In ruminant feeding, one aim is to reduce environmental pollutants, including emissions of N generally and NH3 and N2O especially. While the contribution of manure causes high eutrophication of aquatic environments, NH3 affects air quality and the presence of N2O increases global greenhouse gas concentrations. Recently, it has been shown that in lactating dairy cows, the total dietary N intake determines the total N excretion as manure(Reference Castillo, Kebreab and Beever1). Therefore, there is an opportunity for decreasing N pollution in ruminants by reducing dietary N intake as long as reducing N does not decrease animal health and productivity. Exacerbating the known changes in Ca homeostasis by dietary N and Ca reduction(Reference Muscher and Huber2) could abolish the positive effects of N reduction on environmental pollution. Since ruminants are unique in their ability to save N by endogenous recycling mechanisms, especially at times of protein scarcity(Reference Houpt3, Reference Harmeyer and Martens4), it is expected that these efficient recycling mechanisms help to maintain the animal's health in times of dietary N reduction.
In monogastric animals and humans, metabolic responses to variation in dietary protein supply provided evidence that protein metabolism was closely related to electrolyte homeostasis. Alterations in plasma 25-hydroxyvitamin D3 (calcidiol) and urinary excretion of Ca and inorganic phosphate (Pi) in dietary protein-restricted fed rats confirmed that hypothesis(Reference Orwoll, Ware and Stribrska5). Additionally, a reduction of intestinal Ca absorption occurs in monogastric animals and humans with a low-protein diet(Reference Orwoll, Ware and Stribrska5, Reference Kerstetter, O'Brien and Insogna6). During the reduction of intestinal Ca absorption in humans, an abrupt rise in serum parathyroid hormone (PTH) concentration has been observed(Reference Kerstetter, O'Brien and Insogna6, Reference Kerstetter, O'Brien and Insogna7). A low-protein diet reduces plasma amino acid concentrations(Reference Peng, Meliza and Vavich8, Reference Fujita, Yoshimura and Inoue9), which induces higher secretion of PTH from parathyroid glands(Reference Kerstetter, Caseria and Mitnick10). In vitro studies have shown that in isolated bovine parathyroid gland cells, changes in amino acid concentrations were able to modulate PTH secretion(Reference Conigrave, Mun and Delbridge11). In kidneys, high PTH concentrations induced internalisation of Na-dependent Pi transporters (NaPi II; SLC34A1, renal NaPi IIa) located in proximal tubules, resulting in higher renal Pi excretion(Reference Murer, Forster and Hernando12). Simultaneously, PTH stimulated renal synthesis of 1,25-dihydroxyvitamin D3 (calcitriol), which elevates the intestinal absorption of Ca and Pi(Reference Brown, Dusso and Slatopolsky13). In bones, an increase in PTH concentrations activated the mobilisation of Ca and Pi, which causes a reduction in bone density and increases the risk of osteoporosis(Reference Sahota, Masud and San14).
During periods of protein deficiency, it is expected that in goats, because of their ability to recycle N from the blood and saliva into the gastrointestinal tract, the described interaction between protein and electrolyte metabolism in monogastric animals would be less expressed, and therefore the possible negative effect for the animal's health would be less pronounced.
The first findings showed that when growing goats were fed a diet reduced in N and Ca, hypocalcaemia and further changes in Ca homeostasis occurred(Reference Muscher and Huber2). However, it could not be distinguished to what extent the dietary N reduction elicited these changes. Therefore, the purpose of the present study was to determine the effect of dietary N reduction under normocalcaemia on the relationship between N and electrolyte metabolism in young goats. For this reason, parameters of Ca and Pi homeostasis were determined in goats fed a reduced N diet. In addition, calcitriol and calcidiol concentrations were measured to evaluate whether this relationship is hormonally regulated. Finally, we investigated whether a reduced N diet affects the intestinal absorption of Pi focusing on Na+-dependent Pi transport across the apical membrane using brush-border membrane vesicles (BBMV) and examining the protein amount of Na+-dependent Pi transporters (SLC34A2, intestinal NaPi IIb).
Materials and methods
Animals and feeding regimen
The protocol of the animal treatment was approved, and its conduct was supervised by the Animal Welfare Commissioner of the University of Veterinary Medicine Hannover (Hannover, Germany) according to the German Animal Welfare Law.
A total of twenty male White Saanen goats, about 3 months of age and weighing approximately 24·0 (se 1·0) kg live weight, were subdivided into two different feeding regimens with an adequate or a reduced N supply. Each group of ten animals was housed separately. Water was available at all times. The amounts of all feeds offered and refused was monitored for estimating intake ‘per animal’ based on the intake amounts from pooled group mean values.
The reduction of N supply was maintained for about 7 weeks. The feed content of DM, crude ash, crude fat and crude protein was determined by the standard procedure of VDLUFA (Der Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten; Weender analysis)(Reference Naumann and Basler15). The amount of acid-detergent and neutral-detergent fibres was measured by a method described by Van Soest et al. (Reference Van Soest, Roberstson and Lewis16). The components and composition of the two diets are presented in Table 1. Both diets were isoenergetic, containing 11·35 kJ metabolisable energy/kg diet.
Table 1 Components and composition of the pelleted concentrate diets*

ND, not detected; DCAD, dietary cation–anion differences.
* Composition expressed as fed.
† Per kg mineral–vitamin mix: 180 g Ca, 60 g P, 100 g Na, 30 g Mg, 500 000 IU (525 μmol/l) vitamin A, 80 000 IU vitamin D3 (4992 nmol/l), 300 mg vitamin E (697 μmol/l), 4200 mg Zn, 900 mg Mn, 16 mg Co, 20 mg I and 44 mg Se. Values are determined by Weender analysis.
‡ Sipernat type 22S (Evonik Industries AG, Essen, Germany) is a fine particle silica with high oil absorption capacity. It is widely used as a flow regulator, anti-caking and dusting agent, especially in the food and feed industry.
Sampling
Plasma and saliva samples were obtained shortly before the goats were killed. These samples were stored at − 20°C for subsequent analysis. At the end of the study (4–5 months of age), the animals were slaughtered after captive bolt stunning by exsanguination. Samples of ruminal fluid, abomasal fluid and urine from the bladder were taken. Mid-jejunal segments were removed within 3–5 min after slaughter. After being rinsed with ice-cold saline (0·9 % NaCl, w/v), the mucosa of the jejunum was stripped off. After freezing in liquid N2, the jejunal samples were stored at − 80°C until further structural and functional analyses.
Biochemical determinations
Haematocrit was determined in total blood samples. An aliquot of each blood sample was placed in a microhaematocrit tube and spun to constant packed cell volume at 14 926 g for 5 min at room temperature. Plasma and urine urea concentrations were measured by using a commercial kit (R-Biopharm, Darmstadt, Germany). Creatinine concentration in plasma and urine was analysed by a standard enzymatic method, the creatinine p-amino-phenazone method(Reference Jung, Wesslau and Priem17). Total plasma protein concentration was measured with a commercial Coomassie blue protein assay (Bio-Rad, Munich, Germany) using bovine plasma γ-globulin as a standard protein. Plasma albumin concentrations were detected by a standard dye-binding technique using bromocresol green (Hengler Analytik, Steinbach, Germany). Concentrations of Pi and total Ca were measured colorimetrically in plasma, saliva, urine, abomasal and ruminal fluids by standard spectrometric techniques(Reference Sarkar and Chauhan18, Reference Kruse-Jarres19). The amount of calcidiol was determined by a competitive enzyme-linked immunosorbent assay (Immundiagnostik AG, Bensheim, Germany). The declared inter- and intra-assay CV of the kit were < 13·2 and < 10·7 %. Calcitriol concentrations were measured by a commercial radioreceptor assay (Immundiagnostik AG, Bensheim, Germany). For this process, the plasma was cleaned by Extrelut extraction. The intra-assay CV were < 15 and < 10 % for samples with calcitriol concentrations of 10 and 60 pg/ml, respectively. The inter-assay CV were < 20 and < 15 % for these two concentrations. The detection limit of this assay was 2 pg/ml. The calcitriol assay systems had already been used in other studies to detect caprine hormone concentrations(Reference Schröder and Breves20–Reference Muscher, Hattendorf and Pfeffer22). cAMP concentrations were determined in plasma and urine by a commercial competitive binding assay (Immundiagnostik AG, Bensheim, Germany). The inter- and intra-assay CV of the kit were < 8·8 and < 7·6 %. The concentration of plasma carboxyterminal cross-linked telopeptide of type I collagen (CTX) was determined using the commercial kit Serum CrossLaps® ELISA (Immunodiagnostic Systems, Frankfurt, Germany). The cross-reactivity for goats had been tested by the company(Reference Chavassieux, Garnero and Duboeuf23). The inter- and intra-assay CV of the kit were < 10 and < 6 %. Total alkaline phosphatase (EC 3.1.3.1) activity was determined in plasma samples by measuring the increase in absorbance (405 nm) due to the formation of p-nitrophenol from p-nitrophenyl phosphate ester(Reference Kawade24). For the determination of total plasma insulin-like growth factor 1 (IGF-1), an ACTIVE Insulin-Like Growth Factor-I coated-tube IRMA Kit (DSL-5600; Diagnostic Systems Laboratories, Inc., Webster, TX, USA) was used. IGF-1 was separated from its binding proteins by an acid–ethanol extraction procedure, and IGF-1 concentrations were determined with a two-site immunoradiometric assay. Intra- and inter-assay CV were 1·5–3·5, and 1·5–8·5 %, respectively. The concentration of growth hormone (GH) was measured by an enzyme-linked immunosorbent assay, as described previously(Reference Kawashima, Sakaguchi and Szuki25, Reference Roh, Matsunaga and Miyamoto26), with the following modifications: a rabbit antibody against ovine GH (anti-ovine GH-3, 1:20, AFP-0802210, obtained from the National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney, and Dr Parlow) was distributed in all wells of a ninety-six-well microplate previously coated with anti-rabbit γ-globulin antiserum (D Schams; TU-Munich, Weihenstephan, Germany). After incubation for 24 h at room temperature and decantation of the antibody solution, 100 μl of chicken serum diluted in assay buffer (1 %) was added to each well. Then, 15 μl of a standard GH solution (0·78–100 ng/ml, bovine GH, AFP-9984C, obtained from the National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney, and Dr Parlow) dissolved in assay buffer or plasma samples were added, and the plate was incubated for 24 h at room temperature. After washing the plate, biotin-labelled GH was distributed in all wells and then incubated for 3 h at room temperature. Streptavidin solution (Sigma Aldrich, St Louis, MO, USA) was added and optical density was measured, and concentrations were calculated using Magellan software (Magellan 3.11, Dortmund, Germany). Intra- and inter-assay CV were 9·8 and 12·6 %, respectively. The lowest detection limit was 1·0 ng/ml, and the effective dose 50 in this assay system was 7·6 ng/ml.
Calculation of fractional excretion
The fractional excretion (FE) of urea was determined at the end of the experimental period using the following formula:
The fractional excretion of cAMP, Pi and total Ca was calculated according to the same equation, but with respective cAMP, Pi and total Ca concentrations.
Isolation of intestinal brush-border membrane vesicles, transport studies and Western blot analysis
For preparation, transport studies and Western blot analysis of small-intestinal BBMV have already been described in Muscher et al. (Reference Muscher, Hattendorf and Pfeffer22). Briefly, BBMV were prepared according to the Mg2+ precipitation method, with two precipitation steps. For the uptake of 32P/Pi, BBMV were incubated at 21°C with the uptake medium containing non-labelled Pi and α-32P (Hartmann, Braunschweig, Germany; 0·037 MBq/incubation vessel). Concentration-dependent Pi uptakes were performed over a range of 0·01–1·0 mm-Pi. Extravesicular incubation buffer contained 100 mm-mannitol, 10 mm-HEPES–Tris, pH 7·4, and 100 mm-NaCl. The stop solution contained 150 mm-KCl, 1 mm-KH2PO4 and 10 mm-HEPES–Tris, pH 7·4. The Na+ dependency of Pi transport was established by incubating BBMV in solutions in which KCl replaced NaCl equimolarly. BBMV were washed with stop solution on a 0·65 μm cellulose nitrate filter. The 32P activity of each filter was counted using a Packard Tri-Carb 2500TR scintillation counter. Kinetic parameters V max (nmol × 10 s Pi/mg protein) and K m (mmol Pi/l) were calculated from the Michaelis–Menten kinetic equation of Pi uptake into the BBMV.
Immunoblot assays using brush-border membrane preparations were performed as described previously(Reference Muscher, Hattendorf and Pfeffer22, Reference Huber, Walter and Schröder27). In brief, brush-border membrane protein (50 μg/lane) was separated by 8·5 % SDS–PAGE. Separated proteins were transferred onto nitrocellulose membranes and blocked with 10 % fat-free milk powder. Immunodetection of electrotransferred protein was performed according to standard procedures. The NaPi IIb antibody was used at 1:2000 dilution. Specificity of the murine antibody for goat NaPi IIb protein was validated by blocking the antibody with the respective antigenic peptide. Immunoreactive proteins were visualised using the enhanced chemiluminescence system (Perbio Science GmbH, Bonn, Germany) according to the manufacturer's protocol. The internal standard β-actin was used to semi-quantify relative protein expression amounts. Bands were analysed semi-quantitatively using Quantity One software (Bio-Rad).
Statistical analyses
Results are expressed as means with their pooled standard errors, with n number of animals. Significance of differences was tested by unpaired Student's t test, if appropriate (software GraphPad Prism version 4.0 for Windows, GraphPad Software, San Diego, CA, USA, www.graphpad.com). In all cases, P values < 0·05 were set to be significant, and a tendency was assumed when P < 0·1. To test for a linear relationship between two variables, a simple correlation analysis with Pearson's correlation coefficient was calculated.
Results
Daily weight gain, body weight, daily feed intake, intake of DM, nitrogen, calcium and phosphorus
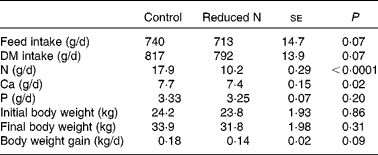
The animals were clinically healthy throughout the study. The daily weight gain of the goats receiving a reduced N diet was tendentially lower than in the control animals (Table 2). However, the mean body weight of all goats at the beginning and at the end of the study was not affected by dietary treatment (Table 2). Mean daily feed, DM, N, Ca and P intake for each animal was estimated from group mean values, and parts of these results have already been published in Muscher et al. (Reference Muscher, Schröder and Breves28) (Table 2).
Table 2 Daily feed, DM, nitrogen, calcium and phosphorus intake, initial and final body weight and daily weight gains on a per animal basis, as estimated from group mean values over the entire experimental period (about 7 weeks)
(Mean values with their pooled standard errors)
Blood parameters
The concentration of urea in plasma decreased significantly by a reduced N feeding regimen, while plasma creatinine concentrations increased simultaneously (Table 3). Both parameters were inversely related (r − 0·604, P < 0·01). Haematocrit, total plasma protein and albumin content remained unchanged throughout the complete experiment, indicating that there was neither haemoconcentration nor haemodilution with the various diets (Table 3). The concentrations of plasma Pi and total Ca (Table 4) were not affected by a reduction of dietary N supply in young goats. Plasma concentrations of calcidiol and calcitriol were significantly reduced in the animals fed a reduced N diet (Table 3). There were no significant differences between control and N-reduced fed goats in the plasma concentrations of cAMP (Table 3). However, a linear correlation between plasma cAMP concentrations and calcidiol could be detected in the goats (r 0·642, P < 0·01). The amount of plasma CTX, as the bone resorption marker, and total alkaline phosphatase was both significantly elevated in the goats of the N-reduced N group.
Table 3 Haematocrit values of young goats fed a reduced nitrogen diet*
(Mean values with their pooled standard errors and number of animals (n))

* Concentrations of urea, creatinine, total protein, albumin, calcidiol, calcitriol, cAMP, CrossLaps, total alkaline phosphatase, growth hormone and insulin-like growth factor 1 in the plasma and urine of goats as affected by a reduced N diet.
Table 4 Effects of a reduced nitrogen diet on calcium and inorganic phosphate (Pi) concentrations in plasma, saliva, ruminal fluid, abomasal fluid and fractional excretion (FE) in young goats
(Mean values with their pooled standard errors and number of animals (n))

The reduction of dietary N content was associated with a decrease in plasma IGF-1 concentration in these animals, while concentrations of GH were not affected (Table 3). Additionally, the concentrations of IGF-1 and plasma urea were positively related (r 0·475, P < 0·05).
Concentrations of inorganic phosphate and total calcium in saliva, ruminal and abomasal fluids
The Pi concentrations of parotid saliva, ruminal and abomasal fluids were all significantly reduced in goats fed a reduced N diet, whereas plasma Pi concentrations were not affected (Table 4). The decline in Pi concentrations in saliva (r 0·684, P < 0·001), ruminal (r 0·503, P < 0·05) and abomasal fluids (r 0·715, P < 0·001) was positively correlated with decreasing plasma urea concentrations.
Salivary total Ca concentrations were significantly elevated at the end of the experiment (Table 4). Ruminal concentrations of total Ca were not affected, whereas total Ca concentrations in the abomasal fluid were significantly diminished in the goats with the reduced N diet (Table 4).
Effects of the reduced nitrogen diet on the renal excretion of urea, total calcium, inorganic phosphate and adenosine 3′,5′-cyclic monophosphate
A reduced dietary N supply was associated with a decrease in the FE of urea (control: 48·4 %, reduced N: 9·34 (se 6·13) %) and an increase in total urine Ca, while FE of Pi (Table 4) and cAMP (control: 232·7 %, reduced N: 262·6 (se 127·7) %) remained unaffected.
Na+-dependent inorganic phosphate transport and type IIb Na+-inorganic phosphate cotransporter expression in the small intestine
Transport capacity (V max; control: 1·38 nmol/mg protein per 10 s, reduced N: 1·48 (se 0·33) nmol/mg protein per 10 s; P = 0·76) and transporter affinity (K m; control: 0·074 mm, reduced N: 0·063 (se 0·01) mm; P = 0·29) of Na+-dependent Pi transport into mid-jejunal BBMV were not affected by a reduced dietary N supply in young goats. However, the protein expression (90 kDa) of NaPi IIb normalised to β-actin in the mid-jejunum was tendentially reduced under a reduced N diet (control: 2·29, reduced N: 1·24 (se 0·51); P = 0·08).
Discussion
The present study demonstrates that the electrolyte homeostasis of young goats was influenced by a reduced N diet despite efficient N recycling mechanisms. These effects of a reduced N diet might be most marked during growth; therefore, a growing goat model was chosen. A slightly reduced daily weight gain indicates that dietary N reduction (8 %) may show the limitation of compensation of N metabolism. Isoenergetic diets were used to detect the effect of N reduction as the single variable on electrolyte homeostasis.
Plasma urea concentrations were determined as a significant indicator of the extent of dietary N supply. The reduction of dietary N by about 44 % caused a marked decrease in plasma urea concentrations. The order of magnitude of this effect is in accordance with data reported for other goats and sheep(Reference Schröder, Schöneberger and Rodehutscord29). The FE of urea in the kidney fell to a very low concentration as one of the N-saving mechanisms to facilitate the recycling of urea for microbial protein synthesis in the rumen. In previous studies in goats, this renal reduction of urea excretion rate could be demonstrated as a consequence of renal mechanisms to conserve urea during N deprivation, whereas glomerular filtration rate (GFR) dropped by about 60 % when N intake was reduced(Reference Eriksson and Valtonen30, Reference Valtonen, Uusi-Rauva and Eriksson31). A decrease in GFR could be caused by a reduction in IGF-1 concentrations, which were able to modulate GFR in rats(Reference Hirschberg and Kopple32). Such a decline in GFR was indicated by an increase in plasma creatinine concentration in the goats fed a reduced N diet in the present study. However, as indicated by the simultaneous increase in urinary creatinine concentrations, it is also likely that creatinine was increasingly released from muscle due to a higher muscle turnover. A higher muscle protein turnover would provide more N for urea synthesis in the N-reduced fed goats to improve N supply to ruminal micro-organisms. Furthermore, a reduction in urine volume, as has been observed in ongoing balance studies in N-reduced fed goats, could contribute to high creatinine concentrations in urine.
Besides a decrease in GFR, reduced IGF-1 concentrations could also cause a decrease in the renal blood flow as in monogastric animals(Reference Rabkin and Schaefer33). Both parameters, a reduced GFR and a decrease in renal blood flow, could diminish substrate delivery to the renal 1-α hydroxylase, which would cause less calcitriol formation in the kidney(Reference Schroeder and Cunningham34). Confirmingly, a correlation between GFR and plasma calcitriol concentrations was shown in humans(Reference Prince, Hutchison and Kent35). Another reason could be that reduced IGF-1 concentrations affect the activity of the renal 1-α hydroxylase as in humans(Reference Bianda, Hussain and Glatz36). A reduction in calcitriol concentrations could be already shown in goats fed a reduced N and Ca diet(Reference Muscher and Huber2) as well as in rats with a low-protein diet(Reference Orwoll, Ware and Stribrska5). In monogastric animals, changes in calcitriol concentrations were able to regulate the intestinal absorption of Pi by the modulation of intestinal Pi transporter function and expression amounts(Reference Yagci, Werner and Murer37, Reference Xu, Bai and Collins38). However, the decrease in calcitriol concentrations in the young goats caused only a tendential reduction in the intestinal transport protein NaPi IIb, which was not reflected in changes in the intestinal absorption of Pi.
Low calcitriol concentrations in plasma are known to decrease bone mineralisation(Reference Dusso, Brown and Slatopolsky39). In this experiment, the bone resorption marker CTX in goats fed a reduced N diet was increased, indicating a higher bone resorption in these animals; this could be shown in goats fed a reduced N and Ca diet, too(Reference Muscher and Huber2). Hypothetically, higher bone resorption could be caused by changes in PTH concentrations because of the intense intertwining of these regulating hormones in Ca and Pi homeostasis(Reference Bergwitz and Juppner40). Humans consuming a low-protein diet expressed an increase in serum PTH(Reference Kerstetter, Caseria and Mitnick10). These elevated PTH concentrations were able to activate Ca and Pi mobilisation from bones. Hence, a link between protein and electrolyte metabolism was postulated for monogastric animals and humans(Reference Conigrave, Mun and Lok41). A possible candidate for such a link could be the Ca-sensing receptor (CaR), which is expressed in many organs(Reference Brown42, Reference Goodman43). The CaR seemed to be able to respond to altered Ca and also to changes in amino acid concentrations due to its binding sites for both(Reference Conigrave, Mun and Lok41). This property of the CaR may be the molecular basis for the crosstalk between protein and Ca and Pi metabolism. Therefore, it is assumed that a low-protein diet was able to stimulate PTH synthesis and secretion via the CaR. Another possibility could be that as in rats, the reduced calcitriol concentrations inhibited less of the CaR, which results in higher PTH secretion by the parathyroid glands(Reference Brown, Zhong and Finch44). Further investigation of the expression and activity of the CaR in young goats fed a reduced N diet is needed.
The elevated activity of total alkaline phosphatase in plasma in the goats of the N-reduced group could be a result of the decreased calcitriol concentrations, which caused perturbed mineralisation of the bones. However, total alkaline phosphatase was not a specific marker of bone formation because its activity in plasma was also contributed to other sources than the bone(Reference Moss45).
The concentration of cAMP in plasma and urine, which were described in the literature as indirect parameters of PTH activation(Reference Drezner, Neelon and Curtis46, Reference Chase and Aurbach47) in humans and monogastric animals, could not confirm that the higher bone resorption detected in the goats fed a reduced N diet was PTH-dependent.
Alterations in amounts of other humoral factors could also be another explanation for the change in bone resorption induced by dietary N reduction. In particular, changes in IGF-1 concentrations were associated with unchanged GH concentrations in the growing N-reduced fed goats. In humans, a positive correlation between protein intake and bone growth factor IGF-1 was observed, while concentrations of CTX decreased at the same time(Reference Dawson-Hughes, Harris and Rasmussen48). Therefore, the effects of N reduction on mineral and bone metabolism observed in the present study may be mediated in part by reduced IGF-1 concentrations. Calcidiol synthesis in the liver might also be affected by reduced IGF-1 concentrations because, in humans, a positive correlation between these two hormones was detected(Reference Gomez, Maravall and Gomez49).
Besides the effects on bone mineralisation, the influence of a dietary N reduction on electrolyte homeostasis was expressed by alterations of Pi and Ca concentrations in body fluids of young goats (Table 4). Changes in Pi homeostasis included a decrease of Pi in saliva starting at the 28th day of the experiment (Fig. 1; Table 4) and low Pi concentrations in ruminal and abomasal fluids (Table 4). Plasma Pi concentrations and extent of urinary Pi excretion were not affected as in goats fed a reduced N and Ca diet(Reference Muscher and Huber2) (Fig. 1; Table 4). The concentrations of Pi in saliva, ruminal and abomasal fluids were all positively correlated with plasma urea concentrations. Since neither the rumen epithelium nor the abomasal mucosa is able to absorb Pi, explaining lower concentrations in these fluids, the modulation of Pi concentrations must be mediated by the capacity of salivary glands to concentrate and secrete Pi into the rumen. In general, it is well known that salivary Pi is adjusted to plasma Pi concentration without any regulation by hormones or other factors(Reference Scott and Beastall50). During a reduced N feeding, this tight adjustment seemed to be uncoupled by mechanisms not known so far. Caprine salivary gland Pi transporter was assessed to be a Pi transporter similar to those which are members of the SLC34 transporter family (NaPi IIb-like)(Reference Huber, Roesler and Muscher51). NaPi IIb protein is ubiquitously expressed in different organs (e.g. small intestine) and tissues(Reference Murer, Forster and Biber52). In monogastric animals, intestinal NaPi IIb was regulated by calcitriol, which stimulated NaPi IIb expression as well as Na+-dependent Pi transport(Reference Hattenhauer, Traebert and Murer53). Hypothetically, the caprine Na+-dependent Pi transporter might be decreased in its function by the lack of calcitriol stimulation due to low calcitriol concentrations in N-reduced goats.

Fig. 1 Concentrations of inorganic phosphate (Pi) in the plasma and saliva of goats fed a reduced N diet for about 7 weeks. Values are means, with standard errors represented by vertical bars (n 10). Mean values were significant for the effect of a dietary reduced N level: * P < 0·05 and ** P < 0·01. ![]() , Pi saliva control;
, Pi saliva control; ![]() , Pi saliva reduced N;
, Pi saliva reduced N; ![]() , Pi plasma control;
, Pi plasma control; ![]() , Pi plasma reduced N.
, Pi plasma reduced N.
In the present study, Ca homeostasis was also affected in growing goats by a reduced N intake. While in plasma and ruminal fluids, total Ca concentrations were not affected, which was in contrast to goats fed a reduced N and Ca diet where plasma Ca concentrations were reduced(Reference Muscher and Huber2), an increase in salivary Ca was found (Table 4). Even though salivary total Ca concentrations were elevated at the end of the experiment, the physiological meaning of the increased Ca concentrations in saliva was not clear. Low abomasal Ca concentrations may be associated with an enhanced ruminal active Ca absorption, which was described for goats and sheep(Reference Schröder, Rittmann and Pfeffer54). The reason for this activated ruminal absorption of Ca during a reduced N diet cannot be explained yet.
The hypercalciuria of the goats receiving a N-reduced diet was not associated with a reduction in plasma total Ca concentrations. In goats fed a reduced N and Ca diet, a reduction in plasma total and ionised Ca concentrations could be observed, caused by a lower Ca intake, which resulted in hypocalciuria(Reference Muscher and Huber2). In the present study, a higher Ca release from bones could be assessed, as indicated by the increase in the bone resorption marker CTX. The majority of Ca is transported in the plasma bound to protein. However, a reduction in binding capacity could be excluded because plasma protein and albumin concentrations did not differ between the two feeding groups. The excess of Ca was finally excreted by the kidneys resulting in a higher FE of Ca. Hypothetically, this could be mediated by lower calcitriol concentrations due to a down-regulation of calcitriol-dependent epithelial Ca channels (transient receptor potential cation channel, subfamily V, member 5) as in rats(Reference Hoenderop, Muller and Van Der Kemp55).
In summary, the present study shows that a reduced N diet in young goats led to decreased calcitriol, calcidiol as well as IGF-1 concentrations and increased bone resorption marker CTX. Furthermore, Pi and Ca homeostasis was perturbed in the N-reduced fed goats despite N recycling mechanisms. In conclusion, an interaction between dietary N and electrolyte metabolism occurred in young goats as in monogastric animals. The dietary requirements of N for young goats must be investigated further regarding the aim to decrease N pollution, especially with a focus on mineral and bone metabolism.
Acknowledgements
The present study was supported by the German Research Foundation. The authors thank K. Hansen, K. Hustedt and B. Leppich for their excellent technical assistance. The NaPi IIb antibody was kindly provided by Dr J. Biber and Professor Dr H. Murer, Institute of Physiology, University of Zurich-Irchel, Zurich, Switzerland. A. S. M., G. B. and K. H. designed the experiments. A. S. M. performed the animal feeding trials, biochemical analyses, molecular analyses and statistical analyses. M. P. performed the IGF-1 and GH measurements. A. S. M. and K. H. wrote the manuscript. All authors discussed the results and commented on the manuscript. There are no conflicts of interest to declare.







