (+) indicates that the Cirsium species in that row emerged significantly earlier than the Cirsium in the column (P < 0.05). An “x” indicates no comparison to be made.
We also modeled time to shoot emergence for perennial Cirsium species, time to new leaf initiation for all biennial species, and shoot emergence/leaf initiation time for all species combined using the Cox proportional hazards model, using pooled data from 2016 through 2019 and including air and soil GDD as independent covariates. The global null hypothesis that air GDD and soil GDD do not affect time to emergence or leaf initiation was tested with a likelihood ratio test and rejected at the 1% significance level.
Soil GDD hazard ratios for perennial, biennial, and all Cirsium species combined, were greater than 1.0 (Table 5). Conversely, hazard ratios for air GDD were less than 1.0. For all Cirsium species combined, for every 1-unit increase in cumulative soil GDD, the “risk” or chance of emergence was increased by 1.2%. For every 1-unit increase in cumulative air GDD, the chance of emergence was increased by 0.5%. Thus, cumulative soil GDD has an approximately 2.4 times larger effect on time to emergence relative to air GDD, which is reasonable, as shoots are under the soil or near the soil surface in the early spring. Hazard ratios for soil GDD for biennial and perennial species were 1.011 and 1.016, respectively (Table 5). Thus, with the accumulation of each additional soil GDD in the spring, the risk, or chance of emergence of Cirsium plants, was higher than with the accumulation of 1 GDD calculated from air temperatures. Soil GDD have been used to accurately predict germination of annual species (Harvey and Forcella Reference Harvey and Forcella1993; Martinson et al. Reference Martinson, Durgan, Forcella, Wiersma, Spokas and Archer2007) and may describe perennial or biennial shoot emergence more accurately than air GDD (Wu et al. Reference Wu, Boyd, Cutler and Olson2013) (Figure 1; Supplementary Material).
Table 5. Cirsium species emergence in the common garden (St Paul, MN), 2016 through 2019. a

a Cox proportional hazards regression of cumulative air and soil growing degree days (GDD) for combined perennial and biennial Cirsium species.
b n = 621.
c n = 319, C. flodmanii, C. arvense, C. undulatum, C. pitcheri.
d n = 302, C. discolor, C. altissimum, C. muticum.
e Percent increase per 1.0 GDD.
In practice, soil temperature data are not always readily available. For this reason we presented our results on a calendar basis (day of the year) rather than soil GDD. Phenological sequences of events can be reliable across years (Herms Reference Herms, Krischik and Davidson2004), and our objective was to determine the relative spring emergence among Cirsium species. We monitored Cirsium shoot emergence across 4 yr, in which spring temperatures, snowfall, and snowmelt varied considerably (Figure 1). Our results showed that C. arvense shoots emerged consistently later than native Cirsium species
Hadroplontus litura Phenology
First adult activity of H. litura was observed in the spring from mid-April (2016) to early May (2018) on caged and overwintered C. arvense plants, coinciding with the emergence of C. arvense vegetative shoots (Table 3). Similar results were reported by Gramig et al. (Reference Gramig, Burns and Prischmann-Voldseth2015), Peschken and Wilkinson (Reference Peschken and Wilkinson1981), and Zwolfer and Harris (Reference Zwolfer and Harris1966).
We first observed H. litura eggs on April 26 (646 air GDD), May 8 (827 air GDD), and May 10 (521 air GDD) in 2016, 2017, and 2018, respectively, approximately 1 to 3 wk after first emergence of C. arvense shoots each year (Table 3). Larvae were first observed on May 19 (1,098 air GDD), May 8 (827 air GDD), and May 24 (930 air GDD) in 2016, 2017, and 2018, respectively. However, different head capsule measurements indicated that multiple instars were present, so larvae development likely began before sampling.
F1 adults are cryptic and very difficult to recover in the field (Gramig et al. Reference Gramig, Burns and Prischmann-Voldseth2015; Peschken and Beecher Reference Peschken and Beecher1973). At our St Paul site, we first collected F1 adults in screen-caged plants on June 15 (2,041 air GDD), June 19 (2,185 air GDD), and June 14 (1,781 air GDD) in 2016, 2017 and 2018, respectively (Table 3). These dates are similar to those reported near Bozeman, MT, near 45.6778°N (Rees Reference Rees1990) and earlier than the August emergence recorded in the most northern location documented, Regina, SK, Canada, near 50.4547°N (Peschken and Wilkinson Reference Peschken and Wilkinson1981).
A logistic regression model created by Gramig et al. (Reference Gramig, Burns and Prischmann-Voldseth2015) predicted that H. litura egg medium development time (when 50% of a cohort were in the egg stage) occurred when greater than 235 air GDDbase 0 C had accumulated after soil temperatures warmed to 9 C. Because we recorded date of first egg observation rather than median egg development, it was not possible to determine whether our results align with those of Gramig et al. (Reference Gramig, Burns and Prischmann-Voldseth2015). At sites in eastern North Dakota (48.7016°N to 46.3628°N), H. litura eggs were found from mid-May to the beginning of June (Prischmann-Voldseth et al. Reference Prischmann-Voldseth, Burns, Swenson and Gramig2016), a period of 2 to 3wk later than what we found at St Paul, MN, a more southerly site. Hadroplontus litura appear to oviposit later at locations farther north, mirroring the later emergence of F1 adults at more northerly sites (Peschken and Wilkinson Reference Peschken and Wilkinson1981).
In conclusion, for all species, soil cumulative GDD was a superior predictor of emergence of shoots of perennial Cirsium species or initiation of leaves in biennial species emergence compared with air GDD. Native Cirsium initiated new leaves or shoots before C. arvense shoot emergence in the spring, even when the native species’ growth was delayed during snow events, blizzards, or cooler temperatures of the springs of 2018 and 2019. In turn, Cirsium arvense shoots emerged approximately 1 to 3 wk before female H. litura adults began to lay eggs. As such, all native Cirsium plants had shoots available for H. litura oviposition. In the spring, there was no phenological separation between native Cirsium and C. arvense shoot emergence or initiation that would render native Cirsium species safe from H. litura attack. Based on the phenology of shoot emergence/initiation in the spring, all tested Cirsium species native to the upper Midwest have the potential to be within the ecological host range of H. litura. As such, we recommend further studies on H. litura search and acceptance behavior be determined in the field, outside a screen cage, to further define the ecological host range of H. litura. Although we are not aware of published accounts of H. litura accepting these native Cirsium species as hosts in the field, it would be prudent for managers to document any observed attack.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/inp.2023.3
Acknowledgments
We would like to acknowledge the following groups and individuals for providing funding, Cirsium seed, expertise, and labor for this project:
-
Chicago Botanic Garden: Kayri Havens
-
Minnesota State Climate Office: Peter Boulay
-
Minnesota Department of Natural Resources: Ross Hier, Welby Smith, Dan Wovcha, and Laura Van Riper
-
Prairie Legacy Inc: Kay Kottas
-
The Nature Conservancy, Ordway Prairie: Matt Graeve and Toni Aguilar
-
University of Minnesota: Mary Marek-Spartz, Brad Kinkaid, and Ryan Mentz, with additional help from Parker Sheaffer, Hugo Dos Santos Oliveira, Aryane Batista, Kylie Rich, and Lewis Sheaffer
-
U.S. Fish and Wildlife Service: Gregg Knutsen, Craig Mowry, and Jordon Young
-
Funding for this project was provided by the Minnesota Environment and Natural Resources Trust Fund as recommended by the Legislative-Citizen Commission on Minnesota Resources (LCCMR). No conflicts of interest have been declared.











