Phylloquinone (vitamin K1) is the predominant form of vitamin K in typical Western diets. Vitamin K also occurs naturally as menaquinones (known collectively as vitamin K2) in a range of foods including fermented soyabean-containing foods, eggs, meat and some cheese (Schurgers et al. Reference Schurgers, Geleijnse, Grobbee, Pols, Hofman, Witteman and Vermeer1999). The intake of menaquinones in the British diet is currently unknown, but is likely to be small. The contribution was only 10 % of vitamin K intake in the Netherlands (Schurgers et al. Reference Schurgers, Geleijnse, Grobbee, Pols, Hofman, Witteman and Vermeer1999).
No recommended intakes currently exist for vitamin K in the UK, only a guideline for adults of ≥ 1 μg/kg body weight per d, set some 15 years ago (Department of Health, 1991). However, this guideline is based on the estimated requirement for phylloquinone to maintain normal blood clotting and does not take into account a probable higher requirement suggested more recently for bone (Szulc et al. Reference Szulc, Chapuy, Meunier and Delmas1996; Shearer, Reference Shearer1997; Binkley et al. Reference Binkley, Krueger, Kawahara, Engelke, Chappell and Suttie2002) and cardiovascular health (Shearer, Reference Shearer2000; Schurgers et al. Reference Schurgers, Dissel, Spronk, Soute, Dhore, Cleutjens and Vermeer2001; Vermeer et al. Reference Vermeer, Shearer, Zittermann, Bolton-Smith, Szulc, Hodges, Walter, Rambeck, Stöcklin and Weber2004), especially in adulthood when age-related changes place men and women at greater risk of deteriorating health in these key areas.
Based on more recent data, particularly median phylloquinone intakes of apparently healthy individuals from the population-based third National Health and Nutrition Examination Survey (NHANES III) of 1988–1994, the adequate intake (AI) for phylloquinone has been set in the USA at 120 μg/d for men and 90 μg/d for women (Institute of Medicine, 2001).
Dietary vitamin K requirements for health cannot be set without detailed assessment of usual population intakes in relation to measurable health outcomes (Shearer & Bolton-Smith, Reference Shearer and Bolton-Smith2000). Nationally representative estimates of phylloquinone intake are scarce and relatively recent in Great Britain (Thane et al. Reference Thane, Paul, Bates, Bolton-Smith, Prentice and Shearer2002), Scotland (Bolton-Smith et al. Reference Bolton-Smith, Price and Shearer2000b), Ireland (Duggan et al. Reference Duggan, Cashman, Flynn, Bolton-Smith and Kiely2004), Germany (Mensink & Beitz, Reference Mensink and Beitz2004) and the USA (Booth et al. Reference Booth, Pennington and Sadowski1996, Reference Booth, Webb and Peters1999b; Institute of Medicine, 2001), owing to a previous lack of comprehensive and reliable data on the phylloquinone content of foods.
The present study uses UK-specific food content data (Bolton-Smith et al. Reference Bolton-Smith, Price, Fenton, Harrington and Shearer2000a) to (1) estimate and compare dietary phylloquinone intake and sources in two nationally representative samples of British adults under 65 years, set 14 years apart, (2) assess the variation in intake according to season and selected socio-demographic and lifestyle factors, and (3) consider the prevalence of potentially inadequate phylloquinone intake.
Subjects and methods
A sample of adults, aged 16–64 years, was drawn from the 1986–7 Dietary and Nutritional Survey of British Adults. A second sample of adults, aged 19–64 years, was drawn from the 2000–1 National Diet and Nutrition Survey.
After obtaining ethical approval for each part of each survey from the National Health Service Local Research Ethics Committees for each of the 120 and 152 sampled areas included in 1986–7 and 2000–1 respectively, participants living in private households were selected by a random stratified selection procedure. Participation in each survey was voluntary, while pregnant and lactating women were excluded from taking part. The survey fieldwork was conducted between October 1986 and August 1987 for the Dietary and Nutritional Survey of British Adults, and between July 2000 and June 2001 for the National Diet and Nutrition Survey, with approximately one-quarter of participants surveyed in each season. Information on participants' health and lifestyle, employment status, occupational social class, food habits and smoking status was obtained by trained fieldworkers. Further details and methods for each survey are provided in survey reports (Gregory et al. Reference Gregory, Foster, Tyler and Wiseman1990; Henderson et al. Reference Henderson, Gregory and Swan2002).
Dietary assessment
For each survey, participants kept a 7 d weighed record of all food and drink consumed, both inside and outside the home. In 1986–7, of 2635 participants in the ‘responding’ sample (i.e. those who cooperated in any aspect of the survey), 83 % (2197 out of the 2635) provided complete 7 d weighed dietary records, comprising the ‘diary’ sample. Dietary records of all participants who were unwell with eating habits affected (7 %, 157 out of 2197) were also excluded, leaving 2040. In addition, dietary records were only included if participants provided information on age, occupation and smoking status, and had height and weight recorded, resulting in 1916 dietary records eligible for inclusion in the final data analyses.
In 2000–1, of 2251 participants from the ‘responding’ sample who took part in a dietary interview, complete 7 d weighed dietary records were obtained for 77 % of respondents (1724 out of 2251). Considering the same inclusion criteria applied in 1986–7, food diaries were analysed for 1423 participants. Factor categories were identical to those in 1986–7, with the following exceptions: (1) the youngest age group in 2000–1 was 19–34 years compared with 16–34 years in 1986–7; (2) for season, periods of assessment in 2000–1 were winter, January to March; spring, April to June; summer, July to September; autumn, October to December. In 1986–7, the corresponding timing of assessments was 5 January to 27 February, 30 March to 29 May, 29 June to 31 August, and 29 September to 28 November.
Estimating phylloquinone intake
Phylloquinone intake is expressed as μg/d, μg/kg body weight, and compared with the UK and US AI guidelines for vitamin K adequacy. Intake and relative contribution of different food groups were estimated using UK-specific food content data (Bolton-Smith et al. Reference Bolton-Smith, Price, Fenton, Harrington and Shearer2000a; Food Standards Agency, 2002; MJ Shearer and C Bolton-Smith, unpublished results) for over 3500 and 4500 foods reported as being consumed in 1986–7 and 2000–1 respectively. For each participant, food phylloquinone contents were assigned to every occurrence of every food consumed during dietary assessment. Individual intakes of phylloquinone were then aggregated, to provide estimates of daily intake and food sources.
As described elsewhere (Bolton-Smith et al. Reference Bolton-Smith, Price, Fenton, Harrington and Shearer2000a), one problem when assigning phylloquinone contents to foods is the inherent variability that exists, particularly with regard to different parts of cooked leafy green vegetables (LGV) consumed (for example, outer v. inner leaves of cabbage) and concerning the use of different fats and oils (with very different phylloquinone contents) in fat spreads, processed foods and for frying. With regard to the ‘fat spreads’ food group, butter was recorded separately from fat spreads. Fat spreads were also recorded separately according to their brand, fat content, whether they were hard or soft (margarines) and, to a limited extent, the type of oil used in their preparation (for example, polyunsaturated, not polyunsaturated, with or without olive oil). For processed foods, when the type of oil or fat used in a recipe was not specified, the phylloquinone content assigned assumed that blended vegetable oil was used. Finally, food descriptions of fried foods generally included the types of oil or fat used, thus enabling a more accurate estimate of phylloquinone content.
Phylloquinone intake is presented from the diet only since contributions from dietary supplements have been shown previously to be negligible. For example, the mean contribution of supplements (mainly as cod-liver oil and to a much lesser extent multivitamin tablets) to phylloquinone intake was only 0·02 % in free-living British adults aged 65 years and over (Thane et al. Reference Thane, Paul, Bates, Bolton-Smith, Prentice and Shearer2002), and about 1 % among Irish adults aged 18–64 years (Duggan et al. Reference Duggan, Cashman, Flynn, Bolton-Smith and Kiely2004). Furthermore, among adults surveyed in 2000–1 and described in the present paper, only 0·3 % of total phylloquinone intake was estimated as being derived from supplements. In 1986–7, insufficient information was available on supplement use to provide an equivalent estimate.
Representativeness of phylloquinone intake data
For each survey, the representativeness of results from participants included in the dietary analyses was examined by comparing their socio-demographic and lifestyle characteristics with those of participants excluded from the analyses (27 % of the ‘responding’ sample (719 out of 2635) in 1986–7; 37 % (828 out of 2251) of participants in 2000–1).
Data have not been weighted to take into account slight differences in the age–sex distribution of responders and non-responders to the invitation to participate in the respective surveys since a weighting factor was not available for 1986–7. However, using data in 2000–1 (Henderson et al. Reference Henderson, Gregory and Swan2002), the impact of applying a weighting factor for other dietary components was not significant, as indicated by only very small differences in summary statistics before and after its application to the dietary record.
Data analysis
The variation in phylloquinone intake was examined by sex, age group (16 or 19–34 years; 35–44 years; 45–54 years; 55–64 years), region of habitation (Scotland and the North; Central, South-West and Wales; London and South-East), season, occupational social class of the participant (non-manual; manual), smoking status (non-smoker; smoker) and adiposity (indicated by BMI). ‘Non-manual’ occupational social class comprised professional, managerial, technical and skilled non-manual occupations, while the ‘manual’ category comprised skilled manual, partly skilled and unskilled occupations, as derived from the Registrar General's Standard Occupational Classification (Great Britain Office of Population Censuses & Surveys, 1991) that was applicable at the time of both surveys. BMI was grouped into three categories ( < 25, ≥ 25 to < 30, and ≥ 30 kg/m2), corresponding to ‘healthy weight’, ‘overweight’ and ‘obese’.
Where necessary, the normality of continuous data was improved by natural logarithmic transformation. Owing to skewed distributions of estimated phylloquinone intake, geometric means (with 95 % CI) are given throughout. These represent better ‘average’ measures for such non-normally distributed data. For the entire samples and for men and women separately, arithmetic means and standard deviations are also provided, for comparison, together with the ranges of phylloquinone intake and inner 95 % of intakes (2·5 to 97·5 percentile range) – providing a better indication of the distribution of values when one or two extreme intakes were reported. Arithmetic means are also reported for daily consumption of selected food groups and their percentage contribution to phylloquinone intake.
ANOVA with Scheffé tests were used for continuous data, while multiple logistic regression and χ2 tests were used for discrete data (percentages of adults with phylloquinone intake below the UK guideline and US AI, and percentages of adults in different BMI categories). ANOVA models included two-factor interactions in order to assess the universality or specificity of findings to factor categories, and apportion more of the overall variance of the dependent factor to specific independent terms rather than being included as residual error. Phylloquinone intake has been analysed in men and women separately although, owing to a lack of difference by sex, food sources were analysed using the entire samples. Multivariate statistical tests were conducted both within and between the two surveys.
Unadjusted summary statistics are presented throughout in order to enable easy comparison with findings elsewhere and because different analyses involved different adjustments which would require different adjusted summary statistics to be reported in the same Tables. P < 0·01 denotes significant differences. Data reduction and analyses were carried out using Excel (Microsoft Corp., Redmond, WA, USA) and SPSS (SPSS Inc., Chicago, IL, USA).
Results
In 1986–7, participants excluded from the current analyses did not differ significantly from included participants with respect to socio-demographic and lifestyle characteristics, region in which participants lived or the season when their diets were assessed. Results obtained from participants included from 1986–7 may therefore be regarded as being representative of participants in that survey as a whole. In 2000–1, significant differences existed in the distribution of participants included in and excluded from the current analyses with respect to age group, region of habitation and smoking habit. Excluded participants were more likely than included participants to be aged < 35 years (35 v. 28 % of the respective sub-samples), live in Scotland or northern England (40 v. 33 %) and report being smokers (39 v. 30 %) (each P < 0·01).
Socio-demographic characteristics and smoking habits of those included in the current analyses are compared in Table 1. Although characteristics of adults from the two surveys were broadly similar, some differences were apparent. Age-group distributions differed significantly between the two surveys (P < 0·001; χ2), with a higher percentage of adults aged < 35 years in 1986–7 v. 2000–1. In 1986–7, a higher percentage of participants also reported having a manual occupation compared with their counterparts in 2000–1 (P < 0·01). Finally, a lower percentage of adults were found to be overweight and/or obese in 1986–7 v. 2000–1 (P < 0·001).
Table 1 Socio-demographic and lifestyle characteristics of British adults in 1986–7 and 2000–1 included in the present study*
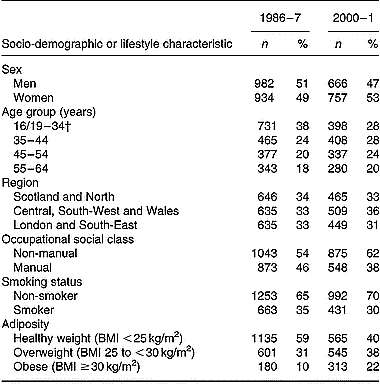
* The Table excludes data for participants who reported being unwell with eating habits affected and those who failed to provide a complete food diary during the 7 d periods of dietary assessment.
† Age range for 1986–7 is 16–34 years; age range for 2000–1 is 19–34 years.
Dietary phylloquinone intake
Values are tabulated by sex in Table 2 (men) and Table 3 (women). For the sexes combined, geometric mean phylloquinone intakes from the diet were 72 (95 % CI 70, 74) μg/d in 1986–7 and 67 (95 % CI 65, 69) μg/d in 2000–1 (P < 0·001). The higher average intake in 1986–7 applied to both sexes, although the difference was greatest among men. In both surveys, intake was significantly higher in men than women when expressed in μg/d, although this difference disappeared when expressed per kg body weight. When expressed per kg body weight per d, geometric mean phylloquinone intake remained significantly higher in 1986–7 v. 2000–1, 1·04 (95 % CI 1·01, 1·07) v. 0·90 (95 % CI 0·87, 0·93) μg/kg per d (P < 0·001), and applied to both sexes.
Table 2 Dietary phylloquinone intake of British men in 1986–7 and 2000–1, by selected socio-demographic and lifestyle factors‡(Geometric means (GM) and 95 % CI)
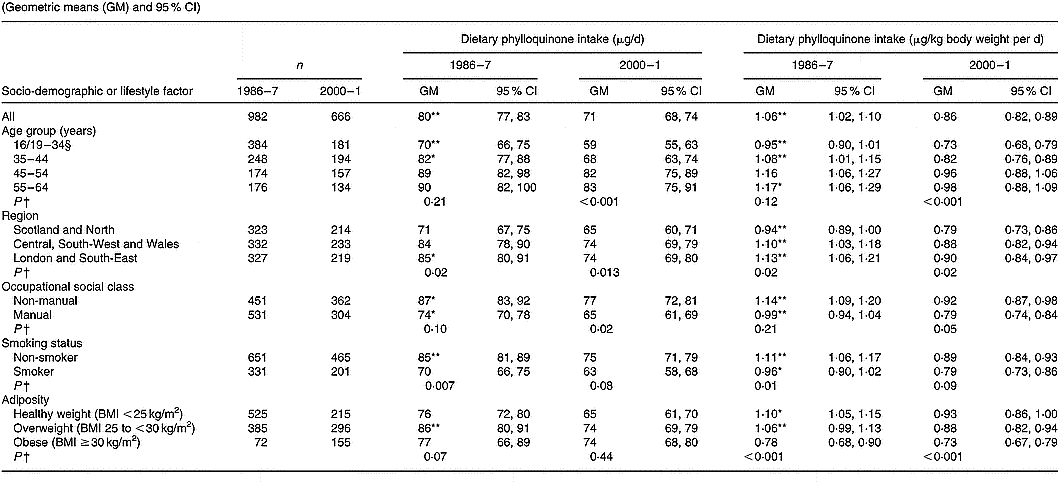
For each category of each factor, mean value for phylloquinone intake was significantly different from that for 2000–1, expressed as μg/d and μg/kg body weight per d respectively: * P < 0·01, ** P < 0·001 (ANOVA, after adjustment for the other socio-demographic or lifestyle factors, season, and their respective two-way interactions with year of survey).
† Significance of difference between factor categories at each time point (by ANOVA, adjusted for the other socio-demographic or lifestyle factors and including two-factor interactions).
‡ The Table excludes data for participants who reported being unwell with eating habits affected and those who failed to provide a complete food diary during the 7 d periods of dietary assessment. Unadjusted summary statistics are presented, while findings by season are not shown since no significant seasonal differences were observed.
§ Age range for 1986–7 is 16–34 years; age range for 2000–1 is 19–34 years.
Table 3 Dietary phylloquinone intake of British women in 1986–7 and 2000–1, by selected socio-demographic and lifestyle factors‡(Geometric means (GM) and 95 % CI)
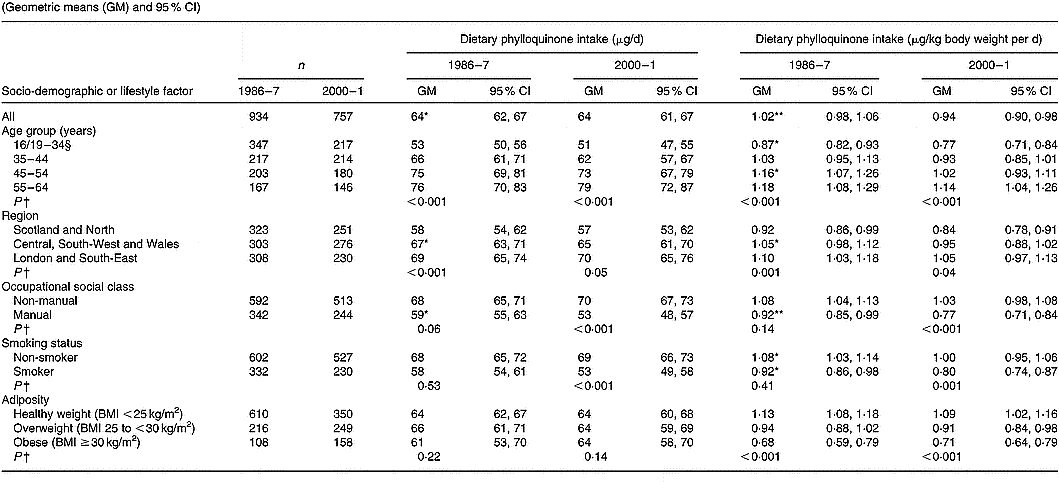
For each category of each factor, mean value for phylloquinone intake was significantly different from that for 2000–1, expressed as μg/d and μg/kg body weight per d respectively: * P < 0·01, ** P < 0·001 (ANOVA, after adjustment for the other socio-demographic or lifestyle factors, season, and their respective two-way interactions with year of survey).
† Significance of difference between factor categories at each time point (by ANOVA, adjusted for the other socio-demographic or lifestyle factors and including two-factor interactions).
‡ The Table excludes data for participants who reported being unwell with eating habits affected and those who failed to provide a complete food diary during the 7 d periods of dietary assessment. Unadjusted summary statistics are presented, while findings by season are not shown since no significant seasonal differences were observed.
§ Age range for 1986–7 is 16–34 years; age range for 2000–1 is 19–34 years.
Corresponding arithmetic mean phylloquinone intakes for the entire samples were somewhat higher than their geometric means – owing to positively skewed distributions; 86 (sd 63) and 80 (sd 60) μg/d in 1986–7 and 2000–1 respectively. The range of phylloquinone intake was wide (1986–7, 0·2–668 μg/d; 2000–1, 3–887 μg/d), and similar for men and women – particularly in 1986–7. The inner 95 % range of values typically showed a 10-fold difference in intake, with no significant difference between 1986–7 (23–237 μg/d) and 2000–1 (22–217 μg/d). When expressed per kg body weight, the range of intake remained wide (0·003–11·41 and 0·02–14·91 μg/kg body weight per d in 1986–7 and 2000–1 respectively), and was similar for men and women. Inner 95 % ranges of phylloquinone intake were also similar for both surveys (0·34–3·61 and 0·29–3·00 μg/kg body weight per d in 1986–7 and 2000–1 respectively).
In 1986–7 and 2000–1, 47 and 59 % of participants had estimated daily phylloquinone intakes below the UK guideline (P < 0·001). Participants in 2000–1 were 62 (95 % CI 48, 76) % more likely to have a ‘low’ phylloquinone intake ( < 1 μg/kg body weight per d) than their counterparts in 1986–7 before adjustment for the potential confounding effects of other socio-demographic and lifestyle factors and season. This increased likelihood was almost identical after adjusting for potential confounders (adjusted odds ratio 1·61, 95 % CI 1·46, 1·76). In 1986–7, percentages of intakes below the UK guideline did not vary by sex (men, 45 %; women, 49 %; P = 0·14), whereas significantly more men than women had low intake in 2000–1 (66 v. 53 %; P < 0·001).
In 1986–7, 75 % of participants had phylloquinone intakes below the US AI, v. 78 % in 2000–1 (P = 0·06). Men were significantly more likely to have an intake below the US AI in 2000–1 compared with 14 years earlier (85 v. 77 %; P < 0·001), whereas corresponding percentages for women did not vary significantly (72 v. 74 %; P = 0·44) in relation to their US AI. Percentages of adults with phylloquinone intake below the US AI did not vary by sex in 1986–7 (men, 77 %; women, 74 %; P = 0·07), although significantly more men than women failed to achieve the US AI in 2000–1 (85 v. 72 %; P < 0·001).
Phylloquinone intake by season, socio-demographic and lifestyle factors
Phylloquinone intake (μg/d and μg/kg body weight per d) was directly associated with age in men (Table 2) and women (Table 3), although associations only achieved statistical significance among men in 2000–1 compared with both times among women (each P < 0·001). Among women, intake varied significantly by region in 1986–7 but not in 2000–1. Men and women aged < 35 years had lower intake than older adults, while those living in Scotland and northern England had lower intake than their counterparts living elsewhere. Smokers also had lower intake than non-smokers, independent of socio-demographic differences including occupational social class. While phylloquinone intake tended to be higher among participants of non-manual occupational social class, this only achieved statistical significance among women in 2000–1 (P < 0·001). Within each survey, phylloquinone intake expressed as μg/d was not associated with adiposity. However, when expressed as μg/kg body weight per d inverse associations were observed between intake and adiposity in both sexes in 1986–7 and 2000–1.
Compared with 1986–7, geometric mean phylloquinone intake in 2000–1 was lower in more categories of socio-demographic factors and smoking status among men than women (Tables 2 and 3). One striking sex difference is the absence of lower daily intakes in 2000–1 v. 1986–7 across age groups in women compared with significantly lower intakes in men aged < 45 years between the surveys.
Findings on the prevalence of low phylloquinone intake (in relation to the UK guideline and US AI) were the inverse of those reported for phylloquinone intake. In relation to the UK guideline, prevalence of low intake was highest among adults aged < 35 years and among those of manual occupational social class, in both surveys. In 1986–7, regional differences existed in the prevalence of low phylloquinone intake (being highest in Scotland and northern England), whereas by 2000–1 these differences were much less pronounced and failed to achieve statistical significance.
Food sources of phylloquinone intake
Vegetables contributed most to phylloquinone intake (Table 4). With about 60 % of intake provided by this food group, 19–23 % was provided by cooked LGV (cabbage, broccoli, Brussels sprouts and spinach), with another 14–18 % provided by raw salad vegetables (mainly lettuce; 9 % in 1986–7, 11 % in 2000–1). Of cooked LGV, cabbage and broccoli contributed most to phylloquinone intake; 13 and 3 % in 1986–7 v. 7 and 8 % in 2000–1. In common with that of cabbage, the contribution of Brussels sprouts was also significantly lower in 2000–1 compared with 14 years earlier (3 v. 7 %; P < 0·001).
Table 4 Mean daily consumption and percentage contribution of food groups to dietary phylloquinone intake of British adults in 1986–7 and 2000–1†
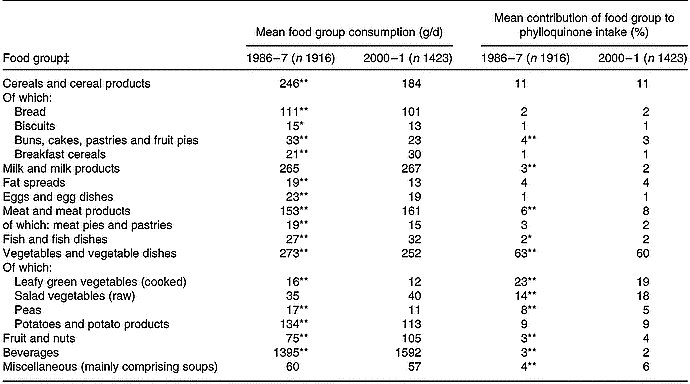
† The Table excludes data for participants who reported being unwell with eating habits affected and those who failed to provide a complete food diary during the 7 d periods of dietary assessment. Unadjusted summary statistics are presented.
‡ For respective food groups, mean value for daily consumption or percentage contribution to dietary phylloquinone intake was significantly different from that for 2000–1: * P < 0·01, ** P < 0·001 (ANOVA, after adjustment for sex, age group, region, occupational social class, smoking status, adiposity and season).
The contribution of cooked LGV to phylloquinone intake was substantially lower in 2000–1 compared with 1986–7, owing to lower mean daily consumption. The same applied to peas. In contrast, although consumption of raw salad vegetables was similar at both times, the mean contribution of this food group to phylloquinone intake was significantly higher in 2000–1. Along with findings for potatoes, cereals and their respective products, this indicates that it is the type of foods consumed within defined food groups and not simply the amount of food group consumption that is important with regard to its contribution to phylloquinone intake.
Differences in the percentage contribution of food groups to phylloquinone intake between the two surveys were minor. Although some differences (for example, for buns, cakes, pastries and fruit pies; milk and milk products, fruit and nuts) achieved statistical significance, the differences were small.
Apart from a higher contribution of raw salad vegetables to phylloquinone intake among women v. men in both surveys, contributions from all vegetables and other sub-groups did not differ significantly by sex (Table 5). The contribution to phylloquinone intake of vegetables and vegetable dishes in toto was lower in 2000–1 v. 1986–7 across all factor categories apart from participants aged 55–64 years and those of non-manual occupational social class. The contribution of all vegetables to phylloquinone intake was directly associated with age. Youngest adults ( < 35 years) derived least phylloquinone intake from cooked LGV and most from potatoes and potato products (mainly comprising chips and potato-based savoury snacks, such as crisps). Adults of manual occupational social class in 2000–1 derived less of their phylloquinone intake from vegetables than those with non-manual occupations. Fourteen years earlier, contributions were identical. At both times, those with manual occupations derived less phylloquinone intake from raw salad vegetables and more from potatoes and potato products than their non-manual counterparts. A similar finding occurred for smokers v. non-smokers, independent of season and other socio-demographic factors including occupational social class.
Table 5 Percentage contribution of vegetables to mean dietary phylloquinone intake of British adults in 1986–7 and 2000–1, by selected socio-demographic or lifestyle factors and season‡
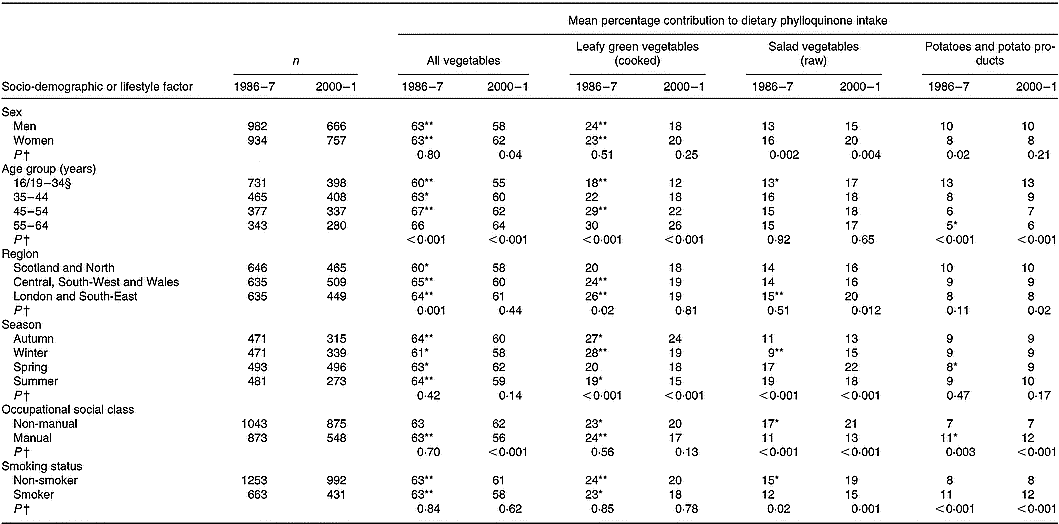
For each category within each factor, mean percentage contribution by the food group was significantly different from that for 2000–1: * P < 0·01, ** P < 0·001 (ANOVA, after adjustment for the other socio-demographic or lifestyle factors and season, and their respective two-way interactions with year of survey).
† Significance of difference between factor categories at each time point (by ANOVA, adjusted for the other socio-demographic or lifestyle factors and including two-factor interactions). Findings by adiposity are not shown since no significant differences were observed.
‡ The Table excludes data for participants who reported being unwell with eating habits affected and those who failed to provide a complete food diary during the 7 d periods of dietary assessment. Unadjusted summary statistics are presented.
§ Age range for 1986–7 is 16–34 years; age range for 2000–1 is 19–34 years.
Regional differences in sources of phylloquinone intake were also noted in 1986–7 but were either absent or attenuated in 2000–1. In 1986–7, adults living in Scotland and northern England derived less of their intake from vegetables and its sub-group of cooked LGV compared with their counterparts living elsewhere. In 2000–1, these respective contributions did not differ by region. While those living outside Scotland and northern England derived significantly less of their phylloquinone intake from this main food group and its sub-group, contributions in Scotland and northern England remained very similar between 1986–7 and 2000–1. At both times, participants living in Scotland also derived proportionately more of their phylloquinone intake from soups. In both surveys, seasonal differences were also observed regarding percentage contribution to phylloquinone intake from cooked LGV (highest in autumn and winter, lowest in summer) and raw salad vegetables (lower in autumn and winter v. spring and summer).
Discussion
Using dietary data collected in 1986–7 and 2000–1, the present study provides the first nationwide estimate of phylloquinone intake and sources among adults aged < 65 years living in mainland Britain. In summary, about one-half of adults had phylloquinone intakes below the UK guideline for adequacy (based on its role in blood clotting), with a higher prevalence of ‘low’ intake in 2000–1 than 14 years earlier. In both surveys, intake was higher in men than women, in non-smokers and those of non-manual occupational social class, and increased with age. About 60 % of intake was provided by vegetables, with about one-quarter provided by cooked LGV such as cabbage and broccoli. The lower phylloquinone intake observed in 2000–1 v. 1986–7 can be attributed to lower consumption of cooked LGV. Intakes, sources and their variability by socio-demographic and lifestyle factors observed in both surveys are comparable with those derived from the National Diet and Nutrition Survey of people aged 65 years and over in 1994–5 (Thane et al. Reference Thane, Paul, Bates, Bolton-Smith, Prentice and Shearer2002).
The reported estimates of phylloquinone intake were made possible using UK-specific phylloquinone contents for a comprehensive range of food and drink (Bolton-Smith et al. Reference Bolton-Smith, Price, Fenton, Harrington and Shearer2000a; Food Standards Agency, 2002; MJ Shearer and C Bolton-Smith, unpublished results). Although phylloquinone contents were assigned as accurately as possible, variation in food content is inevitable (Ferland & Sadowski, Reference Ferland and Sadowski1992; Piironen & Koivu, Reference Piironen and Koivu2000; Damon et al. Reference Damon, Zhang, Haytowitz and Booth2005), and changes in the composition or type of fat used in some processed foods (Davidson et al. Reference Davidson, Booth, Dolnikowski and Sadowski1996) between the two surveys will contribute to errors when estimating phylloquinone intake. However, the robust methodology and similarity of results with those of other published work suggest that estimated intakes are likely to be broadly representative of this age group in the UK.
Findings are also presented in the context of some differences in socio-demographic characteristics of adults from the two surveys. The sub-sample of participants from 1986–7 contained a higher percentage of adults aged < 35 years and of manual occupational social class, compared with their counterparts in 2000–1. However, when comparing phylloquinone intake between 1986–7 and 2000–1, these differences were adjusted for as part of multivariate statistical analyses in order to report differences independent of these and other potential confounders. One difference that could not be adjusted for relates to the representativeness of participants included in the present study compared with those excluded. Significant differences in socio-demographic or lifestyle characteristics were not found in 1986–7 between adults included or excluded from the data analyses, although in 2000–1 those excluded were more likely to be aged < 35 years, live in Scotland or northern England and report being smokers. These latter differences should be borne in mind if findings from the present study are extrapolated to the wider population.
Average phylloquinone intakes from the present study are similar to those reported in adults of a similar age in Scotland (Bolton-Smith et al. Reference Bolton-Smith, Price and Shearer2000b), Ireland (Duggan et al. Reference Duggan, Cashman, Flynn, Bolton-Smith and Kiely2004) and the USA (Booth & Suttie, Reference Booth and Suttie1998; Booth et al. Reference Booth, Webb and Peters1999b), and among older adults living in mainland Britain (Thane et al. Reference Thane, Paul, Bates, Bolton-Smith, Prentice and Shearer2002). Arithmetic mean intakes at both times are comparable with 82 μg/d estimated for free-living British adults aged 65 years and over (Thane et al. Reference Thane, Paul, Bates, Bolton-Smith, Prentice and Shearer2002), but higher than the 69 μg/d reported in American adults in 1990 (Booth et al. Reference Booth, Pennington and Sadowski1996). Intakes in the present study are somewhat lower than those reported by others in the USA (Booth et al. Reference Booth, Sokoll, O'Brien, Tucker, Dawson-Hughes and Sadowski1995; Institute of Medicine, 2001), and considerably lower than estimates from two Dutch studies (Jie et al. Reference Jie, Bots, Vermeer, Witteman and Grobbee1995; Schurgers et al. Reference Schurgers, Geleijnse, Grobbee, Pols, Hofman, Witteman and Vermeer1999). These latter findings may reflect a genuinely higher intake or be merely a reflection of differences in methodology used for dietary assessment, such as multiple questions on vegetable consumption within the food-frequency questionnaires used.
The lower geometric mean phylloquinone intake found in 2000–1, v. 1986–7, has also been reported in a 10-year follow-up of adults living in Scotland in 1985 and 1995 (Bolton-Smith et al. Reference Bolton-Smith, Price and Shearer2000b). The decline in phylloquinone intake over the 10 years was attributed to a decline in fat intake, whereas in the present study lower intake found in 2000–1 can be attributed to lower mean consumption of vegetables in toto, their sub-group of cooked LGV and, to a much lesser extent, cereals and cereal products compared with 1986–7.
The percentages of adults in 1986–7 (47 %) and 2000–1 (59 %) with phylloquinone intakes below the UK Government guideline of ≥ 1 μg/kg body weight per d (Department of Health, 1991) are similar to those reported in free-living British individuals aged 65 years and over (59 %; Thane et al. Reference Thane, Paul, Bates, Bolton-Smith, Prentice and Shearer2002), and Irish adults aged 18–64 years (52 %; Duggan et al. Reference Duggan, Cashman, Flynn, Bolton-Smith and Kiely2004). Only 15 % of men in 2000–1 achieved the US AI, compared with 23 % in 1986–7. Corresponding percentages among women were 28 and 26 %. Percentages found in 2000–1 are similar to those of men (17 %) and women (27 %) from the North/South Ireland Food Consumption Survey of 1997–9 (Duggan et al. Reference Duggan, Cashman, Flynn, Bolton-Smith and Kiely2004).
The change in percentages of participants failing to achieve the UK and US dietary reference intakes for phylloquinone between 1986–7 and 2000–1 is noteworthy (increases of 26 and 4 % respectively). The stark difference (men, increases of 47 and 10 %; women, 8 % increase v. 3 % decrease; in relation to the UK and US dietary reference intakes respectively) can be attributed to the units used (UK, μg/kg body weight per d; USA, μg/d) in the context of a significant increase in the percentage of the population being overweight and/or obese in 2000–1 compared with 14 years earlier.
In agreement with findings for British elderly individuals (Thane et al. Reference Thane, Paul, Bates, Bolton-Smith, Prentice and Shearer2002), lower phylloquinone intakes were found in participants living in Scotland and northern England, those of manual occupational background, and smokers compared with their comparator groups. With regard to adults living in Scotland and northern England having lower phylloquinone intake than those living elsewhere in 1986–7 but not in 2000–1, this may be attributed to a greater decrease in phylloquinone intake of adults living outside Scotland and northern England between 1986–7 and 2000–1. The lower phylloquinone intakes found in smokers v. non-smokers have also been reported in older British adults (Thane et al. Reference Thane, Paul, Bates, Bolton-Smith, Prentice and Shearer2002) and Scottish adults (Fenton et al. Reference Fenton, Price, Bolton-Smith, Harrington and Shearer1997; Bolton-Smith et al. Reference Bolton-Smith, Price and Shearer2000b), although not in a study of Dutch elderly individuals (Schurgers et al. Reference Schurgers, Geleijnse, Grobbee, Pols, Hofman, Witteman and Vermeer1999).
With regard to sources of phylloquinone intake, several studies have also shown vegetables to contribute at least one-half of intake, with cooked LGV alone providing one-quarter to one-third of intake among older adults in Great Britain (Thane et al. Reference Thane, Paul, Bates, Bolton-Smith, Prentice and Shearer2002), and among adults of a similar age in Scotland (Fenton et al. Reference Fenton, Price, Bolton-Smith, Harrington and Shearer1997), Ireland (Duggan et al. Reference Duggan, Cashman, Flynn, Bolton-Smith and Kiely2004) and the USA (Booth et al. Reference Booth, Sokoll, O'Brien, Tucker, Dawson-Hughes and Sadowski1995, Reference Booth, Pennington and Sadowski1996). Of cooked LGV, the largest contributions from cabbage and broccoli observed in the present study were also reported among Irish adults (11 and 7 % respectively; Duggan et al. Reference Duggan, Cashman, Flynn, Bolton-Smith and Kiely2004).
Although only 4 % of phylloquinone intake was derived from fat spreads in both surveys, this masks the fact that intake would also have been derived from phylloquinone-containing fats and oils distributed in many foods not categorised within the main food group of ‘fat spreads’. For example, according to the grouping of foods in both surveys, phylloquinone contained in oils and fats used in the manufacture of baked cereal products such as biscuits, buns, cakes and pastries would have contributed to phylloquinone intake derived from the main food group of ‘cereals and cereal products’. Similarly, oils contained within mixed or predominantly vegetable dishes would have contributed in the current analyses to phylloquinone intake derived from the main food group of ‘vegetables and vegetable dishes’. Therefore, the stated contribution of 4 % greatly under-estimates the total contribution of all fats and oils in the diet to phylloquinone intake, that could not be determined in the present study. In a study of seventy-two adults living in Scotland in the mid-1990s, fats from all sources were estimated to contribute 30 % of phylloquinone intake (Fenton et al. Reference Fenton, Price, Bolton-Smith, Harrington and Shearer1997). In the USA, fats and oils added to mixed dishes and desserts were estimated to provide 12–16 % (Booth et al. Reference Booth, Pennington and Sadowski1996), making them important contributors in the whole diet to phylloquinone intake.
The content of phylloquinone-rich rapeseed and soyabean oils in some fat spreads may also have been underestimated, depending on changes in the blend of oils used for the manufacture of fat spreads before and since similar products were analysed in the late 1990s (Bolton-Smith et al. Reference Bolton-Smith, Price, Fenton, Harrington and Shearer2000a; MJ Shearer and C Bolton-Smith, unpublished results). Therefore, for a food described in the surveys as, for example, ‘commercial blended oil’ its phylloquinone content would depend to a great extent on its content of rapeseed and soyabean oils, which would by itself be under commercial influence. The varying phylloquinone contents of fats and oils would in turn influence the overall estimate of phylloquinone intake.
Through its association with higher under-carboxylation of osteocalcin (Sokoll et al. Reference Sokoll, Booth, O'Brien, Davidson, Tsaioun and Sadowski1997; Binkley et al. Reference Binkley, Krueger, Kawahara, Engelke, Chappell and Suttie2002), it may be speculated that low phylloquinone intakes observed in the present study may have a negative impact on osteoblast activity and bone health. Similarly, low phylloquinone intake associated with under-carboxylation of matrix γ-carboxyglutamic acid protein may contribute to vascular calcification and influence the pathogenesis of CVD (Schurgers et al. Reference Schurgers, Dissel, Spronk, Soute, Dhore, Cleutjens and Vermeer2001), although recent findings from two large American cohort studies suggest that low phylloquinone intake is merely a marker, rather than a causative agent, for increased risk of CHD (Braam et al. Reference Braam, McKeown, Jacques, Lichtenstein, Vermeer, Wilson and Booth2004; Erkkilä et al. Reference Erkkilä, Booth, Hu, Jacques, Manson, Rexrode, Stampfer and Lichtenstein2005). Nevertheless, substantially higher phylloquinone intakes may be needed to maximise carboxylation of bone γ-carboxyglutamic acid proteins than those required for carboxylation of coagulation γ-carboxyglutamic acid proteins synthesised in the liver and involved in blood clotting (Sokoll et al. Reference Sokoll, Booth, O'Brien, Davidson, Tsaioun and Sadowski1997; Booth et al. Reference Booth, O'Brien-Morse, Dallal, Davidson and Gundberg1999a; Binkley et al. Reference Binkley, Krueger, Kawahara, Engelke, Chappell and Suttie2002; Vermeer et al. Reference Vermeer, Shearer, Zittermann, Bolton-Smith, Szulc, Hodges, Walter, Rambeck, Stöcklin and Weber2004). Within the European Union, it has recently been suggested that since under-carboxylation of extrahepatic phylloquinone-dependent Gla proteins seems to be common in apparently healthy adults, the guideline intake of about 1 μg/kg body weight per d is probably insufficient to fully carboxylate these proteins (European Union Scientific Committee on Food, 2003).
Combined with a future investigation of the relationship between phylloquinone intake estimated in 2000–1 and plasma phylloquinone concentration, the impact of these combined findings on future dietary recommendations for phylloquinone intake should be assessed in the light of increasing evidence of the suggested need for higher intakes than those currently recommended (and observed) in the UK.
Acknowledgements
Analysis of data from the Dietary and Nutritional Survey of British Adults was funded by the Medical Research Council. Analysis of data from the National Diet and Nutrition Survey of adults aged 19–64 years was funded by the Food Standards Agency (project number N05050). We thank The UK Data Archive (University of Essex, Colchester CO4 3SQ, UK) for providing electronic copies of the datasets for both surveys. We also thank Dr Martin Shearer for allowing us to use the comprehensive unpublished database of food phylloquinone contents.







