INTRODUCTION
Vaccination against hepatitis B in early childhood is the most effective way of preventing spread of infection. According to published data, available vaccines induce a protective antibody response to hepatitis B surface antigen (anti-HBs) in about 95% of vaccinees and confer long-lasting protection against hepatitis B infection in immunocompetent individuals [Reference Floreani1–Reference Zanetti8]. The magnitude of the anti-HBs response is largely influenced by vaccine regimen, body mass, site and route of injection, immunosuppression, and age [Reference Shaw9–Reference Zuckerman12]. A peak anti-HBs concentration ⩾10 milli international units per millilitre (mIU/ml) measured 1 month after completion of primary vaccination course is considered to provide long-term immunity [Reference Hammitt5]. Even in individuals whose anti-HBs concentration decreases to lower or undetectable levels, protection against disease is likely to persist due to the maintenance of HBsAg specific immunological memory [Reference Dentinger13–Reference Bauer and Jilg16]. Nonetheless, it has not been firmly established whether booster vaccination is necessary and if so, at what intervals [Reference Floreani1–Reference FitzSimons3, Reference Lin6, Reference Zanetti8, Reference Banatvala and Van Damme17].
Germany, a country with a low endemicity for hepatitis B, introduced national recommendations for universal hepatitis B vaccination of infants, children, and adolescents in 1995. In 2000, two hexavalent vaccines, Hexavac® (Sanofi Pasteur MSD), France and Infanrix hexa® (GlaxoSmithKline, Belgium), against diphtheria, tetanus, pertussis, hepatitis B, polio and Haemophilus influenzae type b were approved for use in Germany. Both vaccines were licensed for either a three- or a four-dose primary series. In Germany the routine vaccination schedule for hexavalent vaccines recommends four doses at ages of 2, 3, 4, and 11–14 months, with at least 6 months between the third and fourth dose [18]. The recommendations made no preference for either vaccine.
Hepatitis B vaccination coverage in Germany increased markedly in conjunction with the availability of hexavalent vaccines and data from the German National Health Interview and Examination Survey for Children and Adolescents (KiGGS) shows that hexavalent vaccines practically replaced vaccination with monovalent hepatitis B vaccinees during childhood [Reference Poethko-Müller, Kuhnert and Schlaud19, Reference Kalies20].
In 2005, the European Medicines Agency (EMEA) suspended the marketing authorization for Hexavac due to precautionary concerns about the long-term persistence of immunogenicity of the hepatitis B component of the vaccine [21, 22]. Additionally, there was some evidence that Hexavac-vaccinated children who achieved anti-HBs levels between 10 and 100 mIU/ml post-primary vaccination had no or a lower response to booster vaccinations with monovalent hepatitis B vaccines given at age 7–9 years, compared to children whose initial levels were >100 mIU/ml [22]. Hexavac was licensed in Germany in late October 2000 and between 2001 and the time of its suspension in September 2005, 5·8 million doses of vaccine were prescribed, corresponding to at least 1·5 million vaccinated children (IMS Health, written communication). Hexavac was also licensed in nine other European countries as well as in 19 additional countries worldwide [21].
Current knowledge on the immunogenicity of Hexavac and Infanrix hexa has largely been gained from clinical trials [Reference Kilpi23–Reference Tichmann27]. Questions remain about the short- and long-term hepatitis B immunogenicity in children vaccinated with these hexavalent vaccines during routine immunization. To investigate this, we compared anti-HBs levels in children in Germany fully vaccinated with either Hexavac or Infanrix hexa between 2000 and 2005 using data from a large cross-sectional survey. We also assessed factors associated with anti-HBs concentrations <10 mIU/ml.
METHODS
The German Health Survey for Children and Adolescents (KiGGS)
A nationwide cross-sectional survey, KiGGS, was conducted between 2003 and 2006 [Reference Kurth28]. KiGGS was based on a nationally representative sample of children and adolescents aged 0–17 years with main residence in Germany. Participants were enrolled in two steps: first, 167 study locations (sample points) were chosen; second, subjects were randomly selected from the official resident registers in the 167 sample locations. A total of 17 641 subjects (8656 girls, 8985 boys) participated in the survey (response 66·6%). Analysis of the non-responder questionnaires showed that the collected data gave comprehensive and nationally representative evidence on the health status of children and adolescents. To ensure that estimates derived from KiGGS were representative at the national level, weights were applied in statistical analyses to correct for regional differences in the distribution of age structure, sex, and nationality, and to compensate for oversampling of individuals from Eastern Germany which was undertaken to achieve sufficient statistical power.
Data on age, sex, anthropometric variables, chronic diseases, socio-economic status, and a large number of health indicators were collected through interviews and self-completed questionnaires. A wide range of blood and urine tests were performed. Vaccination status was extracted from hand-held vaccination cards. A detailed description of the sampling method has been published elsewhere [Reference Kurth28, Reference Hölling29].
Hepatitis B testing
Laboratory tests for anti-HBs and antibodies to hepatitis B core antigen (anti-HBc) were performed in the majority of children aged 3–17 years (88%). However, the proportion of children tested for anti-HBs and anti-HBc was smaller in the younger age groups due to more frequent refusals or blood sampling failures. Anti-HBs and anti-HBc were determined by electrochemiluminescence immunoassay (ECLIA, Roche Diagnostics, Switzerland) using automatic ELECSYS 2010 Analyzer. Only samples which were anti-HBc positive were tested for hepatitis B surface antigen (HBsAg). Anti-HBs concentration was determined quantitatively in the range 2–1000 mIU/ml. Levels <2 mIU/ml were classified as undetectable, whereas concentrations >1000 mIU/ml were not further diluted. Tests for anti-HBc and HBsAg were qualitative. Details of the laboratory methods have previously been published [Reference Thierfelder30].
Selection of subjects vaccinated with hexavalent vaccines – subgroup analysis
For the purpose of this study, we selected a subset of children from KiGGS. We included only children who fulfilled the following four criteria: (i) previous vaccination either with only four doses of Hexavac or only four doses of Infanrix hexa; (ii) complete and original vaccination card available; (iii) at least 5 months between the third and fourth vaccination dose, and (iv) results of anti-HBs and anti-HBc testing available. Children who were positive for anti-HBc or HBsAg, or who had been passively or actively immunized at birth were excluded from our dataset. For the selected subgroup we extracted the following from the KiGGS database: data on age, sex, birth weight, results of anti-HBs testing, brand and dates of hexavalent vaccination, information on diseases which may impair immunity, and concomitant administration of other available childhood vaccines [measles, mumps and rubella (MMR), pneumococcal, meningococcal, varicella, influenza, hepatitis A, and tick-borne encephalitis].
Statistical analysis
We grouped children according to anti-HBs levels (<2, 2 to <10, 10 to <100, 100 to <1000, ⩾1000 mIU/ml) and assessed differences in anti-HBs between Hexavac and Infanrix hexa vaccinees using Pearson's χ2 test.
We performed weighted bivariate and multivariable logistic regression analyses to identify factors associated with anti-HBs concentration <10 mIU/ml. The weighted analyses were performed applying the weights developed for KiGGS as described above. For these analyses, we grouped subjects by vaccine (four doses of Hexavac vs. four doses of Infanrix hexa), anti-HBs concentration (<10 mIU/ml vs. ⩾10 mIU/ml), sex (male vs. female), birth weight [<2499 g (underweight), ⩾4500 g (overweight), ⩾2500–4499 g (normal)], concomitantly vaccinated [hexavalent vaccine administered alone vs. hexavalent vaccine administered with other vaccine(s)], and age at first vaccination dose (⩾6 months or <6 months). We included bivariate predictors with P<0·25 in the multivariable model. Using a backward stepwise elimination (P>0·05) in the multivariable analysis, we removed variables not associated with anti-HBs <10 mIU/ml. The prevalence of children with anti-HBs <10 mIU/ml by time elapsed since completed vaccination was estimated for each vaccine group using multivariable logistic regression analysis. All possible interaction terms were tested separately. P values <0·05 were considered statistically significant. All analyses were performed with Stata 9.0 (StataCorp, USA).
RESULTS
Study population
A total of 477 children fulfilled the inclusion criteria; 227 were vaccinated with Infanrix hexa, and 250 with Hexavac. The characteristics of the two vaccine groups were similar in terms of sex, age, time since completion of vaccination and birth weight (Table 1). The only vaccines administered concomitantly with Hexavac or Infanrix hexa were MMR (Hexavac, n=12; Infanrix hexa, n=16) and pneumococcal vaccine (Hexavac, n=6; Infanrix hexa, n=1).
Table 1. Demographic, vaccination, and birth weight characteristics (weighted) of study participants by vaccine received
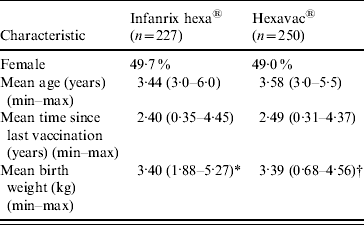
* n=222.
† n=246.
No child was known to have a disease associated with impaired immunity, e.g. nephrotic syndrome, Down's syndrome, or human immunodeficiency virus infection.
We excluded 201 children who had received four doses of Hexavac or Infanrix hexa but did not fulfil the other inclusion criteria. Of these, 183 had not been tested for anti-HBs, 16 had received the fourth dose <5 months after the third dose, and two were positive for anti-HBc.
Excluded children were younger (median age 3·69 years) than those fulfilling all inclusion criteria (median age 3·90 years, Wilcoxon rank sum test 2·53; P=0·011).
Prevalence of protective anti-HBs
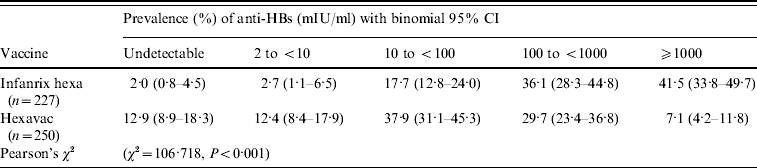
The distribution of anti-HBs levels differed significantly between the two vaccine groups 0·3–4·5 years after the fourth dose (Table 2). For instance, in children vaccinated with Hexavac, 25·3% (95% CI 19·0–32·8) had anti-HBs <10 mIU/ml compared to 4·7% (95% CI 2·4–8·9) of children vaccinated with Infanrix hexa. A significantly larger proportion of children vaccinated with Infanrix hexa (41·5%) had very high anti-HBs levels (⩾1000 mIU/ml) compared with Hexavac vaccinees (7·1%). This difference was also pronounced in children who had completed their vaccination ⩽2 years previously (n=126). In this group, 66·2% of Infanrix hexa vaccinees had an anti-HBs level ⩾1000 mIU/ml compared to 11·9% of Hexavac vaccinees (Pearson's χ2=36·3, P<0·001).
Table 2. Anti-HBs levels in Infanrix hexa® and Hexavac® vaccinees 0·3–4·5 years after fourth vaccine dose
Bivariate logistic regression analysis
In the weighted bivariate logistic regression analysis, vaccine type, birth weight, and years since completion of vaccination were significantly associated with an anti-HBs level <10 mIU/ml (P<0·01, Table 3), while there was no association with sex, age at first vaccination dose, and concomitant vaccinations (P>0·25).
Table 3. Results of weighted bivariate analysis of potential risk factors for anti-HBs <10 mIU/ml
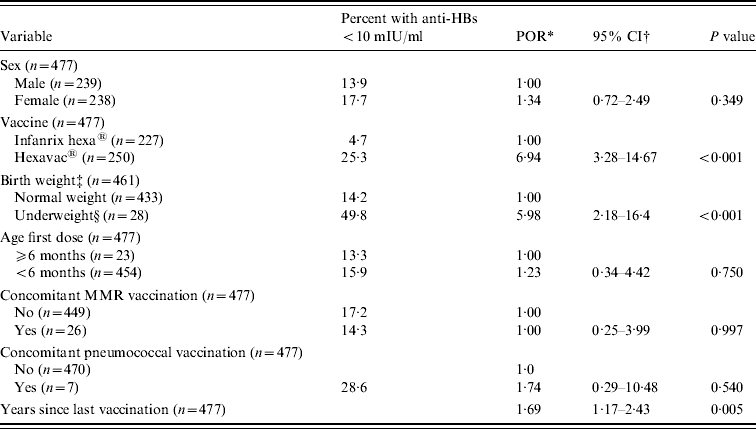
MMR, Measles, mumps and rubella.
* Prevalence odds ratio.
† Binomial 95% confidence intervals.
‡ Normal weight: 2500–4499 g, underweight: ⩽2499 g (according to ICD-10 [48]). Data on birth weight was missing for nine children. Seven children classified as overweight could not be included in the analysis as they all had anti-HBs >10 mIU/ml.
§ Twenty-two were born preterm.
Multivariable logistic regression analysis
In the multivariable logistic regression analysis, vaccine type, birth weight, and years since last vaccination remained as independent risk factors for anti-HBs <10 mIU/ml (Table 4). None of the interaction terms significantly improved the model fit and were thus not considered in the final model. The odds of having an anti-HBs concentration <10 mIU/ml was eight times higher in children vaccinated with Hexavac compared to children vaccinated with Infanrix hexa and increased by 1·8 for every additional year since completion of vaccination. Being underweight at birth was also strongly associated with anti-HBs levels <10 mIU/ml.
Table 4. Results of the weighted multivariable logistic regression analysis, showing factors associated with anti-HBs <10 mIU/ml (n=461)
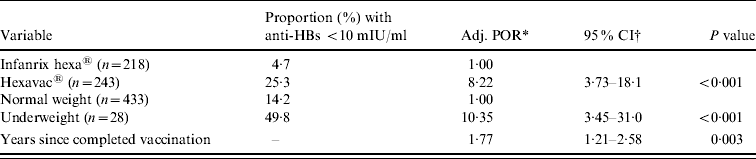
* Adjusted prevalence odds ratio.
† Binominal 95% confidence intervals.
Persistence of anti-HBs by time since completed vaccination
The prevalence of individuals with anti-HBs <10 mIU/ml was significantly higher at any point in time since vaccination in Hexavac vaccinees than in Infanrix hexa vaccinees as modelled using logistic regression (Fig. 1). For example 1 year post-vaccination, the estimated prevalence of anti-HBs <10 mIU/ml was 13·5% (95% CI 7·6–22·9) in children vaccinated with Hexavac vs. 2·3% (95% CI 0·8–6·1) in children vaccinated with Infanrix hexa. For children who completed vaccination 4 years earlier, an estimated 40·7% (95% CI 25·9–57·3) of Hexavac vaccinees had anti-HBs <10 mIU/ml compared to 9·2% (95% CI 4·5–17·9) of Infanrix hexa vaccinees (Fig. 1).
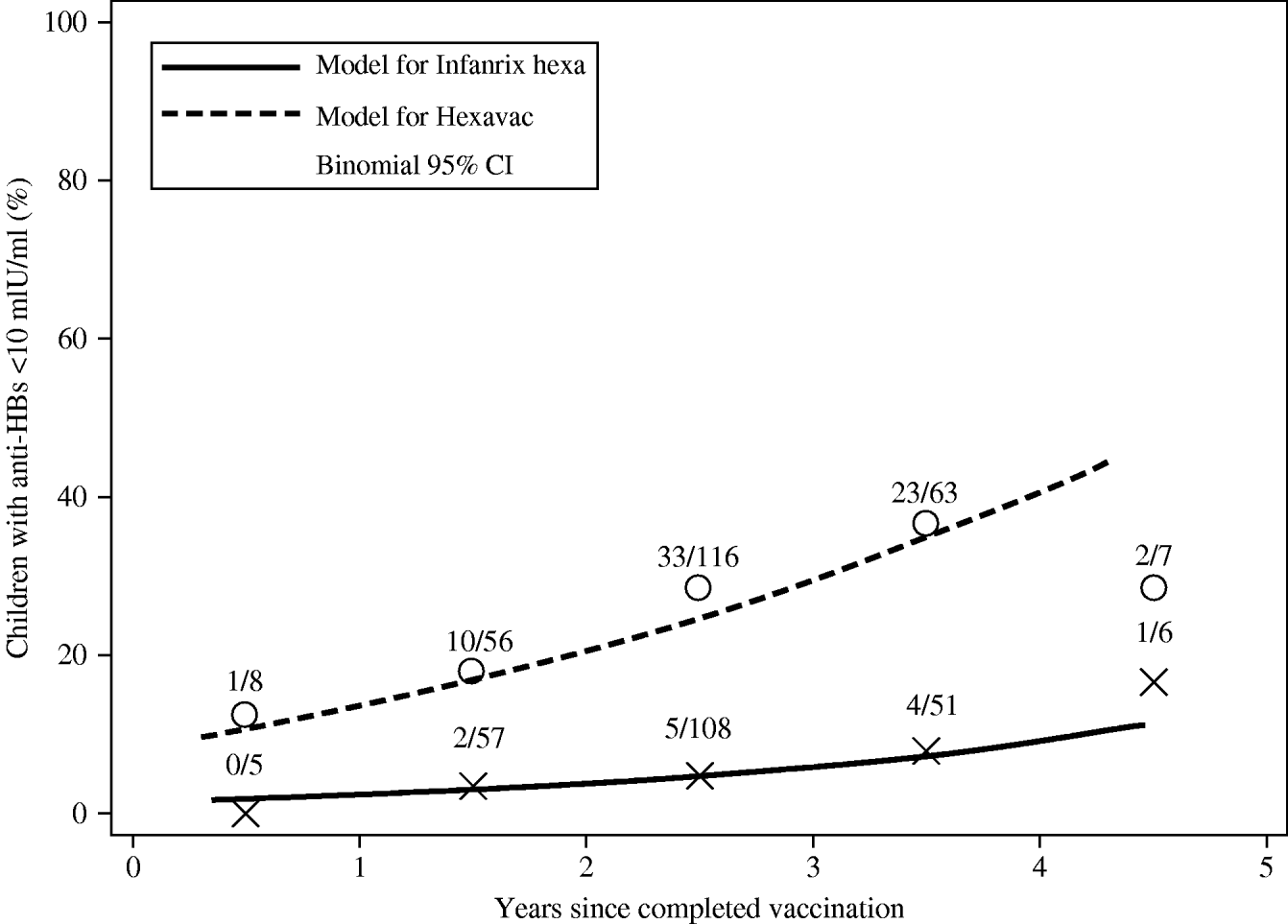
Fig. 1. Estimated prevalence of Hexavac® and Infanrix hexa® vaccinees with anti-HBs <10 mIU/ml according to time since completed vaccination as modelled by weighted logistic regression analysis. Open circles (Hexavac) and crosses (Infanrix hexa) show the number of subjects with anti-HBs levels <10 mIU/ml in all subjects within each successive year after completed vaccination.
DISCUSSION
This study comparing anti-HBs levels in children vaccinated only with Hexavac to those in children vaccinated only with Infanrix hexa provides further evidence on the immunogenicity of the hepatitis B component of Hexavac. The significantly higher proportion of children in our analysis with anti-HBs levels both undetectable (12·9%) and <10 mIU/ml (25·3%) in those vaccinated with Hexavac compared to those vaccinated with Infanrix hexa (2·0% and 4·7%, respectively) suggests that short-term as well as long-term immunogenicity in Hexavac vaccinees may be weaker.
Several studies have shown that the persistence of measurable antibody levels is related to peak anti-HBs levels after immunization [Reference Jilg, Schmidt and Deinhardt31–Reference McMahon33]. The increasingly higher prevalence of anti-HBs <10 mIU/ml with time since completed vaccination in Hexavac vaccinees compared to Infanrix hexa vaccinees as suggested by our model is consistent with lower reported anti-HBs levels post-primary vaccination for Hexavac [Reference Tichmann27]. Similarly, the significantly higher proportion of children vaccinated with Infanrix hexa having anti-HBs levels ⩾1000 mIU/ml within 2 years of the last vaccination compared to those vaccinated with Hexavac implies that Infanrix hexa vaccinees might have responded with substantially higher anti-HBs levels post-vaccination.
According to EMEA, the lower anti-HBs levels in Hexavac vaccinees compared to Infanrix hexa vaccinees may be attributable to variability in the production process of the hepatitis B component of Hexavac [22]. The lower recombinant HBsAg content in the Hexavac vaccine (5 μg HBsAg) compared to that of the Infanrix hexa vaccine (10 μg HBsAg) may also play a role; however, an Italian study showed that a high proportion of children (92·9%) vaccinated in infancy with either 5 μg or 10 μg HBsAg had anti-HBs levels ⩾10 mIU/l 5 years after vaccination [Reference Faustini34]. In that study, simultaneous vaccination with combined diphtheria and tetanus vaccine or with polio vaccine was associated with lower anti-HBs levels. To what extent other differences in hexavalent vaccine composition – particularly differences in the concentration of the adjuvant aluminium hydroxide – might be relevant, is not known. As the children in the two vaccine groups were very similar in terms of a number of factors which might influence response to vaccination, the observed anti-HBs differences in our study are unlikely to be due to differences between the comparison groups.
The findings presented here are consistent with previous reports. In particular, another recent Italian study in children attending local health units found that 31% of 113 Hexavac vaccinees had anti-HBs <10 mIU/ml compared to only 4% of 129 Infanrix hexa vaccinees 15 months post-primary vaccination with three doses [Reference Giambi35]. A clinical trial showed that both the proportion of children with anti-HBs ⩾10 mIU/ml as well as geometric mean titres (GMTs) were significantly higher in children vaccinated with three doses of Infanrix hexa compared to three doses of Hexavac 1 month post-vaccination [Reference Tichmann27]. A follow-up trial reported significantly higher anti-HBs GMTs as well as seroprevalence rates at age 11–23 months in infants primed with Infanrix hexa in the first study [Reference Tichmann26]. However, following a fourth (booster) dose, the proportion of children with anti-HBs ⩾10 mIU/ml was similar in the two groups, although GMTs remained significantly higher in Infanrix hexa vaccinees. Finally, a vaccine trial in Germany showed that >90% of children vaccinated with four doses of Infanrix hexa had persistent protective anti-HBs levels 3·5–4 years after administration of a fourth dose, thus supporting the findings presented here [Reference Heininger36].
Low birth weight was also strongly and independently associated with anti-HBs levels <10 mIU/ml in this study. Since a high proportion (79%) of the children with low birth weight (n=28) were born preterm, these findings are in keeping with other studies which have shown decreased response to hepatitis B vaccines in preterm infants [Reference Kesler37–Reference Lau39].
We found no association between anti-HBs levels <10 mIU/ml and concomitant vaccination with MMR or pneumococcal vaccine; however, the number of concomitantly vaccinated children was small. Previous studies have reported lower geometric mean anti-HBs levels with concomitant administration of hexavalent vaccines and pneumococcal conjugate vaccine [Reference Olivier40, Reference Tichmann-Schumann41].
Our study has two major limitations. The first limitation is inherent in the design of a cross-sectional survey. Because data were only collected at a single point in time for each participant, we could not assess individual anti-HBs levels over time. Thus, we could not firmly establish whether undetectable anti-HBs levels were mainly due to primary vaccine failure, i.e. non-response, or secondary vaccine failure, i.e. waning immunity after seroconversion. Second, data on several factors which might influence the response to vaccination, including non-chronic diseases, medication (e.g. steroids), body weight at the time of vaccination and vaccine administration route were unavailable in this secondary data analysis. However, it appears unlikely that children who received Hexavac would differ from children vaccinated with Infanrix hexa in these aspects.
EMEA's suspension of Hexavac in 2005 was considered a precautionary measure and immediate revaccination of children who completed primary vaccination with Hexavac was not deemed necessary [21]. However, the degree of short- and long-term protection against hepatitis B infection in children vaccinated with Hexavac remains unclear. Overall, the clinical significance of anti-HBs <10 mIU/ml in immunized individuals is not well understood. Studies suggest that long-term persistence of high anti-HBs levels, as well as immunological memory and the degree of protection from infection are associated with peak anti-HBs concentrations after vaccination [Reference Jilg, Schmidt and Deinhardt31, Reference McMahon33, Reference Da Villa42–Reference Whittle44]. Several studies have shown that healthy individuals with an anti-HBs concentration <10 mIU/ml can be infected with hepatitis B, but mostly retain protection against chronic infection provided that they initially responded well to the vaccine [2, Reference FitzSimons3, Reference Banatvala and Van Damme17, Reference McMahon33, Reference Da Villa42, Reference Bialek43].
Most studies investigating long-term immunogenicity of hepatitis B vaccines and related immunological memory followed up relatively small numbers of vaccinees in settings with intermediate or high hepatitis B endemicity [Reference Hammitt5, Reference McMahon33, Reference Da Villa42–Reference Samandari45]. Some or all subjects in these studies were either vaccinated with lower [Reference Bialek43–Reference Samandari45] or higher [Reference Gabbuti4, Reference McMahon33] doses of HBsAg-containing vaccines, were vaccinated at birth [Reference Hammitt5, Reference Bialek43–Reference Samandari45], or were vaccinated with plasma-derived vaccines [Reference McMahon33, Reference Samandari45]. Therefore, results of these studies are not directly applicable to the German setting. However, some of these studies showed a decrease in anti-HBs concentrations to <10 mIU/ml over a period of up to 15 years in up to 60% of vaccinees [Reference Hammitt5, Reference McMahon33, Reference Bialek43, Reference Samandari45], with up to 50% unable to mount an anamnestic immune response [Reference Hammitt5, Reference Bialek43, Reference Samandari45]. Conversely, an Italian study found that 91·2% of adolescents vaccinated with three doses of recombinant vaccine containing a high dose of HBsAg (20 μg) retained anti-HBs-levels of >10 mIU/ml more than 10 years later [Reference Gabbuti4].
According to EMEA, there is some degree of uncertainty as to whether Hexavac vaccinees with an initial immune response of 10–100 mIU/ml anti-HBs after primary vaccination can mount an anamnestic response to a booster dose with monovalent hepatitis B vaccines [22]. A study by Giambi et al. showed that 2/25 (8%) Hexavac vaccinees vs. 0/5 (0%) Infanrix hexa vaccinees with anti-HBs levels <10 mIU/ml 15 months after receipt of three primary doses did not attain anti-HBs levels >10 mIU/ml 1 month after a booster dose with a monovalent hepatitis B vaccine; however, the difference was not statistically significant [Reference Giambi35].
Since hepatitis B booster vaccination is currently not recommended for the general population in Germany [18], the success of the childhood immunization policy relies on long-term immunity into adolescence and beyond, when the risk of hepatitis B infection increases with onset of sexual activity [Reference Walter46]. In countries with low endemicity, where natural exposure to hepatitis B is limited, booster vaccinations may be necessary to sustain immunity.
To establish whether Hexavac-vaccinated children might require re-vaccination or a booster dose, studies should be conducted to assess the ability of children with low anti-HBs levels after completion of the primary series to mount an anamnestic response to booster vaccination. Additional studies to assess the long-term persistence of anti-HBs and investigation of possible breakthrough infections in high-risk populations should also be performed.
In Switzerland, administration of one dose of a monovalent hepatitis B vaccine before the age of 8 years to children who had received four doses of Hexavac was recommended following the suspension of the vaccine [47]. The decision to implement a similar strategy in Germany would largely depend on the results of the above-mentioned studies.
DECLARATION OF INTEREST
The authors were employees at the Robert Koch Institute (P.J., C.P.M., W.H., W.T., C.M., M.H., M.S., D.R.) and University Regensburg (W.J.) at the time of the study. All authors, the Robert Koch Institute, and University Regensburg, report no financial or other conflict of interest.







