I. Introduction
A prevalent symptom among stroke survivors is motor deficits (Basteris et al., Reference Basteris, Nijenhuis, Stienen, Buurke, Prange and Amirabdollahian2014). This motor impairment significantly impedes stroke patients’ ability to perform activities of daily living (ADL) (Timmermans et al., Reference Timmermans, Spooren, Kingma and Seelen2010), subsequently impacting their quality of life (QOL). Notably, approximately 80 percent of stroke survivors encounter post-stroke deficits in upper extremity (UE) motor performance, with approximately half of them facing challenges in performing ADLs (Kwakkel et al., Reference Kwakkel, Kollen, van der Grond and Prevo2003; Duncan et al., Reference Duncan, Bode, Min Lai and Perera2003).
Several stroke survivors who underwent a UE rehabilitation program demonstrated significant recovery of proximal motor functions at the shoulder and elbow joints. However, their progress in regaining hand and wrist joint functionality remained limited (Jonkman et al., Reference Jonkman, de Weerd and Vrijens1998). Hand functions, especially for finger extension, are vital in numerous daily tasks. Nevertheless, developing an effective training device for rehabilitating dexterous hand functions has posed a considerable challenge.
One potential approach to address these challenges is the development of rehabilitation robotic devices (Maciejasz et al., Reference Maciejasz, Eschweiler, Gerlach-Hahn, Jansen-Troy and Leonhardt2014; Song et al., Reference Song, Tong, Hu and Li2008; Wolbrecht et al., Reference Wolbrecht, Rowe, Chan, Ingemanson, Cramer and Reinkensmeyer2018). In recent decades, hand rehabilitation devices have been introduced to aid stroke survivors in recovering hand functionality (Susanto et al., Reference Susanto, Tong, Ockenfeld and Ho2015; Ho et al., Reference Hon.d.; Hu et al., Reference Hu, Tong, Song, Zheng and Leung2009). However, these devices have limitations, notably their bulky and heavy nature resulting from using rigid components like linear motors and rigid linkages connecting the fingers to the motors (Dellon and Matsuoka, Reference Dellon and Matsuoka2007). Consequently, stroke patients often face difficulty raising their paralyzed arms and performing functional tasks (Tong et al., Reference Tong2010). Moreover, these robotic hands seldom account for the individual’s specific hand conditions, such as finger length, palm width, and joint stiffness.
Our prior research presented a 3D-printed soft robotic hand that utilized the soft-elastic composite actuator (Heung et al., Reference Heung, Tong, Lau and Li2019; Heung et al., Reference Heung, Tang, Shi, Tong and Li2020; Shi et al., Reference Shi, Heung, Tang, Li and Tong2021; Tang et al., Reference Tang, Heung, Shi, Tong and Li2022; Shi et al., Reference Shi, Heung, Tang, Tong and Li2020). Building upon this foundation, we have further advanced our work by developing an updated version of the interactive soft wearable robotic hand specifically designed for task-oriented hand-functional training. The new version of the pneumatic actuator in this version offers enhanced actuation torque, while a novel wireless interactive task training board has been designed and seamlessly integrated with the robotic hand. This innovative solution demonstrates our commitment to addressing the unique requirements of stroke rehabilitation, offering a more personalized and interactive approach to therapy.
II. Interactive soft robotic hand system
A. Wireless task training board
The overall set of the hand-task training system is shown in Figure 2. We developed a wireless task training board to achieve interactive assistance for the soft robotic hand. The task board is equipped with an array of Hall effect sensors, and a magnetic component is affixed inside the soft robotic hand.
The wireless task board utilizes magnetic field intensity detection to determine the distance between the soft robotic hand and itself. Once a predetermined threshold is exceeded, the wireless communication module within the task board transmits instructions to the controller, triggering pre-set gesture assistance by the soft robotic hand. To achieve diverse hand functions during task-oriented training, such as five-finger grasps (Cylindrical/Spherical power), three-finger grasp (Tripod pinch), two-finger grasp (Tip/Lateral pinch), and finger extension, the soft robotic hand can connect with multiple task boards. This enables the realization of various task training designs by incorporating different task boards with distinct hand-function interactions.
Figure 3 provides a concise example of a moving objects task, showcasing the functionality of the task boards. The black task board is configured for spherical power grasp assistance, while the white task board is set for hand-opening assistance. In this scenario, a stroke patient wearing the soft robotic hand is instructed to transfer balls (d = 80 mm) from the black task board to the basket placed on the white task board. The training process involves three main steps:
-
a) The patient approaches the black task board with their affected hand, aiming to grasp the ball. As the hand nears the board, the magnetic field intensity reaches a specific threshold, triggering the soft robotic hand to provide grasping assistance. Once the grasp is established, the system allows a brief pause to allow the patient to adjust their grip and lift the hand away from the black task board.
-
b) As the patient’s hand moves away from the black task board and enters the task’s execution phase, the soft robotic hand continues to support and assist in maintaining the grasp, ensuring a stable hand posture throughout the task.
-
c) When the patient’s hand approaches the basket on the white task board, the same detection method as the black task board is employed to assess the distance between the hand and itself. Once the proximity reaches a certain threshold, the soft robotic hand is triggered to provide hand-opening assistance, aiding the patient in releasing the ball into the basket.
B. Actuator design
Our previous studies described the soft-elastic composite actuator (SECA) utilized in the early version of the soft robotic hand (Heung et al., Reference Heung, Tong, Lau and Li2019; Heung et al., Reference Heung, Tang, Shi, Tong and Li2020; Tang et al., Reference Tang, Heung, Tong and Li2021). However, the original design possesses certain limitations. First, wrapping the fibers is time-consuming. Second, prolonged usage tends to loosen the fibers, rendering the actuator susceptible and significantly impacting its performance. To improve the performance of the soft actuator, we have introduced a novel design known as the RSA, shown in Figure 1. The RSA comprises several key components, including an actuator body made of elastomeric materials, plastic ring constraints, and a torque-compensating layer. The operational principle of the RSA closely resembles that of the originally designed SECA. Utilizing a single pressurizing source, the torque-compensating layer enables finger flexion and extension within the same actuator unit.

Figure 1. (a) Prototype of the soft wearable robotic hand, (b) spherical grasp a ball by robotic hand, and (c) tripod pinch a marker pen by robotic hand.
The elastomeric body and soft bottom pad of the RSA are 3D 3D-printed using ACEO Silicone GP Shore A 30 (WACKER Chemie AG), which can offer sufficient elongation (450%) and tensile strength (6 MPa). Since the distal interphalangeal (DIP) joints only play a limited role of 15% of functional gripping (Leibovic and Bowers, Reference Leibovic and Bowers1994), the RSA only covers the two joints of each finger: proximal interphalangeal (PIP) and metacarpophalangeal (MCP) joints. The center of each segment is aligned with a corresponding finger joint. The torque-compensating layer was placed between the elastomeric body and the soft bottom pad. When pressure is applied, the ring constraints effectively prevent radial expansion, while the torque-compensating layer restricts axial elongation at the bottom, leading to a bending motion (Figure 1b and c). Conversely, during depressurization, the torque-compensating layer enables the RSA to return smoothly to its original undeformed position.
C. Actuator performance
Previous studies show the significance of achieving adequate actuation torque (>0.2 Nm) (Bützer et al., Reference Bützer, Lambercy, Arata and Gassert2021) and range of motion (>120°) (Polygerinos et al., Reference Polygerinos, Wang, Galloway, Wood and Walsh2015) when designing a rehabilitation robotic hand. In this study, we focused on assessing the performance of the RSA. To ensure comparability with human finger sizes (Peters et al., Reference Peters, Mackenzie and Bryden2002), the RSA used in our tests had dimensions of 10 cm in length, 1.8 cm in width, and height proportional to human fingers. The force exerted at the fingertip varies based on the lever arm, which changes with different finger positions. Consequently, we evaluated the fingertip force across various overall finger flexion angles (0°, 40°, 80°, and 120°) for increasing input air pressure (from 0 kPa to 300 kPa, with an interval of 25 kPa). To measure the input force, we employed a compression load cell (FC22, Measurement Specialties, U.S.A.). The proximal end of the RSA was clamped to establish a vertical orientation (Figure 4a. The maximum input pressure applied to the RSA was restricted to 300 kPa. Remarkably, the results demonstrated that the RSA achieved an actuation torque of 0.3 Nm when the overall finger flexion angle reached 120° (Figure 4b). No wall rupture or air leakage was observed throughout the bending angle measurement process.
D. Soft robotic hand module
The soft robotic hand module comprises five RSAs seamlessly integrated into a plastic hand brace. To ensure stability and comfort, Velcro straps are utilized to secure the wrist, palm, and finger joints in conjunction with the soft robotic hand (refer to Figure 1a). Both the size of the hand brace and the length of the RSAs are custom-designed and 3D printed to fit the user’s specific hand dimensions accurately. The total weight of the soft robotic hand is 150 grams, which is lightweight enough to avoid placing any additional burden on the impaired hand, thus preserving natural hand movement capabilities.
The interactive pneumatic control system is built to control the soft robotic hand (Figure 2). Air tubes connected the hand to the control box, and five three-way pneumatic solenoid values controlled the actuators individually. Two small air pumps were installed inside the control box, which could provide compressed air pressure up to 320 kPa. An embedded controller is built to control the on–off solenoid valves by regulating the output PWM signal. Pressure sensors (XGZP6847, CF Sensors, Korea) monitor the air pressure inside multiple RSAs to determine whether the RSAs are fully inflated and prevent over-pressurization.
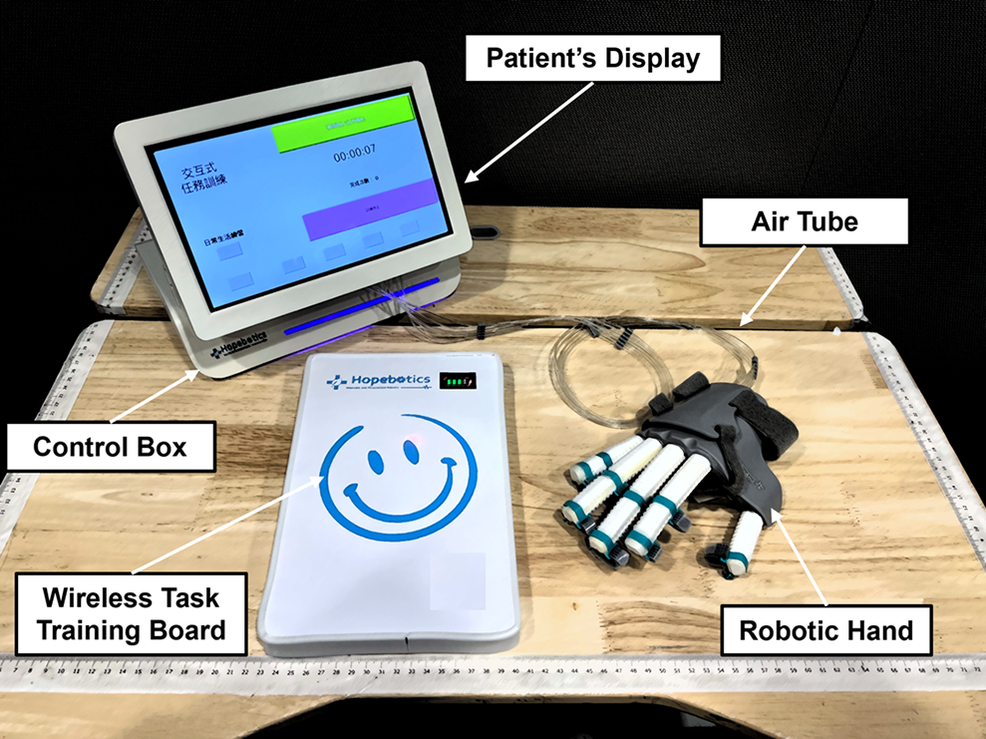
Figure 2. Full set of interactive soft robotic hand-task training systems.

Figure 3. Example of task training process for moving objects task (ball, d = 80 mm). (a) Patient wearing a soft robotic hand approaches the task board (black), and activates interactive grasp mode for ball grasping, (b) Patient’s hand leaves the task board (black), soft robotic hand maintains grasping assistance during the task execution phase.

Figure 4. (a) Measurement setup for fingertip forces in the function of the finger flexion angle, (b) actuation torque (mean and SD.) in the function of the input pressure and the overall finger flexion angle.
III. Evaluation by stroke survivors
A. Stroke subjects
A total of seven chronic stroke subjects were enlisted to assess the effectiveness of the soft robotic hand in conjunction with task-oriented hand-functional training. Each subject participated in 20 sessions of rehabilitation training. The demographic information of the recruited subjects can be found in Table 1.
Table 1. Demographic information

Female (F); Male (M); Hemorrhage (Hemo); and Ischemia (Isch).
B. Task-oriented hand-functional training
In this evaluation, each subject was required to attend 20 training sessions, with an intensity of about 3–5 sessions per week. Clinical outcome measures were assessed before and after the 20 sessions. During each session, the total task execution cycles were recorded and shown on the patient’s display.
During the training sessions, stroke subjects participated in four sets of hand-functional training tasks facilitated by the soft robotic hand. Each training session lasted for 10 min. The training objects were positioned on a task board, with the number of task boards utilized depending on the task requirements (refer to Figure 5). The training encompassed the following tasks:
-
a) Drinking task: One task board with both hand cylindrical grasping (five fingers) and opening assistance was utilized in this task. This task involved the subject gripping a water bottle from the task board (cylindrical grasp assistance), raising the arm, simulating a drinking motion by bringing the water bottle to the mouth, returning the water bottle to the task board, and triggering the hand-opening action.
-
b) Puzzle task: Two task boards with tip pinching (two fingers) and hand-opening were utilized in this task. The therapist placed small object fragments on one task board and the puzzle base on another. The subjects were required to pick up the small objects from the first task board (tip pinch assistance) and attempt to place them in the puzzle base on the other side, aiming to form a complete rectangle.
-
c) Chess game: Three task boards with tripod pinching (three fingers) and hand-opening (use two boards to enlarge the surface area) were utilized in this task. In this task, the subject played chess against the therapist. The subjects picked a chess piece of a particular color from a task board and placed it on a chess cloth (comprising two additional task boards). The objective was to be the first to align three pieces of the same color in a straight line.
-
d) Moving task: Two task boards with spherical grasping (five fingers) and hand-opening were utilized in this task. Subjects were tasked with grasping a ball from one task board and transferring it to a basket positioned on another.

Figure 5. Four different tasks: (a) drinking task (bottle full of water, 330 mL), (b) puzzle task (small blocks with diameters around 2–3 cm), (c) chess game (standard wooden chess pieces), and (d) moving task (Ball with a diameter of 80 mm).
C. Outcome measurements
Multiple clinical assessments were utilized to evaluate motor improvement resulting from the robot-assisted task-oriented hand-functional training immediately after the training and during a follow-up after 3 months. These assessments included the Fugl–Meyer assessment (FMA), action research arm test (ARAT), box and block test (BBT), and modified Ashworth scale (MAS). The FMA evaluates voluntary motor functions of the upper limb and consists of two separate measurements: the shoulder and elbow section (S/E) and the wrist and hand section (W/H). The ARAT and BBT measure hand functions by assessing daily activity tasks. Furthermore, the maximum grip strength (MGS) and maximum pinch strength (MPS) assessments were employed to evaluate improvements in hand functionality. Paired t-tests were applied to examine the changes compared with the pre-training scores.
The evaluation trial consisted of assessments also conducted before training, immediately after training, and during a 3-month follow-up. Each subject was instructed to complete the puzzle task twice within 3 min. In the first trial, subjects utilized the soft robotic hand, while in the second trial, they performed the task without it. The number of successfully grasped small objects transferred into the puzzle base was recorded separately for each trial.
IV. Results
Table 2 presents a comprehensive overview of the training outcomes before and after the 20-session task-oriented hand-functional training. The results reveal significant motor improvements across various clinical assessments, including Fugl-Meyer Assessment (FMA), FMA-W/H (wrist and hand section), FMA-S/E (shoulder and elbow section), action research arm test (ARAT), box and block test (BBT), and maximum grip strength (MGS). The notable enhancements in ARAT and BBT scores indicate improved motor recovery in hand and finger functions. Moreover, the increased FMA scores suggest overall motor improvement in the entire upper limb following the training. The improvement in MGS and MPS signifies enhanced capabilities in performing practical hand-grasp functions.
Table 2. Statistical summary

* Mean value changes with statistical significances (p < .05)
Figure 6 depicts the variations in the number of tasks observed completed during the evaluation trial for all seven stroke subjects. The findings indicate a notable increase in execution numbers, even without wearing the soft robotic hand. This increase signifies the improvement of hand-motor functions following the soft robotic hand training. Furthermore, it is noteworthy that this effect persisted for at least 3 months after completing the training.

Figure 6. Changes of task execution numbers for evaluation trials, with and without wearing a soft robotic hand (mean value with standard deviations). The significant difference is indicated by “*” (independent t-test, p < .05).
V. Conclusions
This study introduces an interactive soft robotic hand system for task-oriented hand-functional stroke rehabilitation. The soft robotic hand could interact with the interactive task board and achieve different hand-functional assistance modes. The clinical outcomes following 20 training sessions involving seven chronic stroke subjects are reported. The soft robotic hand demonstrates practical assistance for stroke patients, enabling them to perform various hand-functional grasping actions and extend their spastic hand. The preliminary data indicates notable improvements in motor functions of the hand and upper limbs after the 20 training sessions, and this effect could persist for at least 3 months after training. However, future clinical studies will require a larger sample size to validate these findings further and explore the full potential of this hand-task training system in stroke rehabilitation.
Data availability statement
The data used to support the findings of the study are included in the article.
Author contribution
XS conceived the presented system concept, designed the system and experiments, collected the data, and wrote the manuscript. CY performed the clinical trial and helped collect the data. PL and ZY designed the circuit of the wireless board, control algorithm, and software. DX helped collect data and contributed to the data analysis. ZL advised on the design of the system concept and contributed to the manuscript revisions. RKT advised on all parts of the project and reviewed the whole manuscript. All authors read and approved the submitted version of the manuscript.
Competing interest
The university encourages knowledge transfer activities, and this robotic hand was licensed by the spin-off company (Hopebotics Limited, Hong Kong SAR, China) in 2021. The authors make every effort to ensure the impartiality, integrity, and scientific rigor of this study. The interpretation of the results and reporting of the findings were conducted objectively and without bias.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The evaluation occurred at the Chinese University of Hong Kong and was registered on ClinicalTrials.gov under the ID NCT03286309. Ethical approval for the study was granted by the Joint Chinese University of Hong Kong-New Territories East Cluster (CUHK-NTEC).
Financial support
This work was supported by the Innovation and Technology Fund of Hong Kong (GHP/061/21GD), the General Research Fund of Hong Kong (CUHK 14216622), and the Guangdong Science and Technology Research Fund (2020B1515120064).











