Introduction
Iodine deficiency (ID) causes a broad range of adverse effects on health resulting from the inadequate production of thyroid hormone that are collectively termed ID disorders (IDD). An ID during pregnancy increases the risk of spontaneous abortion, low birth weight and infant mortalityReference Pharoah, Buttfield and Hetzel1–Reference Dillon and Milliez5 as well as raising the risk of neuromotor, behavioural and cognitive impairment in the offspringReference Fierro-Benitez, Cazar, Stanbury, Rodriguez, Garces, Fierro-Renoy and Estrella6–Reference Azizi, Kalani, Kimiagar, Ghazi, Sarshar, Nafarabadi, Rahbar, Noohi, Mohajer and Yassai8. Even a mild-to-moderate ID during pregnancy can cause transient hypothyroidism, can increase the thyroid volume in mothers and their infants, and may impair cognitionReference Pedersen, Laurberg, Iversen, Knudsen, Gregersen, Rasmussen, Larsen, Eriksen and Johannesen9–Reference Fierro-Benitez, Cazar, Stanbury, Rodriguez, Garces, Fierro-Renoy and Estrella13. During infancy, an adequate amount of iodine is required for normal mental developmentReference Delange, Bourdoux, Chanoine, Ermans and Berger12, Reference Glinoer and Delange13; a meta-analysis estimated that ID in a population lowered mean IQ scores by 13.5 pointsReference Bleichrodt, Born and Stanbury14. It is for these reasons that IDD control programmes should focus on pregnancy, lactation and infancy, the stages in life when iodine requirements are at their greatest.
However, the assessment of iodine status during these life stages is challenging. The main indicator in a population of a response to salt iodisation is the median urinary iodine (UI) concentration15, but there are no established reference values for the median UI concentration during pregnancy, lactation or infancy. A median concentration of iodine in the range of 100–199 μg l− 1 in the urine of school-aged children and in non-pregnant, non-lactating adults indicates an adequate iodine intake and optimal iodine nutrition15. This range has not been validated to reflect an adequate iodine status in pregnant and lactating women or in infants and, as discussed below, if applied to these target groups is likely to underestimate the true degree of ID. Most large systematic surveys of ID have been done in school-aged children or in the general adult population, and have only rarely included pregnant or lactating women, or infants. Many of the studies on pregnant women and infants have comprised only small numbers, and sampling has rarely been representative, making it difficult to draw firm conclusions. Despite this, there is some concern over whether iodised salt programmes meet the needs of these life stages. Therefore, the general objectives of this paper are to:
Review the current recommended iodine intake in pregnancy, lactation and infancy.
Estimate the median UI concentration that reflects an adequate iodine intake during these life stages.
Use the estimates of an adequate UI concentration to assess whether current approaches to salt iodisation and iodine supplementation can control ID in pregnant and lactating women, and in their infants.
Methods used to estimate the requirement for iodine
There are three main ways of estimating how much iodine is required to meet daily needs.
Iodine turnover
The daily uptake and turnover of radioactive iodine can be used to estimate the requirement for iodine, provided that the subjects being tested have an adequate iodine status and are euthyroidReference Fisher and Oddie16–Reference Oddie, Fisher and Long18.
Iodine balance
Several studies have estimated iodine requirements from balance experimentsReference Dworkin, Jacquez and Beierwaltes19–Reference Vought and London23, but they have serious limitations. This is mainly because many ingested substances contain unrecognised iodine, so that a strict control of iodine intake is difficult to achieve. Because of the need to consider the iodine content of the thyroid gland, in addition to iodine intake and excretion, even during prolonged balance studies, an equilibrium may not clearly be establishedReference Dworkin, Jacquez and Beierwaltes19.
Urinary iodine concentration and thyroid size
Since >90% of dietary iodine eventually appears in the urineReference Vought and London23, Reference Nath, Moinier, Thuillier, Rongier and Desjeux24, the concentration of iodine in urine is an excellent indicator of recent iodine intake. An increase in thyroid size is the earliest clinical sign of impaired iodine nutrition and reflects an adaptation to the threat of hypothyroidism.
Definitions of recommendations for iodine intake
The United States Institute of Medicine (IOM) has proposed several categories of reference intake25. The recommended dietary allowance (RDA) is the average daily iodine intake sufficient to meet the iodine requirement of about 97% of healthy individuals in a particular life stage. It is intended to be used as a goal for the daily intake of iodine by individuals.
The RDA is based on the estimated average requirement (EAR) which is the daily iodine intake, defined by specific criteria, that meets the requirement of half of the healthy individuals in a particular life stage. It assumes a normal distribution of intake. The RDA is derived from the EAR after taking into consideration the estimated variability in individual requirements. The EAR is not meant to be used to define the intake of individuals, but can be used for groups.
The adequate intake (AI) can be given if there is not enough scientific evidence to calculate an EAR. For example, the AI of iodine during infancy is based on observed mean iodine intakes by healthy, full-term, breast-fed infants in areas where iodine intake is known to be sufficient. The AI is expected to meet or exceed the amount of iodine needed in almost all individuals in the specified population group, and can be used as a goal for individual intake.
According to the WHO, UNICEF and the ICCIDD, the recommended iodine intake is the amount estimated to cover the needs of nearly all healthy individuals in the specified life stage15.
Iodine requirements
Non-pregnant women
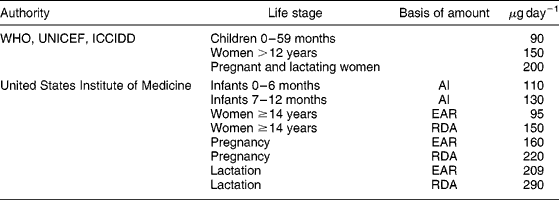
Studies of iodine turnover, radioiodine uptake by the thyroid and balance studies of euthyroid adults have suggested that the average daily requirement for iodine is 91–96 μg per dayReference Fisher and Oddie16, Reference Fisher and Oddie17, Reference Harrison20. There is no evidence to suggest that the average iodine requirement of adults varies with age25. Thus, the IOM have set the EAR of iodine by women ≥ 14 years at 95 μg per day25. The RDA, which is defined as the EAR plus twice the coefficient of variation of intake of the population and then rounded to the nearest 50 μg, is 150 μg day− 1 for females ≥ 14 years25. This amount agrees with the WHO, UNICEF and the ICCIDD recommended daily iodine intake for non-pregnant women of 150 μg per day15. These amounts are summarised in Table 1.
Pregnant women
The iodine requirement during pregnancy increases for three reasons: (1) an increase in the production of thyroxine (T4) by the mother to maintain her euthyroid state and transfer thyroid hormone to the foetus; (2) the transfer of iodine to the foetus, particularly in late gestation and (3) an increase in renal iodine clearance (RIC) by the motherReference Delange26, Reference Glinoer27. The amount of iodine accumulated by the infant at the time of delivery has been used to estimate the daily foetal iodine uptake. The average iodine content of the thyroid gland of a newborn is 50–100 μg, with >95% daily iodine turnoverReference Delange, Delange, Fisher and Glinoer28, Reference Delange, Ermans, Braverman and Utiger29. Balance studies have found that the average iodine retention of full-term infants is 6.7 μg kg− 1 per dayReference Delange, Bourdoux, Vo Thi, Ermans and Senterre30, so the mean retention of iodine by a healthy foetus with a weight of 3 kg would be about 22 μg day− 1. An estimated daily iodine uptake by the thyroid gland of about 75 μg day− 1 by the foetus and an EAR of 95 μg day− 1 for non-pregnant women would yield an EAR of 170 μg day− 1 during pregnancy. The balance studies of Delange et al. suggest an EAR of 22+95 = 117 μg per dayReference Delange, Bourdoux, Vo Thi, Ermans and Senterre30. Dworkin et al. found that five pregnant women were in iodine balance when consuming about 160 μg day− 1, with no significant differences pre- and post-partumReference Dworkin, Jacquez and Beierwaltes19. Several authors have measured the thyroid volume during pregnancy and correlated it with the UI concentration and the effects of iodine supplements. In the studies of Romano et al. Reference Romano, Jannini, Pepe, Grimaldi, Olivieri, Spennati, Cappa and D'Armiento31 and Pedersen et al. Reference Pedersen, Laurberg, Iversen, Knudsen, Gregersen, Rasmussen, Larsen, Eriksen and Johannesen9, total daily iodine intakes of about 200 and 250–280 μg, respectively, during pregnancy prevented an increase in thyroid volume, while in the study of GlinoerReference Glinoer, De Nayer, Delange, Lemone, Toppet, Spehl, Grun, Kinthaert and Lejeune10, a total daily iodine intake of about 150 μg was insufficient to prevent an increase in thyroid size. On the basis of these data, the IOM set the EAR at 160 μg day− 1 for pregnancy in women ≥ 14 years, and set the RDA, estimated to be 140% of the EAR rounded to the nearest 10 μg, at 220 μg per day25. The WHO, UNICEF and the ICCIDD recommend a daily iodine intake of 200 μg day− 1 for pregnant women, a value 10% lower than the RDA15 (see Table 1).
Lactating women
If a lactating woman produces an average of 0.78 and 0.60 l of milk per day during the first and second 6 months of infancy, respectively25, and if the mean breast milk iodine concentration (BMIC) is 146 μg l− 1 in iodine-sufficient women in the USA, the average daily loss of iodine in breast milk has been estimated to be about 114 μg per day25. When this amount is added to the EAR of non-pregnant women of 95 μg day− 1, the EAR for lactating women ≥ 14 years is estimated by the IOM to be 209 μg per day25. The RDA is thus 140% of the EAR rounded to the nearest 10 μg, which is 290 μg day− 1 of iodine. The WHO, UNICEF and the ICCIDD recommend a daily iodine intake of 200 μg day− 1 by lactating women15, which is similar to the EAR but 30% lower than the RDA (see Table 1).
Infancy (0–12 months)
Since no functional criteria are available that reflect iodine intake in infants, recommended intakes are based on the mean iodine intake of healthy full-term infants fed on human milk. The IOM based their recommendation on the median BMIC of women in the United States of America (USA) in the early 1980s, which was 146 μg per l25. Since the iodine intake of the population of the USA was relatively high at this time32, this BMIC is at the upper end of the range of 78–167 μg l− 1 reported for women in iodine-sufficient countries (see later discussion). Based on estimates of the mean daily volume of breast milk, the mean amount of iodine secreted in human milk is estimated to be about 115 μg per day25. Balance studies of full-term infants given 20 μg kg− 1 day− 1 of iodine found that the total amount excreted was 12.7 μg kg− 1 day− 1 and the amount retained was 7.3 μg kg− 1 per dayReference Delange, Bourdoux, Vo Thi, Ermans and Senterre30. If the reference body weight of an infant aged 6 months is 7 kg25, the daily amount of iodine excreted by an infant in positive balance is 90 μg. Considering these data, the recommended AI of iodine for infants aged 0–6 and 6–12 months has been set at 110 and 130 μg day− 1, respectively, by the IOM25. The WHO, UNICEF and the ICCIDD recommend a daily iodine intake of 90 μg day− 1 for infants15, which is 20–30% lower than the RDA (see Table 1).
Iodine intakes in pregnancy and the effects of iodine supplementation
Pregnancy
The WHO, UNICEF and the ICCIDD recommend using the median UI concentration to assess the iodine nutrition of populations15. The daily iodine intake can be extrapolated from the UI if it is assumed that girls aged 7–15 years produce 0.9 ml urine h− 1 per kgReference Hollowell, Staehling, Hannon, Flanders, Gunter, Maberly, Braverman, Pino, Miller and Garbe33, and adult women produce about 1.5 l per day34. If it is assumed that the mean bioavailability of iodine is 92%, the recommended daily iodine intakes during pregnancy of 20015 and 220 μg25 would correspond to a UI concentration of 120–135 μg l− 1 in pregnancy. As pregnancy may occur in adolescence, particularly in developing countries, a 15-year-old girl weighing some 50 kg with a daily iodine intake of 200 or 220 μg would have a UI concentration of 170–190 μg l− 1.
However, during pregnancy, it may be less valid to estimate the iodine intake from the UI concentration due to an increase in the glomerular filtration rateReference Mattsson and Lindstrom35 and, possibly, an increase in RICReference Larsson and Victor36. If the RIC increases during pregnancy, the daily iodine intake extrapolated from the UI concentration in pregnancy would be lower than that in non-pregnancy. However, the evidence for an increase in RIC and a consequent decrease in the plasma inorganic iodide (PII) concentration during pregnancy is equivocal. The study of Aboul-Khair et al. suggested an increase in RIC using an indirect methodReference Larsson and Victor36, while Liberman et al. directly measured the PII concentration and reported no significant difference in PII or UI concentration both pre- and post-partum in 16 women living in a population with a high iodine intakeReference Dafnis and Sabatini37. The iodine balance study of Dworkin et al. also found no differences in the UI concentration both pre- and post-partumReference Dworkin, Jacquez and Beierwaltes19. Owing to the lack of clear data, it is uncertain whether pregnancy per se significantly increases the UI concentration. Therefore, in this review, the above extrapolations from UI concentration to daily iodine intake proposed by the IOM25 were considered valid, and the recommended daily intakes of 200–220 μg of iodine15, 25 were considered to correspond to a median UI concentration of 125–135 μg l− 1 in adult pregnant women, although a higher median of about 170–180 μg l− 1 may be more appropriate if pregnancy occurs in early adolescence. Although more data are clearly needed, a median UI concentration in pregnant women ≥ 140 μg l− 1 has been used in this review as an indicator of adequate iodine intake.
Table 2 compares the UI concentration of pregnant women with the general population in countries with iodine intakes ranging from excessive to deficient. Most studies analysed spot urine samples and few had an adequate power to classify iodine status based on a median UI concentration from such samplesReference Aboul-Khair, Crooks, Turnbull and Hytten38. Few studies have compared the UI concentration of pregnant women with a control group of non-pregnant women. As shown in Table 2, the UI concentration in pregnant women and in the general population generally appear to be similar. However, this may be due to a preponderance of studies in areas where there is low or marginal iodine sufficiency, as several studies in iodine-sufficient countries have found a significantly higher median UI concentration in pregnant womenReference Liberman, Pino, Fang, Braverman and Emerson39–Reference Kung, Lao, Chau, Tam and Low43. All of the large studies in iodine-sufficient countries have reported a median UI concentration in pregnant women of ≥ 140 μg l− 1. This includes countries where all salt is iodised (Switzerland and Iran), and countries where dietary iodine comes from either iodised salt or from other food sources (USA, UK, Sri Lanka and Sweden). If a median UI concentration ≥ 140 μg l− 1 in pregnant women is taken to represent a mean iodine intake ≥ 200 μg day− 1, then it appears that the iodine status of pregnant women is adequate in countries where the general population is adequately covered either wholly or partially by iodised salt programmes.
Table 2 Urinary iodine concentrationReference Pharoah, Buttfield and Hetzel1–Reference Chaouki and Benmiloud3 of pregnant women in longitudinal (L) and cross-sectional (C) studies and, if available, in the general population or in non-pregnant controls. The urinary iodine concentration in the post-partum period is shown if a longitudinal study continued after delivery.

1μg day− 1; 2μg l− 1; 3μg g− 1 creatinine.
Studies of thyroid size in pregnant women support these data. As shown in Table 3, in countries affected by mild or moderate ID (Ireland, Germany, Belgium, Italy and Denmark), the thyroid volume increases 15–31% during pregnancy, while in iodine-sufficient countries (Finland, USA and The Netherlands), there is little or no increase in thyroid volume during pregnancyReference Hess, Zimmermann, Torresani, Bürgi and Hurrell44. This suggests that iodine status is adequate in pregnant women in iodine-sufficient countries with two important caveats. First, the question remains unanswered of whether RIC changes enough during pregnancy to confound the usual estimation of iodine intake based on the median UI concentration. Second, iodine-containing supplements taken during the prenatal period may have led to an increased median UI concentration in pregnant women living in countries with an iodised salt programmeReference Zimmermann, Aeberli and Bürgi45.
Table 3 Changes in thyroid size measured by ultrasound during pregnancy in iodine-sufficient and -deficient countries.

Iodine supplements during pregnancy and their impact on iodine status
In Europe, 13–50% of women receive iodine-containing supplements during pregnancyReference Zimmermann, Aeberli and Bürgi45. In a study of 511 pregnant Swiss women in 1999, although 70% received a prenatal supplement, only 13% received a supplement containing iodine; the median UI concentration in the women taking iodine-containing supplements was 194 vs. 130 μg l− 1 in non-supplemented womenReference Smyth42. After a 33% increase in the concentration of iodine in salt, a study of 276 pregnant Swiss women in 2004 reported a median UI concentration of 254 μg l− 1; there was no significant difference in the median UI concentration of women taking iodine-containing supplements compared with those who were notReference Kung, Lao, Chau, Tam and Low43.
In a recent study of 109 pregnant German women, the median UI concentration was 181 μg g− 1 creatinine (cr.); 58% were taking iodine-containing supplements and had a significantly higher UI concentration (204 μg g− 1 cr.) than women who were not (148 μg g− 1 cr.)Reference Berghout and Wiersinga46.
In earlier studies of women in DenmarkReference Zimmermann and Delange47 and GermanyReference Buhling, Schaff, Bertram, Hansen, Muller, Wascher, Heinze and Dudenhausen48, the median UI concentration was significantly higher in pregnant women supplemented with iodine than women not supplemented: 58 vs. 35 μg day− 1 in Denmark and 85 vs. 59 μg day− 1 in Germany.
In Hungary, about 50% of pregnant women receive a supplement containing iodine. In those women taking supplements containing ≥ 150 μg iodine per day, the median UI concentration was 115–130 μg g− 1 cr., compared with 57–68 μg g− 1 cr. in women who were not supplementedReference Nohr, Laurberg, Borlum, Pedersen, Johannesen, Damm, Fuglsang and Johansen49.
In a cross-sectional study in Denmark, the use of iodine supplements of 150 μg day− 1 and thyroid function were assessed in 144 pregnant women and their newborns50. At full term, the median UI concentration of supplemented mothers was 60 μg l− 1 compared with 35 μg l− 1 in unsupplemented mothers. In the supplement group, the concentration of thyroglobulin (Tg) in both maternal and cord blood was significantly lower, and the free T4 concentration was significantly higher, than in unsupplemented womenReference Hess, Zimmermann, Torresani, Bürgi and Hurrell44. Although the maternal TSH concentration was lower in the supplement group, the cord TSH concentration was 27% higher in the supplement group50, suggesting that in iodine-deficient areas, the foetal thyroid gland may be particularly sensitive to the inhibitory effect of iodine.
Table 4 shows results from six randomised, controlled trials of iodine supplementation involving 450 pregnant European women with mild-to-moderate ID.
Table 4 Randomised, controlled trials of iodine supplementation during pregnancy in mild-to-moderate iodine-deficient countries of Europe and the urinary iodine concentration before and after treatment.

1μg g− 1 cr.; 2μg l− 1; 3μg day− 1.
Romano et al. Reference Romano, Jannini, Pepe, Grimaldi, Olivieri, Spennati, Cappa and D'Armiento31 gave 120–180 μg iodine as iodised salt daily or gave a placebo control beginning in the first trimester to healthy pregnant women (n = 35, median UI concentrations in the two groups 31 or 37 μg l− 1). In the group treated with iodine, the median UI concentration increased threefold and thyroid volume did not changeReference Romano, Jannini, Pepe, Grimaldi, Olivieri, Spennati, Cappa and D'Armiento31. In the controls, there was no change in the UI concentration, and had a 16% increase in thyroid volumeReference Romano, Jannini, Pepe, Grimaldi, Olivieri, Spennati, Cappa and D'Armiento31. Treatment had no effect on the maternal TSH concentration.
Pedersen et al. Reference Pedersen, Laurberg, Iversen, Knudsen, Gregersen, Rasmussen, Larsen, Eriksen and Johannesen9 randomised 54 pregnant women to receive either 200 μg iodine per day as a solution of potassium iodide or no supplement from 17 weeks of pregnancy to full term. The median UI concentration increased from 55 to 90–110 μg l− 1 in the treated group. The thyroid volume of women increased 16% in the treated group compared with 30% in the controlsReference Pedersen, Laurberg, Iversen, Knudsen, Gregersen, Rasmussen, Larsen, Eriksen and Johannesen9. The concentration of Tg and TSH in women, and the concentration of cord Tg, were significantly lower in the treated group than in the controlsReference Pedersen, Laurberg, Iversen, Knudsen, Gregersen, Rasmussen, Larsen, Eriksen and Johannesen9. No significant differences were found between groups in terms of the concentration of T4, T3 and fT4 in maternal or cord bloodReference Pedersen, Laurberg, Iversen, Knudsen, Gregersen, Rasmussen, Larsen, Eriksen and Johannesen9.
In a double-blind, placebo-controlled trial, Glinoer et al. Reference Glinoer, De Nayer, Delange, Lemone, Toppet, Spehl, Grun, Kinthaert and Lejeune10 supplemented 120 pregnant women who had a median UI concentration of 36 μg l− 1 and biochemical criteria of excess thyroid stimulation, with 100 μg iodine per day or gave a placebo control from around 14 weeks of pregnancy to full term. The treatment had no significant effect on the concentration of maternal or cord T3, fT4 and the T3/T4 ratioReference Glinoer, De Nayer, Delange, Lemone, Toppet, Spehl, Grun, Kinthaert and Lejeune10. The treated women had a significantly higher UI concentration, a smaller thyroid volume and a lower concentration of TSH and Tg when compared with controlsReference Glinoer, De Nayer, Delange, Lemone, Toppet, Spehl, Grun, Kinthaert and Lejeune10. The newborn children of the treated group also had significantly higher UI concentration, a smaller thyroid volume and a lower Tg concentrations when compared with controlsReference Glinoer, De Nayer, Delange, Lemone, Toppet, Spehl, Grun, Kinthaert and Lejeune10. There was a 14% increase in the cord serum TSH concentration in the treated group, but it was not statistically significant.
Liesenkötter et al. Reference Mezosi, Molnar, Jakab, Balogh, Karanyi, Pakozdy, Nagy, Gyory, Szabo, Bajnok, Leovey, Kakuk and Nagy51 reported a quasi-randomised, controlled trial of 230 μg iodine per day given from the eleventh week of pregnancy to full term in 108 pregnant women with a median UI concentration of 53 μg g− 1 cr. and a goitre rate of 42.5%. The median UI concentration increased to 104 μg g− 1 cr. in the treated group, and the median thyroid volume was significantly lower in the newborns of the treated women compared with controls (0.7 vs. 1.5 ml, respectively)Reference Mezosi, Molnar, Jakab, Balogh, Karanyi, Pakozdy, Nagy, Gyory, Szabo, Bajnok, Leovey, Kakuk and Nagy51. Treatment had no significant effect on the concentration of maternal TSH, T3, T4, Tg or on the thyroid volume, and had no effect on the concentration of TSH in the newbornReference Mezosi, Molnar, Jakab, Balogh, Karanyi, Pakozdy, Nagy, Gyory, Szabo, Bajnok, Leovey, Kakuk and Nagy51.
In a placebo-controlled, double-blind trial, Nohr et al. Reference Nohr and Laurberg52 gave to 66 pregnant women who had antibodies to anti-thyroid peroxidase (TPO-Ab) a multi-nutrient supplement containing 150 μg iodine per day or a placebo control from the eleventh week of pregnancy to full term. The median UI concentration was significantly higher in the treated women at full term than in the controls, but there were no differences in the concentration of maternal TSH, fT4 or Tg between groupsReference Nohr and Laurberg52. There was no difference in the prevalence of anti-thyroglobulin antibodies (Tg-Ab) or TPO-Ab, and no differences between groups in the prevalence or severity of post-partum thyroid dysfunction (PPTD), defined as an abnormal TSH concentration in the post-partum period. However, 12/20 (60%) treated women developed PPTD compared with 11/24 (46%) controls, and failure to detect a significant difference in the prevalence of PPTD may have been due to a type II error.
In a prospective, randomised, open-label trial, Antonangeli et al. Reference Liesenkötter, Göpel, Bogner, Stach and Grüters53 supplemented 67 pregnant women who had a median UI concentration of 74 μg g− 1 cr. with 50 or 200 μg iodine per day from 18–26 weeks of pregnancy to 29–33 weeks. The median UI concentration was significantly higher in group given 200 μg iodine per day than in the group given 50 μg iodine per day (230 vs. 128 μg g− 1 cr.).Reference Liesenkötter, Göpel, Bogner, Stach and Grüters53 However, there were no differences between groups in the concentration of maternal fT4, fT3, TSH and Tg or in thyroid volume, and no differences in the prevalence of TPO-Ab, Tg-Ab or PPTD.
In summary, in all of these trials, iodine supplements significantly increased the maternal UI concentration. The doses of iodine varied between 50 and 230 μg day− 1, and the data indicate no clear dose–response relationship for the concentration of UI, TSH, Tg, thyroid hormones or in terms of thyroid volume. Iodine supplements during pregnancy generally appear to be safe; there was no increase in the trials the prevalence of thyroid autoimmunity in mothers, or in the prevalence or severity of PPTD. However, the sample sizes were small and more data, particularly from TPO-Ab+ women, would be valuable. In three of the five trials that measured the thyroid volume of mothers, supplementation was associated with a significantly reduced thyroid size. The studies also suggest that an increase in newborn thyroid volume and the concentration of Tg can be prevented or minimised by iodine supplementation. Although less consistent, the data also suggest that the concentration of maternal TSH is generally lower (within the normal reference range) with supplementation. Supplementation has little or no impact on the concentration of total or free thyroid hormones in mothers and newborn children. There are no clinical data on the effect of supplementation on birth weight or prematurity, and no data on long-term outcomes, such as maternal goitre, thyroid autoimmunity or child development.
Iodine intakes during lactation and urinary iodine and breast milk iodine concentrations
Since the mammary gland is able to concentrate iodine, the iodine supply to the newborn via the breast milk may be maintained even in the face of a maternal IDReference Nohr, Jorgensen, Pedersen and Laurberg54, Reference Antonangeli, Maccherini, Cavaliere, Di Giulio, Reinhardt, Pinchera and Aghini-Lombardi55. This may help explain why, in areas where there is an ID, BMIC is often greater than expected based on the UI concentration of lactating mothersReference Antonangeli, Maccherini, Cavaliere, Di Giulio, Reinhardt, Pinchera and Aghini-Lombardi55, Reference Vermiglio, Lo Presti, Finocchiaro, Battiato, Grasso, Ardita, Mancuso and Trimarchi56. The BMIC is strongly influenced by the mothers' iodine intakeReference Kurtoglu, Akcakus, Kocaoglu, Gunes, Budak, Emre Atabek, Karakucuk and Delange58.
In the USA, Gushurst et al. Reference Dorea57 found that the median BMIC in women who consumed uniodised salt was 113 μg l− 1, while the median concentrations in women who consumed low or high amounts of iodised salt were 143 or 270 μg l− 1, respectively.
Several studies have compared the BMIC before and after supplementation with iodised oil or potassium iodide, or with untreated controls.
Pretell et al. Reference Gushurst, Mueller, Green and Sedor59 injected women with 950 mg iodine as iodised oil. The median BMIC at 18–36 months post-partum was 70 μg l− 1 compared with 2 μg l− 1 in mothers not receiving treatment.
In Algeria, Chaouki and BenmiloudReference Semba and Delange60 gave 240 mg iodine as oral iodised oil either 1–3 months before pregnancy or in the first or third trimester. At delivery and 6 month post-partum, the mean BMIC ranged from 520 to 559 μg l− 1 and from 307 to 346 μg l− 1, compared with 307 and 260 μg l− 1 in untreated women.
In 147 Danish mothers, the median BMIC on the fifth day post-partum was significantly higher (57 μg l− 1) in those receiving supplementation with 150 μg day− 1 of oral iodine, compared with women who were not supplemented (34 μg l− 1)Reference Pretell, Moncloa, Salinas, Kawano, Guerra-Garcia, Gutierrez, Beteta, Pretell and Wan61.
In Germany, 60 mothers who received 200 μg day− 1 of oral iodine had a significantly higher mean iodine concentration in breast milk (76 μg l− 1) than untreated controls (55 μg l− 1)Reference Chaouki and Benmiloud62.
Although increasing the iodine intake of iodine-sufficient women can further increase BMICReference Dorea57, an iodine intake by an infant that is greater than requirements will simply be excreted in the urine. Thus, iodine requirements during lactation should be based on infant balance studies rather than on the measured but variable amount of iodine excreted in breast milk by women in iodine-sufficient countries. Based on the balance studies of Delange et al. Reference Delange, Bourdoux, Vo Thi, Ermans and Senterre30, the full-term infant's requirement for iodine is about 90 μg day− 1, and this is the intake recommended by the WHO, UNICEF and the ICCIDD15. Based on a mean breast milk volume of 0.78 l day− 1 in the first 6 months of infancy25, and assuming that 95% of the iodine in breast milk is absorbed, a BMIC of ≥ 120 μg l− 1 should cover the infant's iodine requirement of 90 μg day− 1 until weaning foods are begun. Most infants begin weaning by the second half of the first year of life, and some of the iodine requirement during that period will be met from weaning foods. Semba and DelangeReference Kurtoglu, Akcakus, Kocaoglu, Gunes, Budak, Emre Atabek, Karakucuk and Delange58 proposed that a potential indicator of iodine status in a population could be the proportion of lactating women whose BMIC is ≥ 100 μg l− 1.
Table 5 shows the BMICs of women living in areas where people are iodine sufficient or have mild-to-severe ID. Among the iodine-sufficient countries, the BMICs of women in Switzerland and Sweden are less than the concentration required to meet an infant's needs (100–120 μg l− 1), while they are at, or above, this concentration in the Netherlands, the USA and Japan.
Table 5 The mean or median breast milk iodine concentration of women in iodine-sufficient and -deficient countries.

Although the requirement for iodine of a mother is large at 200–290 μg day− 1, after accounting for iodine losses in breast milk, the median UI concentration of lactating women that indicates adequate iodine nutrition should be similar to that of non-pregnant, non-lactating women, i.e. 100–199 μg per l15. Table 6 shows the UI concentrations reported from studies of lactating women in iodine-sufficient and -deficient countries. There are few data, the numbers of women in most studies are small, and breast-feeding status was often not clearly documented. The studies generally report a median UI concentration in lactating women that is similar to the general population, but almost all reports are from iodine-deficient countries, and show median UI concentrations < 100 μg l− 1.
Table 6 Urinary iodine concentrationReference Pharoah, Buttfield and Hetzel1–Reference Chaouki and Benmiloud3 in lactating women and in the general population or in non-lactating controls in countries of different iodine status.

1μg day− 1; 2μg l− 1; 3μg g− 1 creatinine.
Iodine intakes during infancy
During infancy, the mean volume of breast milk consumed is about 0.78 l day− 1 over the first 0–6 months25 and some 60–65% of ingested water is excreted by the kidneyReference Nohr, Laurberg, Bortum, Pedersen, Johannesen, Damm, Fuglsang and Johansen63. Although there is a large day-to-day variation, the volume of urine daily in early infancy is estimated to be 30–55 ml kg− 1 body weightReference Nohr, Laurberg, Bortum, Pedersen, Johannesen, Damm, Fuglsang and Johansen63–Reference Greenbaum, Behrman, Kliegman and Jensen65 and the reference body weight for a child aged 0–6 months is 7 kg and for a child aged 6–12 months is 9 kg25. Based on these data, the volume of urine is estimated to be 0.4–0.5 l day− 1 in early infancy. Therefore, considering the requirement for iodine of 90 μg per day15, the median UI concentration indicating optimal iodine nutrition in infancy is estimated to be in the range of ≥ 180–220 μg per lReference Delange26.
Delange et al. Reference Thüroff and Schulte-Wissermann66 supplemented healthy infants and children aged 6 months to 3 years in Belgium who had a median UI concentration at baseline of 101 μg l− 1, with an dose of 90 μg day− 1 of iodine. After about 30 days of supplementation, the geometric mean UI concentration was beginning to plateau at 220–240 μg l− 1.
Table 7 shows the median UI in full-term newborns in countries with iodine sufficiency and varying degrees of ID. In general, among the iodine-sufficient countries, the median UI concentration varies from 96 to 167 μg l− 1, well below the estimated median UI concentration of ≥ 180–220 μg l− 1 that indicates an adequate iodine intake. More data are clearly needed, but the evidence available suggests that the iodine intake of many infants, even in countries designated as iodine sufficient, may be suboptimal.
Table 7 The median urinary iodine concentration of full-term infants in iodine-sufficient and -deficient countries (1=Mean).