Introduction
Despite international commitments, global biodiversity loss has accelerated in the past 50 years (Diaz et al. Reference Diaz, Settele, Brondizio, Ngo, Guèze, Agard, Arneth, Balvanera, Brauman, Butchart, Chan, Garibaldi, Ichii, Liu, Subramanian, Midgley, Miloslavich, Molnár, Obura, Pfaff, Polasky, Purvis, Razzaque, Reyers, Roy Chowdhury, Shin, Vissereren-Hamakers, Wills and Zayas2019, Pereira et al. Reference Pereira, Rosa, Martins, Kim, Leadley, Popp, van Vuuren, Hurtt, Anthoni and Arneth2020). Within Europe, changes in land use and overexploitation are considered to be among the main causes for defaunation, with many species experiencing range reductions, decreases in population size, or local extinctions (Diaz et al. Reference Diaz, Settele, Brondizio, Ngo, Guèze, Agard, Arneth, Balvanera, Brauman, Butchart, Chan, Garibaldi, Ichii, Liu, Subramanian, Midgley, Miloslavich, Molnár, Obura, Pfaff, Polasky, Purvis, Razzaque, Reyers, Roy Chowdhury, Shin, Vissereren-Hamakers, Wills and Zayas2019, Henle et al. Reference Henle, Alard, Clitherow, Cobb, Firbank, Kull, McCracken, Moritz, Niemelä, Rebane, Wascher, Watt and Young2008). Such losses can have cascading negative effects, reducing the resilience of ecosystem functions and the stability of ecological communities (Cardinale et al. Reference Cardinale, Duffy, Gonzalez, Hooper, Perrings, Venail, Narwani, Mace, Tilman, Wardle, Kinzig, Daily, Loreau, Grace, Larigauderie, Srivastava and Naeem2012, Oliver et al. Reference Oliver, Heard, Isaac, Roy, Procter, Eigenbrod, Freckleton, Hector, Orme, Petchey, Proença, Raffaelli, Suttle, Mace, Martín-López, Woodcock and Bullock2015). Reintroductions, the intentional movement and release of a species to re-establish a viable population within its native range, is one conservation tool that has been increasingly explored as a solution to Europe’s dramatic biodiversity decline (IUCN 2013), either through re-establishing populations of endangered species (Maran et al. Reference Maran, Põdra, Harrington and Macdonald2017), or through being incorporated into rewilding projects to aid in the restoration of ecosystem function and services (Pereira et al. Reference Pereira, Rosa, Martins, Kim, Leadley, Popp, van Vuuren, Hurtt, Anthoni and Arneth2020, Seddon et al. Reference Seddon, Griffiths, Soorae and Armstrong2014).
Within Great Britain, several of the vertebrate species lost over the last few centuries are either subject to ongoing reintroduction or being considered for future projects (Harrabin Reference Harrabin2020, Stringer and Gaywood Reference Stringer and Gaywood2016, White et al. Reference White, Convery, Eagle, O’Donoghue, Piper, Rowcroft, Smith and van Maanen2015). One such example is the reintroduction of the white stork, Ciconia ciconia, in 2016 through a collaborative effort between several conservation charities and landowners known collectively as the White Stork Project (WSP) (White Stork Project 2021a). Before the reintroduction project began, white storks were last recorded nesting in the wild on St Giles Cathedral in Edinburgh in 1416 with some older archaeological evidence suggesting they were also present in southern England (Parker Reference Parker1988, Serjeantson Reference Serjeantson, O’Connor and Sykes2010). Today the primary reintroduction site is the Knepp Castle Estate in West Sussex, a once intensively farmed estate which turned to rewilding and eco-tourism in 2001 when conventional farming was no longer financially viable (Tree Reference Tree2018). Although not as immediately impactful as beavers and large herbivores, white storks could be considered a more limited ecosystem engineer, with their large nests providing a nesting habitat for passerines and eventually causing tree mortality (Bocheński and Jerzak, Reference Bocheński, Jerzak, Tryjanowski, Sparks and Jerzak2006, Zbyryt et al. Reference Zbyryt, Jakubas and Tobolka2017). In several European countries the white stork has also been recognised as a flagship species for wetland and grassland conservation in part due to the species’ popularity and cultural significance, hence their return may provide an opportunity to re-engage the British public with the natural world (Kronenberg et al Reference Kronenberg, Andersson and Tryjanowski2017, Olsson and Rogers Reference Olsson and Rogers2009, Thomsen and Hötker Reference Thomsen and Hötker2013).
The WSP’s reintroduction strategy has three elements. Firstly, an open-air pen of roughly 30 flightless individuals is managed to act as an “anchor” to encourage flying white storks to return. Secondly, a group of white storks with the ability to fly were retained in this pen for their first two winters to increase the likelihood of them settling and breeding in the area. Lastly, between 2019 and 2023, first-year captive-bred individuals are set to be released in late summer to reinforce the population and encourage migratory behaviour. Whilst intensive management is considered at odds with rewilding’s goal to create a “self-wilded” ecosystem (Sandom and Wynne-Jones Reference Sandom, Wynne-Jones, Nettorellim, Durant and du Toit2019), it is often required in the initial stages of a reintroduction project to successfully establish small populations as they are more vulnerable to stochastic risks (Frankham Reference Frankham2010), which can be seen in other rewilding reintroduction projects (Pouget and Gill Reference Pouget and Gill2021).
Since reintroductions carry some level of risk and uncertainty, it can be valuable to understand what parameters need to be met to achieve a self-sustaining population which no longer requires human interventions to thrive. Here we use population viability analysis (PVA) to incorporate biological and environmental variables to predict this novel population’s trajectory (Beissinger and McCullough Reference Beissinger and McCullough2002). Our study aims to: (i) discern whether the recently reintroduced British white stork population will be viable in the long term as well as (ii) to assess the impact of different management practices and (iii) migratory behaviour on population growth rates. By studying this reintroduction project in its infancy, imminent threats can be identified, and potential solutions can be explored, improving the chances of reintroduction success.
Methods
Population viability analysis
We conducted PVA using the software Vortex 10.5.20 (Lacy Reference Lacy2019). Vortex10 is an individual-based simulation model where a population is subjected to a combination of deterministic environmental, demographic, and genetic stochastic events based on defined probabilities (Brook et al. Reference Brook, Cannon, Lacy, Mirande and Frankham1999). Unless stated otherwise, models were run for 50 years as this time frame would allow for a comparison between the immediate effects of different scenarios while minimising the impacts of errors and uncertainties in the parameter estimates. All models were run for 1,000 iterations where extinction was defined as the point when only a single sex remained.
Baseline model
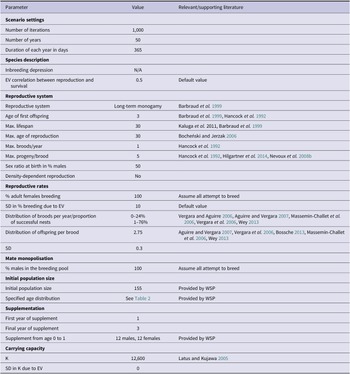
Currently there are minimal data about the British population’s demographic parameters, therefore most of the models’ inputs were derived from relevant literature (Table 1). PVAs can only be as precise as their input values, and this must be taken into consideration when interpreting their results. To improve the accuracy of model estimates, we only extracted parameter values relating to mortality and reproductive rates from papers that fulfilled two criteria. Firstly, only papers with data collected from 1989 onwards were selected to account for the effects of environmental variables on mortality and reproductive rates. For example, during the twentieth century, survival rates of white storks were significantly linked to the amount of rainfall within the Sahel region; this relationship has since weakened (Kanyamibwa et al. Reference Kanyamibwa, Schierer, Pradel and Lebreton1990, Nevoux et al. Reference Nevoux, Barbraud and Barbraud2008a, Schaub et al. Reference Schaub, Kania and Köppen2005). Values produced by older studies would not, therefore, accurately inform models of a present-day British population. Secondly, these populations had to be using a similar migration route to the British storks. European storks migrate using either eastern or western flyways, with the rough dividing line located in central Germany (Shephard et al. Reference Shephard, Ogden, Tryjanowski, Olsson and Galbusera2013). Individuals using the western flyway migrate through the Straits of Gibraltar to overwinter in the Sahel region in West Africa, which is the route the British storks are currently taking (authors’ unpublished data, Barkham Reference Barkham2020). white storks taking this route are anticipated to face similar geographical barriers and risk factors unlike those taking the eastern flyway through Istanbul into the wintering areas in East and South Africa, which is why eastern populations were excluded (Kanyamibwa et al. Reference Kanyamibwa, Bairlein and Schierer1993)
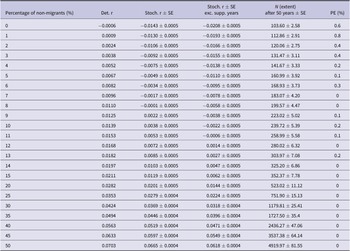
Table 1. Vortex10 parameter inputs for the baseline British white stork population model. EV = environmental variation; N/A = not available; SD = standard deviation; WSP = White Stork Project.

Literature relating to populations prior to 1989 and/or using the eastern flyway were considered for other parameters such as lifespan and maximum brood size (Bocheński and Jerzak Reference Bocheński, Jerzak, Tryjanowski, Sparks and Jerzak2006, Hancock et al. Reference Hancock, Kushlan and Kahl1992, Kaługa et al. Reference Kaługa, Sparks and Tryjanowski2011).
Initial population size
The founder population was derived from three main sources; 74% were wild rescues rehabilitated at Warsaw Zoo, Poland, 7% were from Strasbourg, France, and the remaining 19% were provided by Cotswold Wildlife Park with mostly Polish origins (Groves Reference Groves2021 pers. comm.). The initial population size entered into the PVA consisted of the number of these released white storks and their descendants alive in December 2020, totalling 155 individuals (Table 3, Table 1). This value does not include the flightless storks, which are restricted to a closed pen area maintained by the WSP, due to their greatly different behaviour and mortality rates.
Reproductive system
white storks are known to have infrequent extra pair copulations (e.g. Turjeman et al. Reference Turjeman, Centeno-Cuadros, Eggers, Rotics, Blas, Fiedler, Kaatz, Jeltsch, Wikelski and Nathan2016 found that 73.1% of white stork chicks were fully related siblings), and high rates of social monogamy and nest fidelity (Barbraud et al. Reference Barbraud, Barbraud and Barbraud1999). Due to the absence of genetic information on the British population, white storks were therefore described in Vortex10 as having long-term monogamous relationships. Maximum age of breeding for both males and females was set to 30, with one brood per year (Bocheński and Jerzak Reference Bocheński, Jerzak, Tryjanowski, Sparks and Jerzak2006). Age of first breeding can vary between and within populations, with recorded cases of two-year-old storks attempting to reproduce, although rarely successfully (Barbraud et al. Reference Barbraud, Barbraud and Barbraud1999). For this model the minimum age of successful breeding for males and females was set to three years (Barbraud et al. Reference Barbraud, Barbraud and Barbraud1999, Hancock et al. Reference Hancock, Kushlan and Kahl1992).
Reproductive rate
It was assumed that 100% of adult females aged three years and older would attempt to breed with a 10% standard deviation (SD) due to environmental variation. Based on means taken from studies which met the aforementioned criteria and then weighted based on samples size, it was estimated that 76% of females would successfully fledge their nest at a rate of 2.75 (SD = 0.3) fledglings per successful nest (Aguirre and Vergara Reference Aguirre and Vergara2007, Bossche Reference Bossche2013, Massemin‐Challet et al. Reference Massemin‐Challet, Gendner, Samtmann, Pichegru, Wulgué and Maho2006, Vergara et al. Reference Vergara, Aguirre, Fargallo and Dávila2006, Vergara and Aguirre Reference Vergara and Aguirre2006, Wey Reference Wey and Thomsen2013). Maximum clutch size was set to five (Hancock et al. Reference Hancock, Kushlan and Kahl1992, Hilgartner et al. Reference Hilgartner, Stahl and Zinner2014, Nevoux et al. Reference Nevoux, Barbraud and Barbraud2008b).
Dispersal
As only one population was modelled in Vortex10, dispersal could not be directly considered, nor were there enough data on the British storks to estimate their dispersal rate. Due to their gregarious nature, it is possible some British storks will be attracted to the larger colonies they encounter in Europe, but this information is not yet available, and it will take several years for tracking data on dispersal to be quantified (Bocheński and Jerzak Reference Bocheński, Jerzak, Tryjanowski, Sparks and Jerzak2006). Meanwhile, there have been recorded sightings of storks arriving from the European mainland to Britain since 1958, so it is reasonable to assume that the emigration rate may be similar to the immigration rate (Fraser Reference Fraser2013).
Genetics
The white stork’s long lifespan and slow generation time may result in the presence of deleterious genetic effects within the population initially being hidden. A lethal equivalent (LE) is a unit of deleterious genetic variation, defined as a set of alleles, that, when dispersed amongst a group of individuals, would be lethal in one individual (Kalinowski and Hendrick Reference Kalinowski and Hedrick1998). Due to a lack of data on this population’s genetic background, as well as the means to model gene flow from dispersal and supplementation, all models had 0 LE unless stated otherwise. Additionally, there is evidence to suggest that European white storks did not lose a significant amount of genetic diversity following their twentieth-century decline, suggesting that any negative repercussions from inbreeding depression is unlikely to hinder the success of the reintroduction project in the short term (Shephard et al. Reference Shephard, Ogden, Tryjanowski, Olsson and Galbusera2013). However it is important to consider the genetic background of a reintroduced species due to the impacts of founder effect and inbreeding depression (Frankham Reference Frankham2010, Jamieson Reference Jamieson2011). As such the relationship between the number of LEs within the population and its growth rate across a 100-year timespan were explored separately (see Supplementary Materials S1.1 and S1.2).
Mortality rates
Preliminary data from the released individuals indicated that the majority (>70%) migrated following the western flyway, however, information on survival rates is not yet available (authors’ unpublished data). Mortality rates vary significantly with age; first-year storks have a higher mortality rate due to a lack of experience and less efficient flight strategies (Kanyamibwa et al. Reference Kanyamibwa, Schierer, Pradel and Lebreton1990, Rotics et al. Reference Rotics, Kaatz, Resheff, Turjeman, Zurell, Sapir, Eggers, Flack, Fiedler, Jeltsch, Wikelski and Nathan2016, Schaub and Pradel Reference Schaub and Pradel2004). Collisions with powerlines within the vicinity of the nest can also contribute heavily to post-fledging mortality (Tobolka Reference Tobolka2014). When exploring the impacts of the current management strategy, it was assumed that all the British storks will take the traditional migration along the western flyway towards the Sahel (Kanyamibwa et al. Reference Kanyamibwa, Schierer, Pradel and Lebreton1990). Both sub-adult (storks aged 1–3) and adult (storks aged 3+) mortality was set at 22.16% (SD due to environmental variation = 3) based on the population in the Brouage marshes in west France with rings recovered in Mali and Uganda (Barbraud et al. Reference Barbraud, Barbraud and Barbraud1999, Nevoux et al. Reference Nevoux, Barbraud and Barbraud2008b). Juveniles (age 0–1) were assigned a mortality rate of 65.1% (SD due to environmental variation = 10) based on a mean derived from Cheng et al. (Reference Cheng, Fiedler, Wikelski and Flack2019), Flack et al. (Reference Flack, Fiedler, Blas, Pokrovsky, Kaatz, Mitropolsky, Aghababyan, Fakriadis, Makrigianni, Jerzak, Azafzaf, Feltrup-Azafzaf, Rotics, Mokotjomela, Nathan and Wikelski2016), and Rotics et al. (Reference Rotics, Turjeman, Kaatz, Resheff, Zurell, Sapir, Eggers, Fiedler, Flack, Jeltsch, Wikelski and Nathan2017), and weighted by sample size.
The impact of management
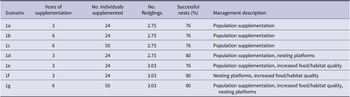
Several management options were tested to compare their impacts on the population’s growth rate and population size up to 50 years. The management options considered included population supplementation, supplementary feeding/habitat improvement, and the provision of nesting platforms. The combination and duration of the management options tested are shown in Table 2.
Table 2. Different management strategies applied in Vortex10.

Population supplementation
Population supplementation has been used in previous white stork reintroductions to reinforce the founder population and increase population size (Olsson Reference Olsson2007, Schaub et al. Reference Schaub, Pradel and Lebreton2004). The WSP’s current management plan is to release a further 24 fledglings over the next three years (see Table 2, scenario 1a). These fledglings are bred by the Cotswold Wildlife Park and due to the success of their captive breeding programme, the authors decided to explore the impacts of increasing the duration of the programme and number of storks supplemented (see Table 2, 1b and 1c), which are both feasible in reality. For all models an equal number of males and females were supplemented.
Provision of nesting platforms
The provision of nesting platforms is a common management option in white stork conservation with evidence to suggest that providing platforms in high-quality suitable habitat can increase reproductive success (Hilgartner et al. Reference Hilgartner, Stahl and Zinner2014, Santopaolo et al. Reference Santopaolo, Godino, Golia, Mancuso, Monterosso, Pucci, Santopaolo and Gustin2013, Zbyryt et al. Reference Zbyryt, Sparks and Tryjanowski2021). To model the provision of nesting platforms, the percentage of successful breeding females was increased from 76% to 80% in scenarios 1d, 1f, and 1g. This increase was based on expert experience and considered plausible.
Supplementary feeding and habitat improvement
Studies have shown that access to food supplementation as well as proximity to high-quality habitat can significantly increase the number of fledglings produced (Barbraud et al. Reference Barbraud, Barbraud and Barbraud1999, Hilgartner et al. Reference Hilgartner, Stahl and Zinner2014, Massemin‐Challet et al. Reference Massemin‐Challet, Gendner, Samtmann, Pichegru, Wulgué and Maho2006, Tortosa et al. Reference Tortosa, Pérez and Hillström2003). In scenarios 1e, 1f, and 1g, improvement in the breeding habitat quality or food supplementation was represented by increasing the number of fledglings produced per successful nest (Jzm) by 10% to 3.03. This was considered feasible as greater productivity is possible without management interventions; for example, 3.82 fledglings per successful nest have been recorded in the Kizilirmak Delta, Turkey (Yavuz et al. Reference Yavuz, Yavuz, Tavares and Barõú2012).
Migratory behaviour
Whilst white storks are regarded as a migratory species, populations where some individuals overwinter on their breeding grounds can be found in several northern European regions and are expected to grow due to increasingly mild winters and year-round food availability (Gilbert et al. Reference Gilbert, Correia, Silva, Pacheco, Catry, Atkinson, Gill and Franco2016, Massemin‐Challet et al. Reference Massemin‐Challet, Gendner, Samtmann, Pichegru, Wulgué and Maho2006, Olsson Reference Olsson2007, Schaub et al. Reference Schaub, Pradel and Lebreton2004). Since migratory behaviour has a large influence on mortality rates, and consequently population growth rates, the relationship between the two were explored (Cheng et al. Reference Cheng, Fiedler, Wikelski and Flack2019, Flack et al. Reference Flack, Fiedler, Blas, Pokrovsky, Kaatz, Mitropolsky, Aghababyan, Fakriadis, Makrigianni, Jerzak, Azafzaf, Feltrup-Azafzaf, Rotics, Mokotjomela, Nathan and Wikelski2016, Rotics et al. Reference Rotics, Kaatz, Resheff, Turjeman, Zurell, Sapir, Eggers, Flack, Fiedler, Jeltsch, Wikelski and Nathan2016, Reference Rotics, Turjeman, Kaatz, Resheff, Zurell, Sapir, Eggers, Fiedler, Flack, Jeltsch, Wikelski and Nathan2017). To represent the mortality rate of the non-migratory individuals, values were taken from populations foraging heavily on Portuguese landfill sites year-round and mortality rates for adults and each sub-adult class were obtained (Table 3) (Rogerson Reference Rogerson2019). To explore the effect non-migratory behaviour has on the population growth rate, the proportion of non-migratory storks was increased from 0% to 50% in increments of 5%. The associated mortality rates entered into Vortex10 were calculated by taking the previous mean migratory values and those reported by Rogerson (Reference Rogerson2019), weighted based on the proportion of non-migrants being modelled (the exact values entered can be seen in the Supplementary Materials S2). The mortality rate of juvenile storks (age 0–1) remained at the original value (65.1%) as juvenile storks of non-migratory populations still tend to migrate in their first year (Rogerson Reference Rogerson2019, Chernetsov et al. Reference Chernetsov, Berthold and Querner2004).
Table 3. The British white stork initial population size and mortality rates for migratory and non-migratory birds entered into Vortex10. EV = environmental variation; SD = standard deviation.

Results
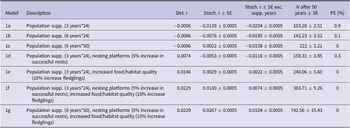
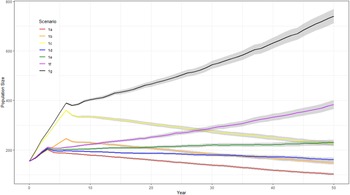
The probability of extinction within 50 years for the British population under the WSP’s current management strategy (1a) was very low (<1%) with additional management reducing the probability further (Table 4). Models 1a–1c had negative deterministic growth rate whilst 1d–1g had a positive deterministic growth rate. When the population is assumed to be fully migratory, maintaining the current WSP management plan (1a) would lead to a negative white stork growth rate (r = −0.0204) (Figure 1, Table 4), suggesting the current management actions would not be sufficient to sustain a viable long-term population. Increasing the length and intensity of the supplementation (1b–1c) would increase population size but would not overcome the negative growth rate once the supplementation ended (r = −0.0185 and −0.0158, respectively (Figure 1, Table 4). Combining the current WSP management plan with nest platforms and the associated increase in number of successful nests (1d) would still not be sufficient to overcome the negative population growth rate (r = −0.0116) (Figure 1, Table 4). Alternatively, increasing food/habitat quality (1e) with the associated higher fledging survival rates would result in a positive growth rate (r = 0.0022) (Figure 1, Table 4). Combining both these management options with the current management plan (1f) increased the growth rate further (r = 0.0074) (Figure 1, Table 4). Combining the greatest length and intensity of population supplementation with nesting platforms and increased food/habitat quality (1g) resulted in the greatest increase in growth rate and population size after 50 years (r = 0.0104) (Figure 1, Table 4).
Table 4. Population viability model results from Vortex10 comparing different management strategies on the British white stork population. Det. r = deterministic growth rate; Stoch. r = stochastic growth rate; SE = standard error; Exc. supp. years = excluding years where population supplementation occurred; N = population size; PE = probability of extinction in 50 years.


Figure 1. Predicted trends in population size of white storks (Ciconia ciconia) in Great Britain over 50 years using Vortex10 under different management strategies (1a–1g). The initial population size in all scenarios was 155 and a carrying capacity of 12,600. The grey shading represents 95 CI based on the distribution of iterations.
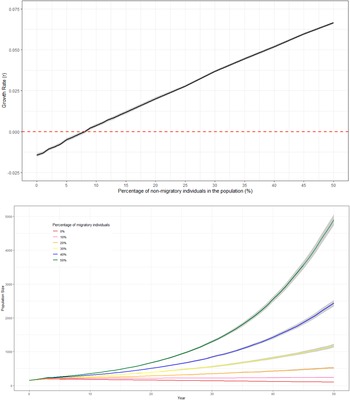
Since the survival rate of non-migratory birds was higher than that of migratory birds, there was a positive relationship between the percentage of non-migratory individuals within the populations and mean growth rate (Figure 2a and b). The deterministic growth rate was only negative when there were no non-migrating individuals in the population (Table 5). With the current management strategy, a positive stochastic growth rate could be achieved if 9% of individuals within the population were non-migratory (Figure 2a, Table 5).
Table 5. Population viability model results from Vortex10 modelling different proportions of non-migratory individuals in the British white stork population. Det. r = deterministic growth rate; Stoch. r = stochastic growth rate; SE = standard error; Exc. supp. years = excluding years where population supplementation occurred; N = population size; PE = probability of extinction of the population in 50 years.


Figure 2. A: Predicted trends in population size of white storks (Ciconia ciconia) in Great Britain over 50 years using Vortex10 where different percentages of non-migratory individuals within the population were modelled. The initial population size in all scenarios was 155 and the carrying capacity was set to 12,600. The grey shading represents 95% CI from the distribution of values from all iterations. B: The relationship between the percentage of non-migratory/resident white stork (Ciconia ciconia)overwintering in Great Britain and the mean stochastic growth rate of the population across all years. Models were produced using Vortex10 and were run for 50 years and 1000 iterations. The initial population size in all scenarios was 155 and a carrying capacity of 12,600. The grey shading represents 95% CI from the distribution of values from all iterations.
Discussion
This study has shown that under the current management strategy, our PVA models predict a decline in the population size if the entire British white stork population chose to migrate along traditional routes. However, the decline would be slow, with only a 0.9% chance of the population going extinct within 50 years. Supplementing the population with a greater number of juveniles, across a longer time period, did further reduce the probability of extinction and delayed when the population would start to decline but could not prevent it entirely. Instead, management actions which increased reproductive output were generally more effective. The provision of suitable nesting sites reduced the rate of the decline compared with the current management strategy, whilst providing additional food or improving habitat quality did achieve a positive growth rate. Combining all the management actions achieved the greatest positive growth rate. Alternatively, if a minimum of 9% of adults overwintered in the UK as residents, additional management would not be required due to the associated lower mortality rate with this behaviour. Overall, this study suggests a promising future for white storks in the UK, and hopefully this reintroduction will follow upon the successes of previous white stork reintroductions undertaken across Western Europe (Thomsen and Hötker Reference Thomsen and Hötker2013).
Many migratory birds, including white storks, are exposed to an array of threats whilst migrating, including hunting pressure and electrocution from overhead powerlines (Cheng et al. Reference Cheng, Fiedler, Wikelski and Flack2019, Kaługa et al. Reference Kaługa, Sparks and Tryjanowski2011, Klaassen et al. Reference Klaassen, Hake, Strandberg, Koks, Trierweiler, Exo, Bairlein and Alerstam2014, Lok et al. Reference Lok, Overdijk and Piersma2015, Raine et al. Reference Raine, Gauci and Barbara2016). Whilst actions such as modifying electrical poles are being implemented to minimise these risks and the associated mortality (Kaługa et al. Reference Kaługa, Sparks and Tryjanowski2011), local management actions which improve reproductive output can also be used to counter the high mortality rates (Schaub et al. Reference Schaub, Pradel and Lebreton2004). Ensuring access to reliable food supplies close to the nesting sites, from either anthropogenic or natural sources, can be an important factor for the successful rearing of fledglings as the foraging range of breeding storks is restricted in the chick rearing period (Hilgartner et al. Reference Hilgartner, Stahl and Zinner2014, Massemin‐Challet et al. Reference Massemin‐Challet, Gendner, Samtmann, Pichegru, Wulgué and Maho2006, Tortosa et al. Reference Tortosa, Pérez and Hillström2003). Food provisions can also minimise detrimental impacts of heavy rainfall in the early chick rearing period when chicks are particularly vulnerable, and this stochastic mortality due to extreme weather may increase in frequency with climate change (Kosicki Reference Kosicki2012, Olsson Reference Olsson2007, Tobolka et al. Reference Tobolka, Zolnierowicz and Reeve2015).
If the British population did require intensive prolonged management, such as directly providing food to prevent a decline, the reintroduction is unlikely to be considered a success (IUCN 2013). Furthermore, such human intervention is often at odds with the values of rewilding, with which this particular project is associated, due to the primary reintroduction site being located on the Knepp Castle Estate (Perino et al. Reference Perino, Pereira, Navarro, Fernández, Bullock, Ceaușu, Cortés-Avizanda, van Klink, Kuemmerle, Lomba, Pe’er, Plieninger, Rey Benayas, Sandom, Svenning and Wheeler2019, White Stork Project 2021a). However, this study shows that a positive population growth rate could be achieved if a small proportion of adults overwintered in the UK as residents due to the lower mortality rate associated with avoiding migration. The trend for white storks to take shorter migrations or remain at their breeding grounds all year has been increasing in the past few decades, particularly around the Iberian Peninsula where large proportions of the population stay year-round (Catry et al. Reference Catry, Encarnação, Pacheco, Catry, Tenreiro, Leão, Bally, Roda, Capela, Alonso, Urbano, Saraiva, Sequeira, Mendes, Monteiro and Elias2017, Cuadrado et al. Reference Cuadrado, Sánchez, Barcell and Armario2016). This is thought to be due to milder climates and the emergence of reliable year-round food sources such as landfills and invasive crayfish (Archaux et al. Reference Archaux, Balança, Henry and Zapata2004, Cheng et al. Reference Cheng, Fiedler, Wikelski and Flack2019, Ferreira et al. Reference Ferreira, Grilo, Mendes, Lourenço, Santos and Petrucci-Fonseca2019, Gilbert et al. Reference Gilbert, Correia, Silva, Pacheco, Catry, Atkinson, Gill and Franco2016).
Unlike in Portugal where studies have reported up to 75% of the white stork population remaining all year round (Andrade et al. submitted), this study only considered the effects of up to 50% of the population remaining as residents. Whilst white storks can tolerate colder temperatures, the associated lack of consistent food can make year-round residency difficult without human intervention (Mata et al. Reference Mata, Caloin, Michard-Picamelot, Ancel and Le Maho2001). This was seen during the early stages of white stork reintroductions in Sweden and Switzerland, where individuals were kept in captivity until they reached sexual maturity, but subsequently did not exhibit migratory behaviour once released and had to rely on feeding stations to survive the winter (Olsson and Rogers Reference Olsson and Rogers2009, Schaub et al. Reference Schaub, Pradel and Lebreton2004). It is interesting to note that the mortality rates calculated for the resident birds in Switzerland were similar to the values adopted by this study, which were derived from a Portuguese population residing on a landfill (Schaub et al. Reference Schaub, Pradel and Lebreton2004, Rogerson, Reference Rogerson2019). This implies that if a reliable food source is available, overwintering British storks may possess mortality rates similar to those recorded in the Swiss population despite the difference in climate.
Since the UK has considerably milder winters in comparison to Sweden and Switzerland, it is expected that food supplementation will not be necessary to support an overwintering population (Gow et al. Reference Gow, Campbell-Palmer, Edgcumbe, Mackrill, Girling, Meech, Dennis and Burrell2016). It is also possible that this population may start to utilise landfills, as seen across Europe, which would provide extra resources for overwintering individuals (Massemin‐Challet et al. Reference Massemin‐Challet, Gendner, Samtmann, Pichegru, Wulgué and Maho2006). However, municipal biological waste (of which food waste is contained) has been steadily decreasing over the past decade across the UK and Europe more widely, and is anticipated to continue in line with EU waste legislation thus minimising landfills effectiveness as a food source (DEFRA 2021, Wang et al. Reference Wang, Tang, Long, Higgitt, He and Robinson2020).
In terms of non-anthropogenic food sources, there may be sufficient high-quality semi-natural habitat to support overwintering individuals as is the case for other wetland species in Britain (Amaral-Rogers Reference Amaral-Rogers2021). Storks are opportunistic feeders, and whilst the composition of their diet varies depending on availability, it often consists of insects, small mammals, and worms (Antczak et al. Reference Antczak, Konwerski, Grobelny and Tryjanowski2002, Chenchouni Reference Chenchouni2017). Since this species’ decline in Europe is party attributed to the loss of high-quality habitat and its associated food sources due to agricultural intensification in the twentieth century (Donald et al. Reference Donald, Green and Heath2001, Luthin Reference Luthin1987, Verhoeven Reference Verhoeven2014), habitat restoration projects have been implemented across Europe to restore white stork populations (ESVN 2021, Thomsen and Hötker Reference Thomsen and Hötker2013). Mirroring such efforts in the UK would not only support overwintering and breeding white storks but could also have wider biodiversity benefits for other vulnerable farmland and wetland species (Carrascal et al. Reference Carrascal, Bautista and Lázaro1993, DEFRA 2020). Therefore, monitoring how the UK storks utilise the landscape across the year could help focus such efforts and aid in the efficient allocation of conservation resources to further increase the reintroductions’ probability of success (Olsson and Rogers Reference Olsson and Rogers2009).
Modelling caveats and recommendations for future research
This study uses a modelling approach to provide guidance for conservation management, however there are several parameters that could be improved to increase the PVA’s performance as a management tool. Firstly, one significant limitation of these models is the lack of consideration of density-dependent effects such as dispersal and the Allee effect. Dispersal is a highly influential parameter for which data are not yet available as the majority of the population are still sub-adults which exhibit more exploratory behaviours than breeding adults, and so it may still be a few years before the general trend in dispersal is understood (Chernetsov et al. Reference Chernetsov, Chromik, Dolata, Profus and Tryjanowski2006, Itonaga Reference Itonaga2009, Vergara et al. Reference Vergara, Aguirre and Fernández-Cruz2007). In white storks natal dispersal occurs more commonly than breeding dispersal, and often occurs along their migration routes, although the availability of suitable nesting habitats is also influential (Chernetsov et al. Reference Chernetsov, Chromik, Dolata, Profus and Tryjanowski2006, Itonaga et al. Reference Itonaga, Köppen, Plath and Wallschläger2010, Rojas et al. Reference Rojas, Sueur, Henry, Doligez, Wey, Dehorter, Massemin and France2016, Ječmenica and Kralj Reference Ječmenica and Kralj2017). This suggests that some of the storks released in the UK may be attracted to joining the larger French and Iberian colonies that are found along the western flyway which would reduce the British population’s growth rate (Thomsen and Hötker Reference Thomsen and Hötker2013). However, the rate of this dispersal is difficult to predict as the influence of population density varies across different populations (Itonaga Reference Itonaga2009, Rojas et al. Reference Rojas, Sueur, Henry, Doligez, Wey, Dehorter, Massemin and France2016). Furthermore, some loss from dispersal could be mitigated by incoming birds; both the British and Swedish populations have attracted immigrating storks even though the populations are small and at the edge of the species’ range (Olsson Reference Olsson and Thomsen2013, White Stork Project 2021b).
The influence of the Allee effect on white stork populations is highly variable and difficult to predict as it is significantly affected by factors such as resource detection probability, colonisation behaviour, and home range selection strategy (Zurell et al. Reference Zurell, Eggers, Kaatz, Rotics, Sapir, Wikelski, Nathan and Jeltsch2015). Even within landscapes with similar carrying capacities, the interaction between breeding pair density and reproductive output can vary considerably (Zurell et al. Reference Zurell, Eggers, Kaatz, Rotics, Sapir, Wikelski, Nathan and Jeltsch2015). Further research into the interactions between individual behaviour and local environmental factors would allow for more meaningful predictions on the influence of the Allee effect on this population (Zurell et al. Reference Zurell, Eggers, Kaatz, Rotics, Sapir, Wikelski, Nathan and Jeltsch2015), particularly within a highly dynamic landscape such as the Knepp Castle Estate (Tree Reference Tree2018). As the British population gains greater numbers of breeding adults and increases in population size, the impact of both these density-dependent processes will become clearer and should be incorporated to produce a more robust model.
Additionally, the inclusion of genetic parameters would also affect the models’ outputs. If included, the population’s growth rate would likely decline whilst the probability of extinction would increase as reintroductions can create population bottlenecks leading to the loss of genetic diversity and/or inbreeding depression (Frankham et al. Reference Frankham, Ballou and Briscoe2002, Jamieson and Lacy Reference Jamieson, Lacy, Ewen, Armstrong, Parker and Seddon2012). The significance of these impacts would be dependent on the genetic diversity of the founder population, reproductive skew, and the mitigating influence of gene flow through the supplementation of individuals and dispersal behaviour (Heber et al. Reference Heber, Varsani, Kuhn, Girg, Kempenaers and Briskie2013, Jamieson Reference Jamieson2011, Le Gouar et al. Reference Le Gouar, Rigal, Boisselier-Dubayle, Sarrazin, Arthur, Choisy, Hatzofe, Henriquet, Lécuyer, Tessier, Susic and Samadi2008).
Even with the uncertainty associated with the aforementioned population parameters, this reintroduction project has a high probability of creating a viable British white stork population, particularly if a residential population forms. Many of the models’ weaknesses are due to a lack of data on this novel population on account of the reintroduction project’s infancy. We recommend that as more data on this population become available, the models’ inputs should be updated and developed to improve their accuracy and effectiveness in assisting with management decisions.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1017/S0959270922000466.