INTRODUCTION
Duke (Reference Duke2005) and Duke et al. (Reference Duke, Zea-Flores and Gannon1990, Reference Duke, Marty, Peet, Pardon, Pion, Kamgno and Boussinesq2002) describe pleomorphic neoplasms of female Onchocerca volvulus filariae. These tumors are of medical significance because their incidence increases several months after treatment with ivermectin (IVM), the widely used drug for mass treatment of onchocerciasis (river blindness). This disease is still a public health problem in several endemic areas in Africa (Basañez et al. Reference Basañez, Pion, Churcher, Breitling, Little and Boussinesq2006; WHO, 2008) and therefore further biological research is recommended (Boussinesq, Reference Boussinesq2008). The macrofilaricidal efficacy of IVM may depend in part on the neoplasms. Duke et al. (Reference Duke, Marty, Peet, Pardon, Pion, Kamgno and Boussinesq2002), assumed that the tumors might originate from filarial oocytes, although other cells such as spermatocytes, zygotes, or embryonic cells could not be excluded, and the origin from filarial cells has not yet definitely been proven. Germ cell tumor formation is known from various animals and man (Jessberger, Reference Jessberger2008) but so far not from parasitic nematodes other than O. volvulus. Tumor-like formations were induced experimentally in germ-line cells in the model nematode Caenorhabditis elegans (Berry et al. Reference Berry, Westlund and Sched1997; McGovern et al. Reference McGovern, Voutev, Maciejowski, Corsi and Hubbard2009) and in mutants of C. elegans worms (Subramaniam and Seydoux, Reference Subramaniam and Seydoux2003; Pinkston et al. Reference Pinkston, Garigan, Hansen and Kenyon2006). Homologous genes that are associated with tumors such as those of the Notch family (e.g. gld-1), have been identified in the genome of filarial nematodes (Ghedin et al. Reference Ghedin, Wang, Spiro, Caler, Zhao, Crabtree, Allen, Delcher, Guiliano, Miranda-Saavedra, Angiuoli, Creasy, Amedeo, Haas, El-Sayed, Wortman, Feldblyum, Tallon, Schatz, Shumway, Koo, Salzberg, Schobel, Pertea, Pop, White, Barton, Carlow, Crawford, Daub, Dimmic, Estes, Foster, Ganatra, Gregory, Johnson, Jin, Komuniecki, Korf, Kumar, Laney, Li, Li, Lindblom, Lustigman, Ma, Maina, Martin, McCarter, McReynolds, Mitreva, Nutman, Parkinson, Peregrín-Alvarez, Poole, Ren, Saunders, Sluder, Smith, Stanke, Unnasch, Ware, Wie, Weil, Williams, Zhang, Williams, Fraser-Liggett, Slatko, Blaxter and Scott2007).
Most filarial nematodes, including O. volvulus, harbour Wolbachia endobacteria in the hypodermis, oocytes and all embryos, which are essential for embryogenesis (Hoerauf et al. Reference Hoerauf, Mand, Volkmann, Büttner, Marfo-Debrekyei, Taylor, Adjei and Büttner2003). The sperm cells do not contain endobacteria. However, an indirect influence on spermiogenesis by compounds secreted by Wolbachia in the hypodermis of male worms cannot be excluded. These endobacteria have a tropism for the stem cell niche (Frydman et al. Reference Frydman, Li, Robson and Wieschaus2006) and at the same time stem cells are considered to play a major role in tumor formation (McGovern et al. Reference McGovern, Voutev, Maciejowski, Corsi and Hubbard2009). So far it is not known whether the endobacteria play a role in the tumor formation of O. volvulus. Our previous drug trials eliminating Wolbachia from the filariae using doxycycline treatment provided an opportunity to study the occurrence of the endobacteria in the neoplasms of O. volvulus (Hoerauf et al. Reference Hoerauf, Mand, Volkmann, Büttner, Marfo-Debrekyei, Taylor, Adjei and Büttner2003 Reference Hoerauf, Specht, Büttner, Pfarr, Mand, Fimmers, Marfo-Debrekyei, Konadu, Debrah, Bandi, Brattig, Albers, Larbi, Batsa, Adjei and Büttner2008a, Reference Hoerauf, Specht, Marfo-Debrekyei, Büttner, Debrah, Mand, Batsa, Brattig, Konadu, Bandi, Fimmers, Adjei and Büttner2009).
We used immunohistology and antibodies specific to filarial or Wolbachia proteins to characterize the neoplasms more closely and to compare their expression pattern with that of the adjacent non-tumorous filarial tissues. The objective of the present study was to answer the following questions. (i) Do filarial proteins labelled in the neoplasms further prove the filarial origin? (ii) Do the tissues of neoplasms-containing filariae harbour Wolbachia? (iii) Do the cells of the neoplasms harbour Wolbachia? (iv) How frequently are neoplasms found in worms collected in 1977–78 before IVM became available? (v) How frequently are neoplasms found after treatment with different antibiotics and anthelminthics?
PATIENTS, MATERIALS AND METHODS
Patients and onchocercomas
Onchocercomas from 494 patients in Liberia, Burkina Faso, Mali, Ghana, and western Uganda were selected from the material of previous studies (Table 1). The nodules had been surgically removed from the patients using local anaesthesia and aseptic conditions. The Ethics Commission of the Medical Board in Hamburg had approved nodulectomies for research purposes. The protocol of the different drug studies had been approved by the authorities and by the ethic committees of the respective African countries (see References). The procedures used were in accordance with the Declaration of Helsinki (1975 and its revisions in 1983, 2000 and 2002).
Table 1. Frequencies and rates of female Onchocerca volvulus filariae that harboured pleomorphic neoplasms, which were identified using histological examination of onchocercomas from various groups of patients
(See text for statistically significant differences.)

a IVM=ivermectin. b Fixation with formaldehyde. c Fixation with ethanol and large nodules one portion with formaldehyde. d Samples of living worms had previously been shown to contain many Wolbachia, but the precise data are no longer available. e w=weeks. f m=months. g These many Wolbachia containing female worms had all been newly acquired after doxycycline treatment (Specht et al. Reference Specht, Hoerauf, Adjei, Debrah and Büttner2009b). Therefore there were zero treated worms with many bacteria. h This worm harboured still few Wolbachia after doxycycline treatment. j Nodulectomy 3, 8, 10, 20, 22, 27, or 34 months after IVM. k DEC=diethylcarbamazine.
Treatment of patients
The treatment has been described in detail in previous reports (references in Table 1). Briefly, the antibiotic doxycycline (100 or 200 mg/day) was applied for 2, 3, 4, 5, 6 or 6+3×2 weeks alone or followed by IVM (standard dose of 1×0·15 mg/kg 2–3 or 5–6 months after the start of doxycycline treatment). Some patients had taken IVM before registration, a few patients 1 dose during the year before doxycycline and others 1 or rarely 2–3 doses several years before registration. The antibiotic azithromycin (250 mg/day or 1200 mg/week) was given for 6 weeks (Hoerauf et al. Reference Hoerauf, Marfo-Debrekyei, Büttner, Debrah, Konadu, Mand, Adjei and Büttner2008b). Among the latter group 10 of 12 patients had taken IVM 33 months before azithromycin. The antibiotic rifampicin (10 mg/day) was given either for 2 or 4 weeks (Specht et al. Reference Specht, Brattig, Büttner and Büttner2008). Six of the rifampicin patients had taken IVM 10 months before recruitment. The anthelminthic IVM was applied in the usual single dose of 0·15 or 0·2 mg/kg in Liberia and Ghana. In Uganda higher doses of IVM were applied in 3 villages in cooperation with the Basic Health Service of Kabarole District. The participants had received 2 annual standard doses in 1992 and 1993 and then every 3 months a total of 7 doses of 0·4 mg/kg before they were nodulectomised early in 1996. A control group had received 5 or 6 annual doses of 0·15 mg/kg IVM within the frame of mass treatment. The anthelminthic suramin was applied in 5 weekly doses of 17 mg/kg in Burkina Faso in 1977. In a WHO-supported study in Liberia a group of patients had received high doses of the anthelminthic diethylcarbamazine (DEC): 1 week of low doses and then 30 mg/kg/day for 8 days. A patient with a neoplasm-harbouring male worm had been treated with 3 doses of 800 mg of the anthelminthic albendazole 3 months before nodulectomy (Awadzi et al. Reference Awadzi, Hero, Opoku, Büttner and Gilles1991). The time-intervals between the start of treatment and nodulectomy are provided in Table 1, in the text and legends.
Histology and immunohistology
The onchocercomas were fixed in 4% phosphate-buffered formaldehyde solution and in Ghana in 70–80% ethanol and larger nodules were divided in 2 portions and fixed with both fixatives (Table 1). The nodules were embedded in paraffin and stained with haematoxylin and eosin (Merck, Darmstadt, Germany). Movat and trichrome stains and Gomori's iron method were applied for selected nodules. For the immunohistology the alkaline phosphatase anti-alkaline phosphatase (APAAP) technique was applied according to the recommendations of the manufacturer (DakoCytomation, Hamburg, Germany). As primary antibodies polyclonal rabbit sera or monoclonal mouse antibodies against the proteins or other compounds listed in Table 2 were used as described in the respective references. For the determination of the endobacteria loads in 1999–2000 an antiserum against Brugia malayi WSP had been used. As secondary antibodies either anti-rabbit mouse immunoglobulin (clone MR12/53, DakoCytomation) or an anti-mouse rabbit antibody (DakoCytomation) were applied. Fast Red TR salt (Sigma, Deisenhofen, Germany) served as chromogen with haematoxylin as counter stain. The specificity of the primary antibodies was verified using buffer with serum albumin (0·1% w/v), the respective pre-immune serum or an irrelevant instead of the selected antibody. Purified antibodies against the secretory omega-class glutathione transferase OvGST3 in the filarial eggshells were used further as a control (Liebau et al. Reference Liebau, Höppner, Mühlmeister, Burmeister, Lüersen, Perbandt, Schmetz, Büttner and Brattig2008).
Table 2. Filarial and bacterial proteins and other compounds detected by antibodies were used to characterize neoplasms of Onchocerca volvulus
(All of them reacted either with the pleomorphic neoplasms (PN) or with Wolbachia endobacteria (Wol).)
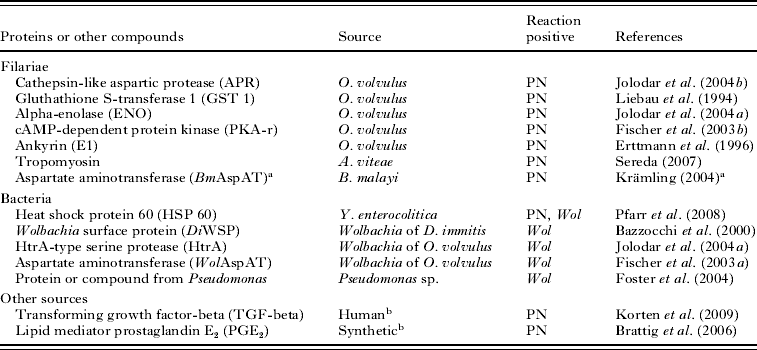
a Krämling, M. (2004). Characterisation of aspartate aminotransferases of intracellular Wolbachia bacteria and their host cells. (In German). Dissertation for Biology Diploma, Rheinisch Westfälische Technische Hochschule Aachen, Germany.
a GenBank Accession number AF411604.1 GI:15723304; Brugia malayi aspartate aminotransferase.
b These antibodies labelled filarial TGF-beta and PGE2 in the hypodermis.
Examination of worms
Following the description of pleomorphic neoplasms by Duke et al. (Reference Duke, Zea-Flores and Gannon1990), tumors were also discovered in previously examined material (Fig. 10D in Büttner et al. Reference Büttner, Albiez, Essen and Erichsen1988). Büttner discussed the neoplasms with Duke and consequently worms with neoplasms were specifically examined whenever a new anti-filarial antibody for immunohistology was available. Different antibodies were often tested with different worms, because sometimes antisera were limited and they reacted only with either ethanol- or formaldehyde-fixed worms. However, more than 10 tumor-harbouring worms were tested with 7 different antibodies. A total of 3587 female worms from 494 patients were examined that harboured 112 neoplasms. Many tumors are found in moribund and dead worms (Duke, Reference Duke2005; Duke et al. Reference Duke, Marty, Peet, Pardon, Pion, Kamgno and Boussinesq2002). The diagnosis of neoplasms in moribund but living filariae was easy, but it was more difficult in dead worms. We decided not to count doubtful neoplasms. Also we only counted 2 neoplasms in 1 nodule provided they lay in clearly different areas of the nodule.

Fig. 1. Identification of filarial proteins in neoplasms (arrows) of living female Onchocerca volvulus using specific antibodies for immunohistochemistry (see Table 1). Note the pleomorphism of the cells. (A–D) Consecutive cross-sections of an untreated female filaria show tumor cells in the pseudocoeloma cavity labelled for (A) tropomyosin; (B) heat shock protein 60 in tumor cells and in many endobacteria in the hypodermis; (C) alpha-enolase in tumor cells and body wall muscles; and (D) B. malayi amino transferase in tumor cells. Further sections from different filariae show cells labelled by a cAMP-dependent protein kinase (E, untreated); cells and muscle fibres in the tumor expressing tropomyosin (F, 12 months after 6×1200 mg/week azithromycin) and muscle fibres in the tumor stained by trichrome (G, 10 months after 1×0·15 mg/kg IVM); expression of ankyrin E1 (H, untreated, after 11 years of vector control); presence of aspartic protease in a living (I) and a dead (J) untreated worm; labelling of glutathione S-transferase 1 (K, azithromycin as F); staining of tumor cells and hypodermis for transforming growth factor-beta (L, untreated), and the lipid mediator prostaglandin E2 (M untreated) showing infiltration of the uterus epithelium (N azithromycin as F). ba, Wolbachia endobacteria; cu, cuticle; hy, hypodermis; mu, body wall muscles; ut, uterus. Scale bars=30 μm.
Statistical analysis
For the analysis of differences in frequencies of neoplasms in various patient groups the chi square test was applied (http://math.hws.edu/javamath/ryan/ChiSquare.html), defining P<0·05 to be a significant difference and P<0·10 to be a trend.
RESULTS
Filarial proteins in the neoplasms
Table 2 lists 8 proteins and 2 other compounds of filariae that we observed in the neoplasms of O. volvulus, and Fig. 1 shows tumors expressing these proteins thus confirming the filarial origin of the neoplasms. Consecutive sections of 1 female worm showed localization of 4 proteins in different cell types of the tumor (Fig. 1A–D) indicating the pleomorphism of the cells. Naturally, the larger cells were more frequently labelled than the smaller ones (Fig. 1B, E, I, L). Tropomyosin was found in epithelial cells (Figs 1A, 2C and 4F), in muscle cells of healthy filariae and in muscle-like cells of the tumors (Fig. 1F). The muscles were also stained by trichrome stain (Fig. 1G) and by antibodies against alpha-enolase (Fig. 1C). Strong expression in different tumor cell types was seen of proteins that were usually observed in the hypodermis and the epithelia of the filariae: heat shock protein 60 (Figs 1B, 2D and G, 3D and 4H), aspartic protease (Figs 1I–J, 2A and 3F), B. malayi aspartate aminotransferase (Figs 1D and 2F), glutathione S-transferase 1 (Figs 1K and 3E) and the polypeptide transforming growth factor-beta (Fig. 1L). Antibodies against heat shock protein 60 labelled O. volvulus cells as well as the Wolbachia endobacteria. Ankyrin E1 (Fig. 1H) and the cAMP-dependent protein kinase (Figs 1E, 2B and 4E) had previously been strongly labelled by the respective antibodies in nerve cells of O. volvulus but also weakly in the hypodermis. It could not be decided, whether the cells positive for these antigens in the neoplasms were altered hypodermal or nerve cells. The hypodermis and different tumor cells were also labelled by the lipid mediator prostaglandin E2 (Fig. 1M–N). Antibodies against the secretory glutathione S-transferase 3 of O. volvulus label the eggshells of intrauterine microfilariae (Liebau et al. Reference Liebau, Höppner, Mühlmeister, Burmeister, Lüersen, Perbandt, Schmetz, Büttner and Brattig2008). No eggshell-like structures were detected in any of the neoplasms and antibodies against the glutathione S-transferase 3 did not label any of the screened 9 tumors, whereas the eggshells in the uterus of tumor-containing female worms were strongly positive (not shown). Negative control staining using buffer or specific antibodies directed against distinct human proteins instead of the specific antibodies showed no labelling of the neoplasms. These antibodies were against human tryptase, elastase of neutrophils, peroxidase of eosinophils, CD68 of macrophages, and CD34 of endothelia (not shown). These results prove the filarial origin and demonstrate the polymorphism of cells and tissues of the filarial neoplasms.

Fig. 2. Histological signs indicating malignancy of the neoplasms in living female Onchocerca volvulus from untreated patients. (A) Large cells with much enlarged nuclei (arrows) and with several nuclei infiltrating the hypodermis (arrowhead, aspartic protease). (B) Large unstained (arrow) and large red stained cells occur among numerous small basophilic cells (protein kinase). (C) Small basophilic cells infiltrate and destroy body wall muscles (arrows) and hypodermis (arrowheads; tropomyosin). (D) Infiltration of the enlarged uterus epithelium by tumor cells (arrows; the arrowheads show the basal lamina of the uterus; heat shock protein 60). (E) A pathological mitosis with several abnormal chromosomes (arrow; Wolbachia serine protease). (F) Many small basophilic, some medium-sized red and 3 large cells with abnormally enlarged nuclei (arrows) have nearly completely destroyed the muscles and hypodermis (arrowheads; B. malayi aspartate aminotransferase). (G) Hypodermis and body wall muscles are no longer present (arrowheads; heat shock protein 60). ba, Wolbachia; cu, cuticle; hy, hypodermis; in, intestine; mu, muscles; ut, uterus. Scale bars=30 μm (A–D and F–G), 10 μm (E).

Fig. 3. Immunohistochemistry of the pleomorphic neoplasms of two living male Onchocerca volvulus filariae with the typical narrow annulations of the cuticle (arrowheads). (A–C, worm # 1) Overview of the worm with a tumor (A, arrow); a few sections show normal spermiogenesis (B, arrow), whereas most sections present typical pleomorphic tumor cells in the pseudocoeloma cavity (C, arrows). Endobacteria stained by an antiserum against a Pseudomonas compound are seen in the hypodermis but not in the tumor. Three months after 3×800 mg albendazole. (D–F, worm # 2). A male worm only 6 months after 4 weeks 200 mg/day doxycycline shows tumor cells in the testis labelled (arrows) by heat shock protein 60 (D); glutathione S-transferase 1 (E) and aspartic protease (F). ba, Wolbachia; in, intestine; sp, sperms; te, testis. Scale bars=300 μm (A), 30 μm (B–F).

Fig. 4. The ovaries of healthy Onchocerca volvulus (arrows in A–B, D) are compared with the rosette formations in neoplasms (arrowheads in C, E–F) examined by immunohistochemistry and intact embryogenesis of a tumor-harbouring female filaria (G–H). The oocytes in the ovary possess nuclei all of the same size (A–B, D) and contain Wolbachia (A–B) whereas the cells of the tumor rosettes vary in form and size (C, E–F, H) and they do not contain endobacteria (C). Ovarian oocytes and rachis are usually negative for protein kinase (D) and tropomyosin (not shown) in contrast to the tumor rosettes with stained cells (E, protein kinase; F, tropomyosin). A tumor-harbouring female worm is fully productive with Wolbachia-positive morulae (G, arrows) and mature microfilariae (H) 12 months after 6×1200 mg/week azithromycin treatment without IVM. Stained with antisera against DiWSP (A–C); heat shock protein 60 (G–H). cu, cuticle; hy, hypodermis; lc, lateral chord; mu, body wall muscle; ov, ovary; tu, tumor; ut, uterus. Scale bars=30 μm.
Histological signs indicating malignancy of the neoplasms
The morphology of the tumor cells varied greatly. Most frequently small basophilic cells were seen with a high nuclear-to-cytoplasm ratio (Figs 1A and L, 2B and F and 5). Many large cells were observed with large, often highly polymorphic nuclei and irregular blocks of chromatin (Figs 1B–D, I and K–M, 2A, B and F and 3C and E). Pathological mitoses occurred (Fig. 2E). Usually a mixture of several cell types was seen (e.g. Figs 1B–D, 2D, F and G, 5B and G). Further observed were muscle fibres (Fig. 1F and G) and rosettes of fibrous cells (Figs 2G, 4C, E and F and 5F) otherwise not seen in healthy worms. It is supposed that the nematode O. volvulus is a eutelic organism with a fixed number of somatic cells as an adult worm (or nuclei in the case of syncytia). It may be possible that there is sometimes a small increase in cell numbers but not such an enormous, ~ thousand-fold increase as was observed in the final stages of the neoplasms. This unlimited cell multiplication and the destructive infiltration into the hypodermis and body wall muscles (Fig. 2A and C) and the uterus epithelium (Figs 1N and 2D) damage and finally destroy the worm tissues (Figs 1A and 2F and G). One or several of these signs may occur in non-tumorous, otherwise damaged worms. However, the usually observed combination of these signs in 1 tumor-containing worm indicates with high probability the malignancy of these neoplasms.
Neoplasms in male O. volvulus
We identified neoplasms in 2 male worms. Fig. 3A shows many sections of a male worm excised 3 months after albendazole treatment. These sections present the typical narrow annulations of the male cuticle (Fig. 3B and C), which excludes the possibility that these rings are narrow ridges at the anterior end of a female worm. Fig. 3B shows a worm section with spermiogenesis in contrast to Fig. 3C that shows typical tumor cells in the pseudocoeloma cavity of this male worm. Different stages of spermiogenesis are only found in male worms. Fig. 3D–F presents another male worm, excised already 6 months after 4 weeks of insufficient doxycycline treatment, again showing the typical narrow annulations of the male cuticle. This worm contained few degenerated sperms and abundant tumor cells. A total number of 1570 intranodular male worms were examined and 0·13% harboured neoplasms. We conclude that the type of pleomorphic neoplasm of O. volvulus also occurs in male worms. The observation may further support the hypothesis for the presence of germ cell tumors originating from testicular tissue.
Neoplasms and filarial reproduction
Duke et al. (Reference Duke, Marty, Peet, Pardon, Pion, Kamgno and Boussinesq2002) reported that the rosette formations resemble the growth portion of the ovary providing perhaps a clue to the origin of the neoplasms. A more detailed examination by immunohistology in the present study showed some differences (Fig. 4A–F). All healthy oocytes harboured Wolbachia but the rosettes did not (compare Fig. 4A and B with 4C). Several filarial proteins such as aspartic protease and tropomyosin were not observed in the ovary (Fig. 4D) but in the rosettes (Fig. 4E and F). The oocytes contained rather large nuclei (Fig. 4A, B and D) whereas the nuclei in the rosettes were polymorphic and smaller (Figs 4C, E and F and 5F). These differences do not exclude the ovarian oocytes as the origin of the neoplasms, but the significance of the rosette formations is not known.
In living filariae, neoplasms were usually seen in middle-aged or old worms with degenerating embryos or with empty uteri. Occasionally we observed a few fecund worms producing embryos of all stages up to microfilariae (Fig. 4G and H). However, we cannot exclude that these tumors may already have originated from a previous cycle of reproduction. We did not observe neoplasms in worms showing the criteria of young filariae, such as those in more than 98 female worms newly acquired 1–3 years after treatment (Specht et al. Reference Specht, Hoerauf, Adjei, Debrah and Büttner2009b) and in 24 worms from a 4-year-old child (Büttner et al. Reference Büttner, Albiez, Essen and Erichsen1988). Among the worms from Burkina Faso fewer tumors were observed in older children and younger teenagers, who were still in the phase of building-up their worm load.
Absence of endobacteria from neoplasm cells
Previous authors assumed that the neoplasms originate from oocytes. Since the oocytes of untreated healthy O. volvulus always harbour numerous Wolbachia, we searched for these bacteria in the neoplasms by applying antibodies that label the endobacteria (Table 1; Fig. 5). All examined worms harbouring tumors presented Wolbachia in the hypodermis (Fig. 3C, 4C, 5A, C and E–G) or in the oocytes in uterus (Fig. 5B and D) or ovary. The endobacteria were numerous and morphologically intact in worms from untreated patients (Figs 1B and 5A and G) or treated with different anthelminthics (Figs 3C, 4A and 5C and F). After treatment with the antibiotics azithromycin, rifampicin or insufficient doses of doxycycline (see next paragraph), the endobacteria were often scanty and more or less degenerated (Fig. 5B, D and E; see also Figs 5–6 in Specht et al. Reference Specht, Hoerauf, Adjei, Debrah and Büttner2009b), but a few bacteria were detected at least in the anterior portions of the worms. In contrast, we did not detect any endobacteria in the cells of 51 neoplasms using the antibody against Dirofilaria Wolbachia surface protein (Figs 4C and 5A–D). In agreement, no endobacteria were found, applying antisera against Wolbachia serine protease (Fig. 3E–F; in 11 tumors), Wolbachia aspartate aminotransferase (Fig. 5G; 3 tumors), and the less specific serum against heat shock protein 60 (Fig. 1B; 38 tumors). We conclude that the tumor-harbouring filariae contain Wolbachia in their hypodermis, oocytes and embryos. However, the cells of the neoplasm were free from endobacteria.

Fig. 5. Wolbachia endobacteria (arrows) are seen in the hypodermis or oocytes of neoplasm-harbouring female Onchocerca volvulus using specific antibacterial antibodies for immunohistochemistry (see Table 1), but no endobacteria are found in the tumor cells. (A) The same untreated worm as shown in Figs 1A–D with bacteria in the hypodermis. (B) Intact Wolbachia in uterine oocytes 6 months after 6×1200 mg/week azithromycin. (C) Bacteria in hypodermis and oocytes (arrowheads) after 7 high doses of IVM. (D) Degenerated bacteria in uterine oocytes 18 months after insufficient doxycycline treatment for 4 weeks. (E) Hypodermal bacteria 18 months after rifampicin treatment for only 2 weeks. (F) Hypodermal bacteria 32 months after 1×0·15 mg/kg IVM. (G) Untreated worm with hypodermal bacteria. Labelling of Wolbachia surface protein (A–D), Wolbachia serine protease (E–F) and Wolbachia aspartate aminotransferase (G). cu, cuticle; hy, hypodermis; ro, rosette formation; tu, tumor; ut, uterus. Scale bars=30 μm.
Absence of neoplasms after doxycycline treatment
Sufficient doses of doxycycline for 4, or better, for 5 or 6 weeks led to degeneration of the Wolbachia and slowly to their elimination during the next few months. Analysing the worms from doxycycline trials, we observed less neoplasms and we decided to re-examine the nodules and search for tumors. To assess the frequency of tumors in untreated worms, we re-examined the nodules of our Ghanaian placebo and control patients as well as those who had only received IVM (Table 1). We could not exclude that a few of the control patients had previously taken IVM but did not report this, which might have caused higher tumor rates. Therefore we examined nodules excised in 1977 before IVM was available for human patients in Burkina Faso (Table 1) and in Liberia in 1976–1978 (126 worms in 61 nodules from 19 patients). We observed the same rates of 2·4% female worms containing neoplasms in both countries (P>0·50). The rate of 4·6% in Ghana was not different from the rate of 2·4% in Liberia (P>0·10), but there was a trend for a lower rate in Burkina Faso (P<0·10). The rate of 6·1% neoplasms observed after a single dose of 0·15 mg/kg IVM in Ghana was not different from the 4·6% observed in untreated Ghanaians (P>0·10) 3, 8, 10, 20, 22, 27, or 34 months after IVM.
We summarized the results of the worms excised 11, 18 and 30 months after only 2 weeks doxycycline treatment, because the rates of tumor-containing females were not different (P>0·10). The rates of female worms with neoplasms from patients, who had received insufficient doxycycline treatment for only 2 or 3 weeks, were 5·2 and 1·4%, respectively. Both rates are not different from that seen in worms from untreated patients (P>0·10). These worms harboured numerous Wolbachia (Table 1). Filariae that had been exposed to sufficient doxycycline treatment but had been nodulectomised already after 6 months, showed a trend for a lower rate of 2·0% neoplasms (P<0·10). There was no difference between the two 6-month groups (P>0·50). In Table 1 we separated the patient groups treated only with doxycycline from those with additional IVM treatment to demonstrate that the reduction or elimination of Wolbachia and the absence of neoplasms was independent of prior IVM treatment. More than 10 months after sufficient doxycycline treatment no neoplasms were observed in the filariae without Wolbachia. No tumors had developed in the worms from patients treated 6 weeks with 100 or 200 mg/day doxycycline (Table 1) or 4 weeks with 200 mg/day. The differences to the rates of 4·6% of untreated (P<0·001 and P<0·02, respectively) and 3·6% of insufficiently treated filariae (P<0·001) were significant. One of 32 worms from patients treated with 100 mg/day for 5 weeks plus IVM still presented a few endobacteria and a tumor (Table 1). Obviously this patient had not received enough doxycycline, since 4 more worms contained few bacteria. The rate of 0·7%, observed in the total 5-week group, was lower than that seen in untreated Ghanaians (P<0·05). We conclude that the pleomorphic neoplasms do not develop after sufficient doxycycline treatment.
Frequency of neoplasms after treatment with anthelminthics
In 1977, ten years before the registration of IVM, we observed a rate of 2·4% tumor-containing worms from untreated patients in Hemkoa south of the Bougouriba River (Table 1) and in 1976–1978 we saw the same rate of 2·4% of 126 untreated worms in Liberia (Albiez et al. Reference Albiez, Büttner and Schulz-Key1984). In Liberia in 1986, and 2 months after a single standard dose of IVM, the tumor rate of 0·8% was low, but after 10 months it had increased to 7·7% (Table 1; P<0·01), which was higher than the rate of 2·4% of untreated worms in Burkina Faso (P<0·05). In Uganda in 1996 the 313 worms from patients treated with 5 or 9 doses of IVM showed a significantly higher rate of 7·3% than the rate of 2·4% of untreated worms in Burkina Faso (Table 1; P<0·001). Suramin and high doses of diethylcarbamazine did not lead to higher tumor rates (Table 1). None of these anthelminthics showed any activity against the Wolbachia (for IVM see Fig. 5C and F), neither did albendazole or metrifonate. Our results confirm that repeated doses of IVM lead to an increased formation of neoplasms.
DISCUSSION
The rates of neoplasms in worms from untreated patients with onchocerciasis were low. From untreated patients we observed worm frequencies of 2·4% in Burkina Faso in 1977 and 4·6% in Ghana in 1999–2005. These rates were not different from the rate of 3·7% of 1422 worms reported by Duke et al. (Reference Duke, Marty, Peet, Pardon, Pion, Kamgno and Boussinesq2002) in Cameroon in 1994 (both P>0·10). The rates reported by Duke et al. (Reference Duke, Marty, Peet, Pardon, Pion, Kamgno and Boussinesq2002) and in the present study for worms from patients treated with anthelminthics other than IVM were also in the same range. This agreement indicates that both groups used a comparable technique for the diagnosis of tumors in worms from untreated patients, who harboured mostly live worms before nodulectomy.
In worms from IVM-treated patients, we observed higher rates compared to worms from untreated patients, which is also in agreement with previous reports (Duke, Reference Duke2005; Duke et al. Reference Duke, Marty, Peet, Pardon, Pion, Kamgno and Boussinesq2002). However, the rates found in the present study were not as high as the 17·5% reported from Cameroon (P<0·001 for the difference between repeated IVM in Uganda and Cameroon). There may be several reasons for this. We may have been too hesitant to count 2 tumors in 1 nodule or in diagnosing neoplasms in dead, degenerated or disintegrated worms not presenting pleomorphism of cells (e.g. Fig. 1G of Duke et al. Reference Duke, Marty, Peet, Pardon, Pion, Kamgno and Boussinesq2002). Possibly the real rates of neoplasms induced by repeated IVM treatment were higher than those observed in Uganda and Cameroon. Using histology instead of the examination of total worms (Büttner et al. Reference Büttner, Albiez, Essen and Erichsen1988), portions of the 20–50 cm long female worms with early developing neoplasms may have been missed in a few cases. Further, some worms may already have been resorbed after treatment. The higher rates seen 10 months after IVM compared to 2 months, in Cameroon and by us, support the recommendation for longer observation periods in drug trials searching for macrofilaricidal activity (Hoerauf et al. Reference Hoerauf, Specht, Büttner, Pfarr, Mand, Fimmers, Marfo-Debrekyei, Konadu, Debrah, Bandi, Brattig, Albers, Larbi, Batsa, Adjei and Büttner2008a, Reference Hoerauf, Specht, Marfo-Debrekyei, Büttner, Debrah, Mand, Batsa, Brattig, Konadu, Bandi, Fimmers, Adjei and Büttner2009).
The proteins and other compounds, that had been previously found in O. volvulus and that we have examined now, occurred also in the neoplasms. An exception was glutathione S-transferase 3 that is bound exclusively to the eggshells, which do not occur in the tumors. Among these detected proteins are proteins that occur in hypodermis, endothelia, muscles, oocytes, spermatocytes or embryos of the worms. The differential localization of proteins in different tumorous cell types demonstrated the pleomorphism of these tumors, which may be germ cell tumors that are characterized by the presence of cells of different tissue types. The absence of human immune cell proteins and the presence of the filarial proteins prove further the assumption of Duke et al. (Reference Duke, Marty, Peet, Pardon, Pion, Kamgno and Boussinesq2002) that filarial tissue is the origin of these tumors.
Duke (Reference Duke2005) and Duke et al. (Reference Duke, Marty, Peet, Pardon, Pion, Kamgno and Boussinesq2002) assumed that the neoplasms originate from cells of the ovary. Our observations showed that they might, alternatively, originate from cells of the testis. For Wolbachia-dependent filarial nematodes it is not clear whether meiosis or mitosis or both depend on the presence of Wolbachia. However, for Drosophila it was shown that zygote killing of uninfected female flies by Wolbachia-infected males is due to defects in chromosome replication/segregation and associated centrosome/microtubule-based processes (Lassy and Karr, Reference Lassy and Karr1996). It is likely that in filarial nematodes, Wolbachia or their products may influence cell division in both reproduction and neoplasm. All stages of filarial sperms do not contain Wolbachia. However, that does not exclude the activity of bacterial compounds secreted by Wolbachia. IVM has probably no direct influence on cell division since it effects primarily the stretched microfilariae in the uterus and, to a lesser extent, other embryos or germ cells. To our present knowledge, oocytes, spermatocytes, zygotes, and different embryonal cells may be involved in tumor formation, as they are in teratomas. Further, the influence of older worm age (see below) and possibly genetic factors have to be considered (Jessberger, Reference Jessberger2008).
Interestingly, in the free-living nematode C. elegans, the gene gld-1 encodes a protein that contains a K homology RNA-binding domain required for meiotic cell cycle progression during oogenesis, also affecting spermatogenesis. In C. elegans gld-1 mutants, germ cells in the early stages of oogenesis re-enter the mitotic cell cycle and over-proliferate. Eventually these cells break out of the gonad and fill the body, killing the worm early in life (Pinkston et al. Reference Pinkston, Garigan, Hansen and Kenyon2006). As described in the Results section, the outbreak of the tumor cells from the gonads and the filling of the complete body is what we observed in the neoplasms of O. volvulus. Furthermore, primary spermatocytes lacking the pumilio-like protein PUF-8, dedifferentiate back into mitotically cycling germ cells that form rapidly growing tumors in mutants of C. elegans worms (Subramaniam and Seydoux, Reference Subramaniam and Seydoux2003).
Why did we observe only 2 male worms with tumors and Duke et al. (Reference Duke, Marty, Peet, Pardon, Pion, Kamgno and Boussinesq2002) observed none? (1) Males are much smaller than females weighing only approximately 1% of the weight of females and the small dead worms are quickly resorbed, as the low percentages of dead males show compared to those of females (Büttner et al. Reference Büttner, Albiez, Essen and Erichsen1988). The macrofilaricidal drug suramin kills male O. volvulus faster than females and the death caused by doxycycline or repeated doses of IVM may also occur faster (Awadzi et al. Reference Awadzi, Hero, Opoku, Addy, Büttner and Ginger1995). (2) Neoplasms may develop less often in male than in female filariae. If it would be true that endobacterial compounds may have an influence on cell division leading to tumor formation, the low frequency in male worms may be due only to the indirect influence by hypodermal Wolbachia in contrast to the direct activity of bacteria in oocytes.
We did not observe neoplasms in young worms and we estimate (Specht et al. Reference Specht, Brattig, Büttner and Büttner2009a) that the tumor-containing filariae may have been older than 4 years. This observation is in accordance with those of Duke et al. (Reference Duke, Zea-Flores and Gannon1990, Reference Duke, Marty, Peet, Pardon, Pion, Kamgno and Boussinesq2002). They found only 1 female with a tumor among 446 female worms (0·22%) in an area with repeated nodulectomies in Guatemala where nearly all worms were newly acquired. This implies that any potential macrofilaricidal effect due to tumors induced by IVM would never have been observed in animal models, because the worms are not old enough. We conclude that the older age of the worms is a co-factor for the development of the tumors.
Worms containing neoplasms were not observed more than 10 months after the start of effective treatment with doxycycline or with doxycycline plus ivermectin for 4 weeks or longer. One explanation may be that the filariae damaged by the tumors were rapidly killed by doxycycline and absorbed. Another explanation may be that an effect of doxycycline on cell division or other tumor-generating processes prevents the formation or the growth of neoplasms. Doxycycline is used for the treatment of cancer metastases (Sapadin and Fleischmajer, Reference Sapadin and Fleischmajer2006) and it also may affect the filarial tumors directly. Furthermore, the loss of the endosymbionts caused by the doxycycline treatment may have an influence on the development of tumors.
The following conclusions have been drawn from this study. (1) The findings confirmed the filarial origin of the neoplasms. (2) The histology of the neoplasms indicated malignancy. (3) The male worms showed that the neoplasms can also originate from filarial cells, other than ovary cells, e.g. possibly spermatocytes. (4) No Wolbachia were found in the cells of the neoplasms. (5) Further, worms with neoplasms were not observed after more than 10 months following treatment with sufficient doxycycline or doxycycline plus ivermectin.
ACKNOWLEDGEMENTS
We thank Ingeborg Albrecht, Marlies Badusche, and Frank Geisinger for assistance in the histology laboratory. We are grateful to Dr Kwablah Awadzi, Onchocerciasis Control and Research Centre, Hohoe, Ghana, for onchocercomas from albendazole-treated patients and to Dr Sabine Mand, University of Bonn, for some nodules from patients briefly treated with doxycycline. We are obliged to Professor I. B. Autenrieth, University of Tübingen; Professor K. Brune, University of Erlangen; Dr Klaus D. Erttmann, Bernhard Nocht Institute; Dr Kim Henkle-Dührsen, previously of the Bernhard Nocht Institute; Professor Richard Lucius, Humboldt University, Berlin; Professor Peter Zipfel, Leibniz Institute for Natural Product Research and Infection Biology, Jena, all of whom supplied antibodies. The clinical doxycycline studies in Ghana had been supported by the European Commission (A. H., EU grants ICA4-CT-2002-10051 and INCO-CT-2006-032321).









