Introduction
Clostridium difficile infection (CDI) is the most common cause of diarrhoea in hospitalised patients and the most common cause of death due to gastroenteritis in the USA [Reference Kelly and LaMont1, Reference Hall2].
The pathogenesis of CDI includes disruption of the host microbiota, usually with broad-spectrum antibiotics, proliferation of toxins after germination of C. difficile in the colon and lack of antibody response to the infection [Reference Abt, McKenney and Pamer3]. Patients with chronic liver disease (CLD) are especially prone to CDI due to altered immune dysfunction and frequent antibiotic use causing disturbances in gut microbiota [Reference Garcia-Tsao and Surawicz4, Reference Bajaj5]. Patients with CLD tend to display less overt signs and symptoms of infection most likely due to underlying immune dysfunction [Reference Trifan6, Reference Bonnel, Bunchorntavakul and Reddy7]. This may complicate treatment decisions as most severity risk stratification measures for CDI are based on host response to infection such as fever or leucocytosis [Reference Singal, Salameh and Kamath8–Reference Cohen10]. Two previous studies have shown that CDI increases mortality, length of stay and hospitalisation costs in CLD patients [Reference Bajaj5, Reference Sundaram11]. However, a comparator CDI population without CLD were not included in these previous studies. The purpose of this study was to assess resource utilisation, mortality and disease presentation among CLD patients with CDI. To accomplish these aims, we used data available from the nationwide inpatient sample and conducted a medical chart review at a tertiary care medical centre with a large CLD population. In separate analyses, we assessed outcomes of CLD patients with CDI to comparator groups including CLD patients without CDI and CDI patients without CLD to better understand the impact of CDI on the CLD population.
Materials and methods
Nationwide Inpatient Samples (NIS)
Data sources
Data from the 2009 NIS, the largest publicly available all-payer inpatient database in the USA was used to provide nationally representative estimates of CDI incidence, healthcare resource utilisation and mortality in the CLD population [Reference Garber12]. The NIS includes all discharges from 20% of community hospitals from participating short-term, non-Federal, general and other hospitals. The sample is weighted to produce national estimates and represents over 97% of the US population. All data from NIS are de-identified.
Patient identification
The ICD-9 code 008.45 was used to identify patients with CDI [Reference Pechal13]. Patients with CLD were identified using a previously validated set of ICD-9 codes and included one of the following diagnoses: hepatitis B virus, hepatitis C virus, alcohol-induced liver disease, Wilson's disease, autoimmune hepatitis and non-alcoholic fatty liver disease [Reference Nehra14]. The Deyo Modification of the Charlson Comorbidity Index was used as a measure of chronic disease status [Reference Chu, Ng and Wu15, Reference Deyo, Cherkin and Ciol16].
Case and comparison groups
In separate analyses, patients with CDI and underlying CLD (study group) were compared with those with underlying CLD only (control group 1) and patients with CDI only (control group 2).
NIS dataset analysis plan
For statistical analysis of the NIS dataset, incidence rates and 95% confidence intervals for CLD and CDI vs. the entire NIS dataset population were calculated. Separate analyses were conducted to compare CLD patients with CDI vs. the two comparator groups (CLD patients without CDI and CDI patients without CLD). Demographic and comorbidity risk factors were assessed for patients with CLD and CDI vs. comparators. To determine the contribution of CDI to in-patient mortality, length of hospital stay and hospital costs in patients with CLD vs. comparators, mixed-effect general linear models accounting for the hospital as a first-level variable and adjusting for demographic (age in decade-long intervals, gender, race) and socioeconomic characteristics (primary payer and income level) were constructed. In two separate analyses, patients with CLD and CDI were matched with each comparator group 1:1 based on age and Charlson Comorbidity Index using a nearest-neighbour greedy matching algorithm [Reference Austin17]. In this cohort, mixed effect general linear models were constructed as above and included CDI and an interaction term for CDI and CLD as independent variables. Adjusted odds ratios (aOR) were reported adjusting for demographic and socioeconomic differences. The reported difference refers to the β-coefficient for the interaction term.
Tertiary care evaluation of patient presentation
Study design
As the NIS dataset does not include certain types of granular data including disease presentation and treatment, a retrospective case–control observational analysis of patients with CDI, CLD or both who were admitted to a 650-plus bed university-affiliated tertiary care hospital between 2006 and 2016 was conducted. Patients were identified utilising pre-existing hospital and research databases [Reference Garey18]. Patient medical records were reviewed for demographic and hospitalisation variables with a specific focus on disease presentation and treatment.
Tertiary care patient population
The study population consisted of adult hospitalised patients (⩾18 years of age) with CLD and CDI. CDI was defined as diarrhoea (⩾3 stools in a 24-h period) plus a positive C. difficile diagnostic test plus at least one of the following clinical parameters (diarrhoea, fever, leucocytosis (WBC >10 000 cells/ml3), nausea, anorexia or abdominal pain). C. difficile diagnostic test was ordered due to suspicion of CDI by the primary medical team. CLD was classified as at least one of the following: hepatitis B virus, hepatitis C virus, alcohol-induced liver disease, Wilson's disease, autoimmune hepatitis and non-alcoholic fatty liver disease. Patients with CDI and underlying CLD (study group) were compared with those with underlying CLD only (control group 1) and patients with CDI only (control group 2). Patients were excluded if they had CDI following liver transplantation, CLD due to drug-induced causes (except for alcohol consumption), or haemochromatosis. For patients with multiple occurrences of CDI, only data from the first episode were gathered. Leftover stool samples ordered as part of normal clinical care were collected after all clinical tests had been performed from all patients with CDI as previously described [Reference Aitken19]. Briefly, C. difficile toxin-positive stool samples were plated onto cefoxitin–cycloserine–fructose agar plates and incubated anaerobically for 48–72 h. The growth of toxigenic C. difficile was confirmed using multiplex PCR to determine the presence of toxins A (tcdA) and B (tcdB) and strain typed using fluorescent PCR ribotyping [Reference Alam20]. The study including analysis of both datasets was approved by the Committee for the Protection of Research Subjects at the University of Houston.
Statistical analysis
SAS version 9.3 (SAS Institute, Cary, NC), Stata v13.1 (StataCorp, College Station, TX), or SPSS 24.0 software were used for all analyses. Continuous variables were expressed as means ± s.d. (normal distribution) or median and quartiles (non-normal distribution such as the Charlson Comorbidity index) and analysed with the Student t-test/ANOVA or Mann–Whitney U/Kruskal–Wallis test, as appropriate. χ 2 or Fisher exact tests were utilised for categorical data. Univariate analysis was performed for each variable and those variables found to have a P-value of <0.2 were then included in the multiple regression analysis. Odds ratio (OR) with 95% confidence intervals were calculated from the regression analysis and P-value of <0.05 was considered to be statistically significant.
Results
NIS dataset results
Comparison of CLD patients with CDI vs. other patient populations with CDI
A total of 7 802 351 discharges were analysed, of whom 114 108 (1.46%, 95% CI 1.39–1.53) had CLD. The overall incidence rate of CDI was 85.2/10 000 discharges (95% CI 81.3–89.3). Among CLD patients, the CDI incidence rate was 189.4/10 000 discharges (95% CI 175.4–204.5) as compared with 83.7/10 000 (95% CI 79.9–87.7) in patients without CLD (P < 0.001). CLD patients with CDI had higher likelihood of in-hospital mortality (18.6% vs. 8.8%; aOR 2.02, 95% CI 1.50–2.73; P = 0.003), longer hospital length of stay (1.19 days, 95% CI 0.39–2.00; P = 0.004) and increased total costs ($8632, 95% CI $6097–11 167; P < 0.001) compared with matched non-CLD with CDI.
Comparison of CLD patients with CDI vs. CLD patients without CDI
Compared with patients with CLD without CDI, patients with CLD and CDI were older (58.3 ± 13.2 vs. 60.4 ± 14.7; P < 0.001), more likely to be female (44.0% vs. 38.6%; P < 0.001) and had a statistically higher Charlson Comorbidity Index (4 (2–5) vs. 4 (3–5); median (IQR); P < 0.001). CLD patients with CDI had significantly higher likelihood of in-hospital mortality compared with matched CLD patients without CDI (aOR 2.29, 95% CI 1.90–2.76; P < 0.001). An average attributable increase in length of stay of 9.10 days (95% CI 8.55–9.65; P < 0.001) and $28 940 (95% CI $26 900–30 979; P < 0.001) in additional total cost were observed in CLD patients with CDI.
Tertiary care results
Comparison of CLD patients with CDI vs. other patient populations with CDI
A total of 225 hospitalised patients were identified including patients with both CLD and CDI (n = 41), patients with CLD without CDI (n = 73) and patients with CDI but not CLD (n = 111). Patients with CLD averaged 59 ± 10 years (64% male) of which the most common aetiology for the liver disease were hepatitis B or C virus (44%), alcohol-induced (31%) or non-alcoholic fatty liver disease (25%). The majority of patients had CLD-related complications including overt or medically-treated hepatic encephalopathy (70%), ascites (64%), oesophageal varices (57%), or prior spontaneous bacterial peritonitis (26%). The majority of CLD patients had risk factors related to CDI including previous use of antibiotics within the last 30-days (100%), current use of proton pump inhibitors (72%), and continued use of non-C. difficile antibiotics after diagnosis of CDI (69%). Demographics, aetiology, CLD-related complications, or CDI-related risk factors did not differ between patients with CLD regardless of CDI status.
Comparison of CLD patients with CDI vs. CLD patients without CDI
Demographic, CDI-related risk factors, CDI-related severity variables and CDI-related outcomes are shown in Table 1. Compared with patients with CDI but without CLD, patients with CDI and CLD had a significantly higher Charlson Comorbidity Index (P < 0.0001). CDI-related risk factors were similar between the two groups. Ribotype data were available from 66 patients. Ribotype distribution was similar between the two groups which were most commonly ribotypes F014–020 (23%), F106 (14%), F002 (11%), F027 (5%), F056 (5%) and F103 (5%). CDI severity outcomes were also similar between the two groups with no differences noted for ICU admissions, increased creatinine, leucocytosis, or fever. Although not powered to show statistical significance, 30-day mortality was increased by approximately 2 fold in CLD patients who experienced CDI.
Table 1. Patient demographics and baseline characteristics from tertiary care medical centre analysis of patients with CDI and chronic liver disease (CLD) compared with other hospitalised patients with CDI
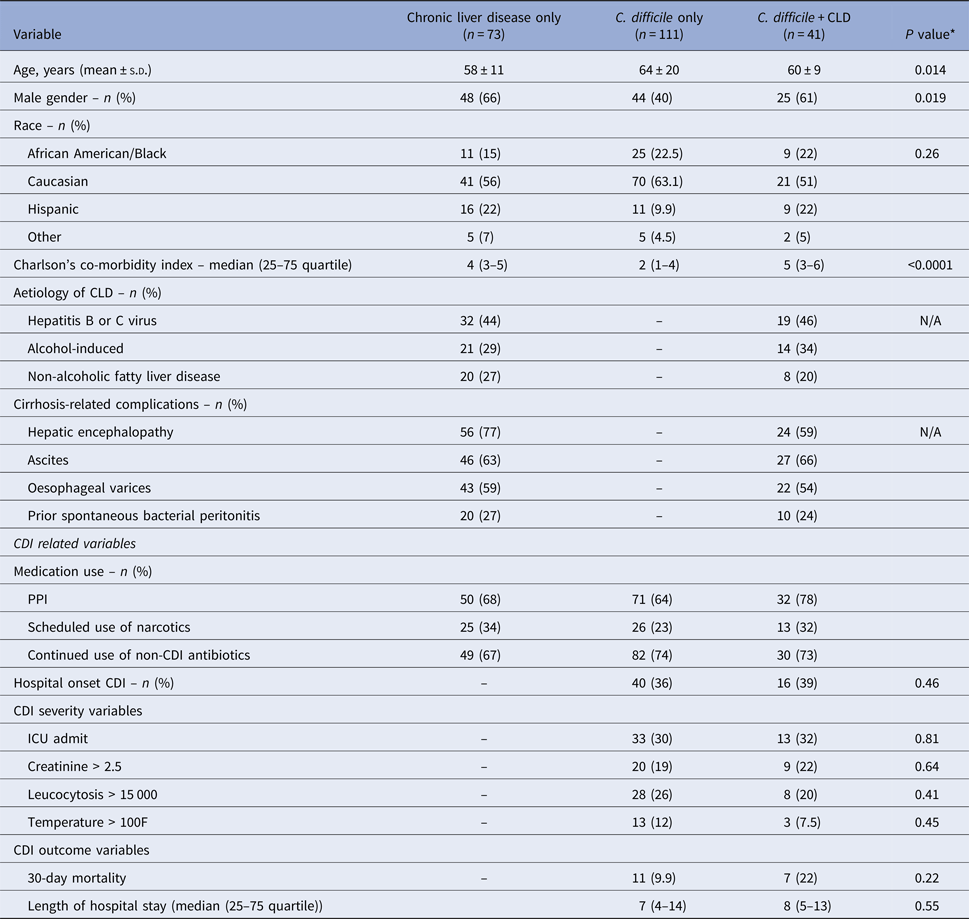
* P value comparison between patients with C. difficile only and C. difficile + Chronic liver disease. Tests between patients with Chronic Liver Disease only and C. difficile + Chronic liver disease were non-significant.
Discussion
CLD patients are at increased risk of bacterial infections including C. difficile infection due to underlying immunodeficiency and frequent hospitalisations. For CDI specifically, CLD associated immune dysfunction leads to an immunocompromised state due to altered immune response and dysbiosis of gut microbiota [Reference Trifan6, Reference Bonnel, Bunchorntavakul and Reddy7]. Previous studies have shown that CDI in patients with CLD results in increases mortality, morbidity and cost [Reference Bajaj5, Reference Trifan6]. This study confirms and extends these findings by demonstrating that mortality and cost are increased significantly in this patient population compared with patients with CDI without CLD. The observed worse outcomes in patients with CLD are likely due to a higher severity of underlying illness in this patient population compared with other chronically ill patients.
In a prior study using the NIS dataset from 2005, comparing CLD patients with or without CDI, CDI was associated with an approximate twofold increase in mortality, and significantly increased length of hospital stay and hospitalisation charges [Reference Bajaj5]. In another study of the NIS datasets from 2008 to 2011. In patients with CLD due to alcoholic hepatitis, CDI was associated with mortality rates similar to hospitalised patients with urinary tract infections but CDI patients experienced longer length of stay and hospitalisation costs [Reference Sundaram11]. CDI proportions in these two studies were 1.42% and 1.62%. Accounting for differences in the underlying patient population and time, similar results were observed in our study. The novel findings from our study centred on comparisons of CDI in patients with or without CLD. Using the NIS dataset and matching patients on comorbidity and age, CLD in patients with CDI was associated with higher likelihood of in-hospital mortality (8.8% vs. 18.6%), longer length of stay (1 day) and increased cost ($8632). From our study, using our tertiary care database of patients with CDI, CLD patients had a high rate of CDI-related risk factors including previous antibiotic exposure and proton pump inhibitor use. Disease presentation was similar regardless of liver disease status suggesting that the immune dysfunction with CLD does not dampen the presenting signs and symptoms of disease. Although underpowered, similar differences in mortality rates compared with the national-level NIS data were observed. Taken together, these data suggest that patients with CLD are (a) at high risk for CDI and (b) are especially prone to severe CDI disease consequences. These data can be used to help justify novel prevention or treatment approaches in this vulnerable patient population [Reference Louie21–Reference Wilcox23].
This study has limitations. We used the NIS dataset to get national estimates of disease burden in this patient population. However, the NIS is limited to discharge characteristics and does not include granular data such as disease presentation, treatment, testing conditions, amongst others. We chose to use the NIS year 2009 as this was before wide-scale adoption of PCR diagnostics for CDI which may detect C. difficile colonisation and not infection, potentially blunting the adverse impact of true CDI in this patient population [Reference Koo24]. Limitations of our single-centre database include generalisability to other centres including different treatment and diagnostic approaches. However, the uniqueness of combining both datasets with each inherent limitation adds overall strength of applicability to this study. Due to inherent limitations with culture-based methodologies, we were not able to obtain ribotyping data on many of the patients in this study. Future analyses with a larger ribotyping database are planned. Moreover, optimal prevention and treatment strategies in this patient population will require further study.
In conclusion, CDI-related risk factors were almost universally present in the CLD population. In this population, worse CDI-related outcomes including mortality were observed compared with CDI patients without CLD.
Financial support
This work was supported by the National Institutes of Health (U01AI124290-01) and Merck and Co (C110473).
Author contributions
KMD: acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis.
SLA: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis.
AS: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
DNS: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
RA: acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
KWG: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; administrative, study supervision.




