Introduction
There are hundreds of systematic reviews on attention deficit hyperactivity disorder (ADHD) of variable quality and with partly or fully overlapping scope. This literature is increasingly difficult to navigate for clinicians, researchers, and policymakers. We aim to make this large literature on ADHD more available by systematically reviewing the published systematic reviews on ADHD and highlighting recent reviews of high quality where there are overlaps.
Methods
We performed a meta-review [Reference Closs, Dowding and Allcock1] to systematically appraise systematic reviews and meta-analyses published on ADHD-related topics by adopting Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [Reference Moher, Liberati, Tetzlaff and Altman2] (Supplementary Table S1) and the Joanna Briggs Institute (JBI) methodology for umbrella review [Reference Aromataris, Fernandez, Godfrey, Holly, Khalil and Tungpunkom3]. The study protocol was pre-registered with the International Prospective Register of Systematic Reviews (PROSPERO; CRD42020165638).
Search strategy and selection criteria Table 1
We searched MEDLINE, PubMed, PsycINFO, Cochrane Library, and Web of Science for studies, using specific keywords (ADHD, systematic review, meta-analysis, see Supplementary 1 for a description of the search strategy) with no language restrictions. The search included all years and final search was completed in December 2021. Reference lists of included publications were also searched. All references from the literature search were imported to Endnote X7.2 [4] and then to Covidence [5]. Two reviewers (A.C. with I.L. or O.N.) independently performed title and abstract screening of all articles identified through database search and full-text screening of more than 95% of articles. Any discrepancies in assessments were resolved in consensus or by consulting the third reviewer or the last author (A.M.). To avoid overlap, when systematic reviews studied identical topics and included more than 50% of the same primary articles, we included only the latest reviews with more studies. The included latest systematic reviews and meta-analysis were of similar or high quality compared to the older reviews of same topics (Supplementary Table S2).
Table 1. Selection criteria

Quality appraisal and data extraction
The JBI guideline for quality appraisal and form for data extraction of systematic review and meta-analysis [Reference Aromataris, Godfrey, Holly, Khalil, Tungpunkom and Aromataris6] was amended and piloted for the purpose of this meta-review (Supplementary 1). The quality appraisal checklist consists of nine items. Reviews that scored less than six on low risk of bias were categorized as low quality and excluded. Reviews scoring six to seven, and eight to nine were categorized as moderate and high quality reviews, respectively, and were included. Two reviewers (A.C., I.L.) independently assessed the quality of 35% of the systematic reviews to ensure consistency in the quality assessment rating. There was good agreement in quality assessment, and consequently, the remaining 65% of included studies were scored for quality by one author only. A similar process was followed for data extraction, where three reviewers were involved (A.C. with I.L. or O.N.). For each eligible article, pre-defined information was extracted, including topic studied, objective, timeframe of database search, main findings with key estimates, implications for clinical practice and future research, and conclusions. Further details are in Supplementary 1.
Data presentation
We present the objective, main findings, and conclusions of each included systematic reviews and meta-analyses in tables dividing the literature into nine topics of ADHD. In the text, we describe the literature in terms of a narrative synthesis, where for some reviews we have also presented effect estimates with 95% confidence intervals (CI) for some key findings. For overlapping reviews, we highlight results based on recency and quality. As the result section is dense, we have also included summary table that include major findings, limitations, and recommendation for future systematic reviews and meta-analyses for each topic.
Results
A total of 1,161 systematic reviews and meta-analyses were identified, where 231 were eligible for inclusion (Figure 1). The reasons for exclusions of each article selected for full-text review are presented in Supplementary Table S3.
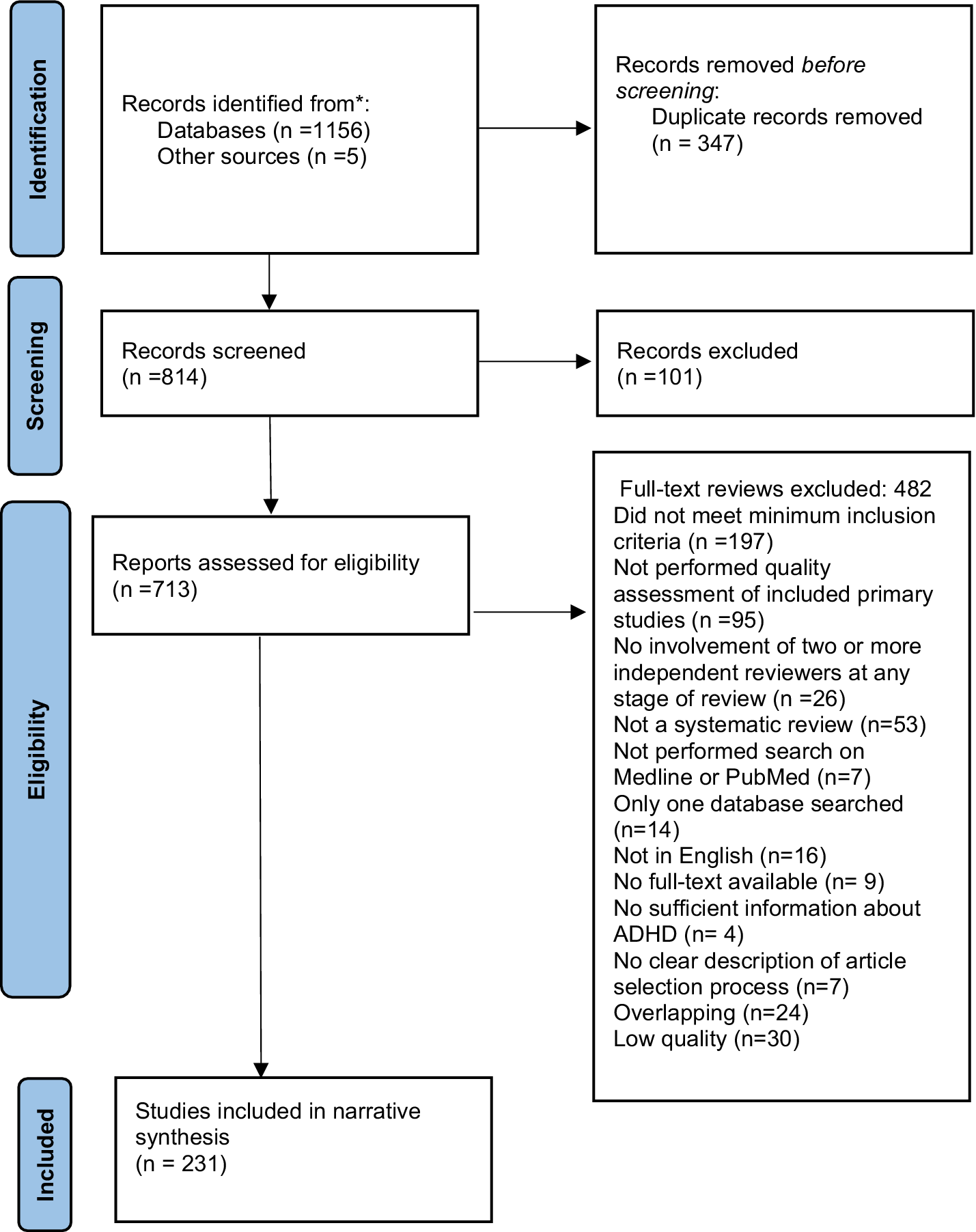
Figure 1. PRISMA flow diagram.
Characteristics and quality of included systematic reviews and meta-analyses
There has been an increase in published reviews annually with a very high number of reviews published in 2021. The most common topic for reviews was pharmacological interventions (28%) (Figure 2). Most of the studies included in reviews were conducted in Europe and North America (34 and 33%, respectively). Of the total included reviews, 59% were of high quality (Tables 3–11).

Figure 2. Number of systematic reviews and meta-analyses published per year on different topics.
Overview of findings
The included reviews were categorized into nine different topics and the major findings for each topic are presented in Table 2.
Table 2. Summary table of major findings
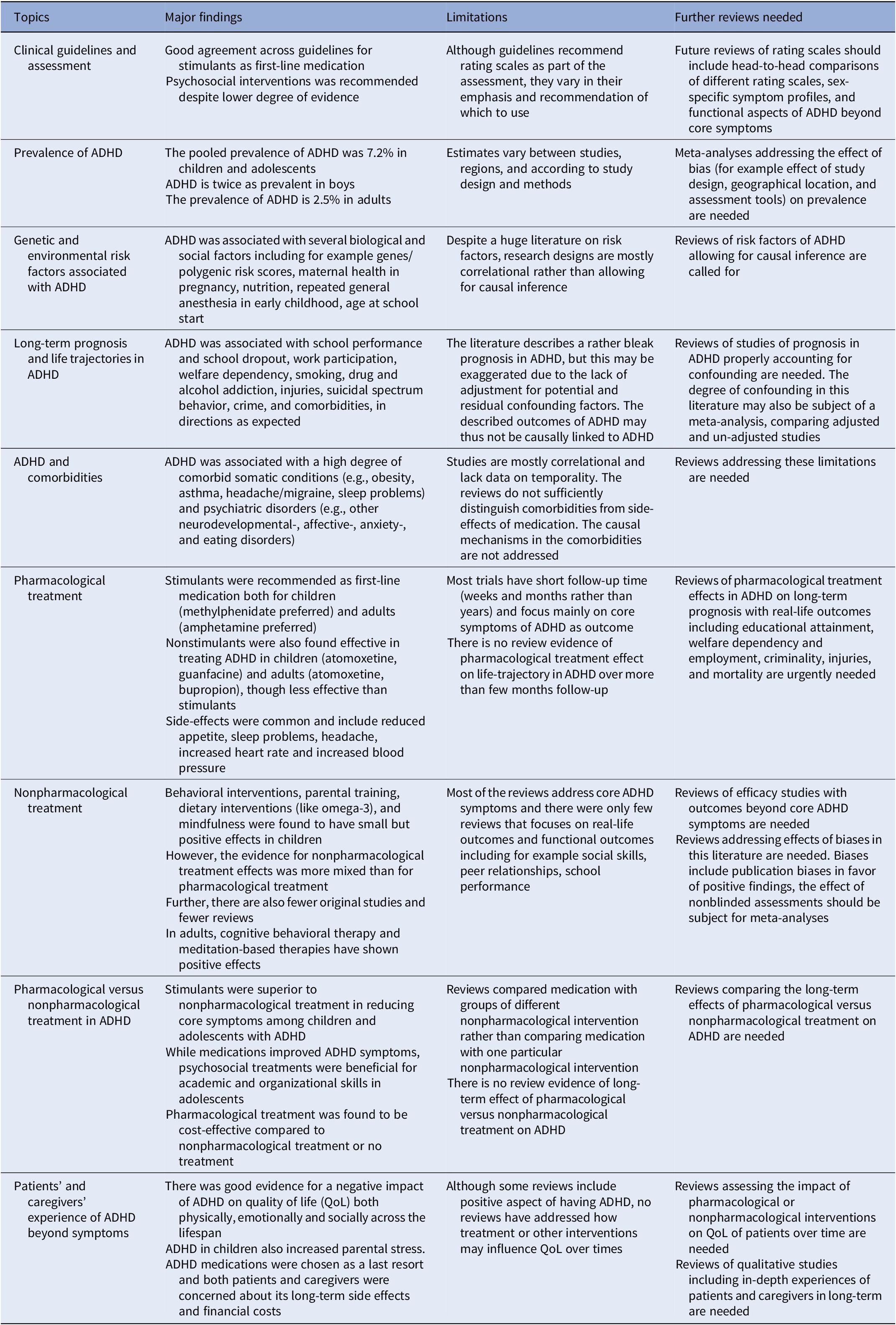
Narrative synthesis
Clinical guidelines and assessment (number of studies, n = 5, Table 3)
In a recent systematic review of five clinical practice guidelines, all guidelines rated stimulants as the first-line pharmacological intervention and recommended the inclusion of psychosocial intervention in the treatment [Reference Razzak, Ghader, Qureshi, Zafar, Shaijan and Al Kuwari7].
A meta-analysis of sex differences in ADHD symptoms showed that boys with ADHD are more hyperactive than girls and have more difficulties in terms of motor response inhibition and cognitive flexibility [Reference Loyer Carbonneau, Demers, Bigras and Guay8].
For screening for ADHD in children, the Child Behavior Checklist-Attention Problem and the Conner’s Rating Scale–Revised had moderate sensitivity and specificity [Reference Chang, Wang and Tsai9]. Conner’s Rating Scale and Strengths and Weaknesses of ADHD – Symptoms and Normal-Behaviors were found as valid and time-efficient measures to assess ADHD symptoms in the classroom [Reference Staff, Oosterlaan, van der Oord, Hoekstra, Vertessen and de Vries10].
In adults, the Conners Adult ADHD Rating Scale and the Wender Utah Rating Scale short version showed the best screening properties [Reference Taylor, Deb and Unwin11].
Table 3. Clinical guidelines and assessment

Abbreviations: ASQ, Abbreviated Symptom Questionnaire; CAARS, The Conners Adult ADHD Rating scale; CBCL-AP, Child Behavior Checklist-Attention Problem; CPRS-R, Conners Parent Rating Scale – Revised; CRS-R, Conners Rating Scale – Revised; CTRS-R, Conners Teacher Rating Scale-Revised; k, Total number of primary studies included; NA, not applicable; SWAN, strengths and weaknesses of ADHD – Symptoms and Normal-Behaviors; WURS, Wender Utah Rating Scale.
a Total participants included in the systematic review and meta-analysis unless otherwise indicated.
b For findings from meta-analysis, if given effect estimates with 95% CI are presented unless otherwise indicated.
Prevalence of ADHD (n = 8, Table 4)
Prevalence of ADHD in children and adolescents
The prevalence of ADHD among children and adolescents was assessed in four different meta-analyses [Reference Thomas, Sanders, Doust, Beller and Glasziou12–Reference Cenat, Blais-Rochette, Morse, Vandette, Noorishad and Kogan15] and one systematic review [Reference Hakim Shooshtari, Shariati, Kamalzadeh, Naserbakht, Tayefi and Taban16]. Internationally, the pooled prevalence of ADHD in children and adolescents was estimated to be 7.2% (95% CI: 6.7–7.8) in a meta-analysis of 175 studies including more than 1 million participants [Reference Thomas, Sanders, Doust, Beller and Glasziou12]. Most of the included studies were conducted within school populations (74%), and few used a whole-population approach (10%). In a multi-variable analysis, the prevalence estimate was 2 percentage points lower in studies conducted in Europe compared to North America after adjusting for the edition of diagnostic manual and measurement tools which included clinical interviews, symptom-only criteria, and reports of ADHD diagnosis [Reference Thomas, Sanders, Doust, Beller and Glasziou12]. Further, a meta-analysis of prevalence studies from Africa reported a pooled prevalence of 7.47% (6.0–9.26). As expected, gender differences were found with a male: female ratio of 2.0:1.0 [Reference Ayano, Yohannes and Abraha13]. The prevalence in China was 6.26% (5.36–7.22) [Reference Wang, Liu, Li, Xu, Liu and Shi14], while it was 13.87% (9.59–19.64) among black individuals in the USA [Reference Cenat, Blais-Rochette, Morse, Vandette, Noorishad and Kogan15]. All included reviews reported that significant heterogeneity in the prevalence attributed to the source of study population, geographical location, and source of data was found across included studies.
A systematic review found substantial evidence of overdiagnosis of ADHD. The authors reported that ADHD diagnoses have consistently increased between 1989 and 2017, and that the majority of new cases were on the milder end of the ADHD spectrum [Reference Kazda, Bell, Thomas, McGeechan, Sims and Barratt17].
Prevalence of ADHD in adults
The worldwide pooled prevalence among adults was 4.61% for persistent adult ADHD and 8.83% for symptomatic adult ADHD [Reference Song, Zha, Yang, Zhang, Li and Rudan18]. By adjusting for the “global demographic structure,” the prevalence of persistent adult ADHD was 2.58% (95% CI:1.51–4.45) and symptomatic adult ADHD 6.76% (4.31–10.61), translating to 139.84 and 366.33 million affected adults in 2020 globally. The meta-analysis found that the prevalence of ADHD decreased with age [Reference Song, Zha, Yang, Zhang, Li and Rudan18]. The prevalence among adults aged ≥50 was 2.2% based on validated scales applied in the general population, and 0.2% when based on clinical diagnosis [Reference Dobrosavljevic, Solares, Cortese, Andershed and Larsson19].
Table 4. Prevalence of ADHD

Abbreviations: DSM, diagnostic statistic model; HICs, high-income countries; k, total number of primary studies included; LMICs, lower and middle-income countries; WHO, World Health Organization.
a Total participants included in the systematic review and meta-analysis or otherwise specified.
b For findings from meta-analysis, if given effect estimates with 95% CI are presented or otherwise specified.
Genetic and environmental risk factors associated with ADHD (n = 41, Table 5)
In this section, we use terms like risk, correlation, protective, and association according to reports in the systematic reviews, without indicating causality. Generally, this literature did not bring evidence for conclusions on causality, which will be discussed later.
Table 5. Genetics and environmental risk factors associated with ADHD

Abbreviations: AA, arachidonic acid; CrI, credible interval; CS, cesarean section; CSA, child sexual abuse; d, effect size; EA, eicosapentaenoic acid; ID, iron deficiency; k, total number of included studies; MSDP, maternal smoking during pregnancy; Nox, nitrogen oxides; OR, odds ratio; PAH, polycyclic aromatic hydrocarbon; PB, preterm birth; PBDE, polybrominated diphenyl ether; PM, particulate matter; POE, prenatal opioid exposure; RR, relative risk; Rrat, risk ratio; SES, socioeconomic status; SHS, second-hand smoke; SMD, standardized mean difference; TBI, traumatic brain injury; VLBW/ELBW, very/extreme low birth weight; VP/EP, very and extreme preterm; WMD, weighted mean difference.
a Total participants included in the systematic review and meta-analysis or otherwise specified.
b For findings from meta-analysis, if given effect estimates with 95% CI are presented or otherwise specified.
Genetic factors
One systematic review found strong evidence that the common genetic variants underlying ADHD, as measured by the ADHD polygenic risk score, were associated not only with diagnosed ADHD but also with more dimensional ADHD traits [Reference Ronald, de Bode and Polderman20].
Maternal factors
Maternal pre-pregnancy overweight [Reference Li, Lagerberg, Chang, Cortese, Rosenqvist and Almqvist21], use of antibiotics [Reference Ai, Zhao, Shi and Zhu22], acetaminophen [Reference Gou, Wang, Tang, Qu, Tang and Shi23], and antidepressants [Reference Man, Chan, Ip, Coghill, Simonoff and Chan24] during pregnancy, and maternal pregestational diabetes [Reference Guo, Ju, Zhou, Mao, Tao and Jing25], but not gestational diabetes [Reference Rowland and Wilson26], were associated with increased rates of ADHD in offspring. However, the authors stated that associations may be due to unmeasured confounding and thus not causal (e.g., the association with maternal overweight was explained by familial confounding). Maternal smoking during pregnancy (odds ratio (OR) = 1.56, 95% CI: 1.41–1.72) or smoking cessation during the first trimester was associated with ADHD in offspring [Reference Dong, Hu, Zhou, Lin, Lan and Hang27]. Similarly, prenatal opioid exposure was associated with higher ADHD symptom scores (standardized mean difference (SMD) =1.27, 0.79–1.75) [Reference Schwartz, Reyes, Meschke and Kintziger28]. Maternal exposure to perfluoroalkyl substances was not associated with ADHD in their children [Reference Qu, Cao, Li, Wang, Liu and Wang29].
Perinatal complications like maternal preeclampsia [Reference Zhu, Gan, Huang, Li, Qu and Mu30], very preterm birth/very low birth weight (OR = 3.04, 95% CI: 2.19–4.21) [Reference Franz, Bolat, Bolat, Matijasevich, Santos and Silveira31], or low birth weight [Reference Serati, Barkin, Orsenigo, Altamura and Buoli32] were associated with offspring ADHD. Likewise, the caesarian section was reported to be associated with later ADHD diagnosis in unadjusted analyses [Reference Curran, O’Neill, Cryan, Kenny, Dinan and Khashan33], but a later review reported this association to be partly or entirely accounted for by residual confounding [Reference Xu, Zhang, Zhou, Jiang, Jiang and Zhou34].
A nonlinear relation between parental age and risk for ADHD in the offspring was found with the highest risk for parents below 20 years and lowest risk for parents in the mid-thirties [Reference Min, Li and Yan35].
While maternal breastfeeding was associated with a reduced risk of ADHD in children [Reference Tseng, Yen, Chen, Stubbs, Carvalho and Whiteley36, Reference Zeng, Tang, Tang, Shi, Zhang and Zhu37], postnatal exposure to second-hand smoking was associated with increased risk (OR = 1.60, 95% CI: 1.30–1.80) [Reference Huang, Wu, Cai, Lin, Zhang and Huang38].
Compared to children with other injuries or without injuries, children with severe traumatic brain injuries had an increased risk of being diagnosed with ADHD at less or more than 1 year, respectively, after the injuries [Reference Asarnow, Newman, Weiss and Su39]. Likewise, two or more exposures to general anesthesia were associated with an increased risk of ADHD in later life (relative risk RR = 1.84, 1.14–2.97) [Reference Sun, Zhu and Jiang40]. Blood lead level was associated with higher ADHD rates in children and adolescents [Reference Daneshparvar, Mostafavi, Zare Jeddi, Yunesian, Mesdaghinia and Mahvi41]. No significant association was found between polycyclic aromatic hydrocarbon exposure and ADHD in children [Reference Kalantary, Jaffarzadeh, Rezapour and Arani42, Reference Zhang, Wang, Zhang, Song and Li43], and there were inconclusive evidence for an association between exposure to air pollution [Reference Aghaei, Janjani, Yousefian, Jamal and Yunesian44] or polybrominated diphenyl ethers [Reference Lam, Lanphear, Bellinger, Axelrad, McPartland and Sutton45] and ADHD.
According to two included reviews, children being relatively younger than classmates had higher rates of ADHD diagnosis [Reference Kazda, Bell, Thomas, McGeechan, Sims and Barratt17, Reference Caye, Petresco, de Barros, Bressan, Gadelha and Goncalves46], with one reporting the relative risk of 1.34 (95% CI: 1.26–1.43) for the youngest children [Reference Caye, Petresco, de Barros, Bressan, Gadelha and Goncalves46]. Further, two reviews suggested an association between socioeconomic disadvantage and risk of ADHD [Reference Cenat, Blais-Rochette, Morse, Vandette, Noorishad and Kogan15, Reference Russell, Ford, Williams and Russell47], with one suggesting it to be mediated by factors such as parental mental health and maternal smoking during pregnancy [Reference Russell, Ford, Williams and Russell47]. The evidence regarding child sexual abuse as a predictor for ADHD was unclear [Reference Langevin, Marshall, Wallace, Gagné, Kingsland and Temcheff48].
Dietary pattern, nutrition, and trace elements
Children and adolescents consuming healthy diet had lower risk of having ADHD compared to those consuming unhealthy diet [Reference Del-Ponte, Quinte, Cruz, Grellert and Santos49]. Positive relationship was indicated between total sugar intake from soft drinks and dietary sources and ADHD symptoms in children and adolescents [Reference Farsad-Naeimi, Asjodi, Omidian, Askari, Nouri and Pizarro50]. Other nutritional factors associated with higher rates of ADHD among children and adolescents were low serum concentration of 25-hydroxyvitamin D, lower perinatal and childhood vitamin D status [Reference Khoshbakht, Bidaki and Salehi-Abargouei51], elevated ratios of both blood omega-6 to omega-3 and arachidonic acid to eicosapentaenoic acid fatty acids [Reference LaChance, McKenzie, Taylor and Vigod52]. Two systematic reviews reported significantly lower serum manganese levels in children with ADHD [Reference Huang, Zeng, Li, Cheng, Chen and Liang53, Reference Effatpanah, Rezaei, Effatpanah, Effatpanah, Varkaneh and Mousavi54]. Another review revealed higher peripheral manganese levels in both blood and hair in children and adolescents with ADHD compared to healthy controls [Reference Shih, Zeng, Lin, Chen, Chen and Wu55].
A review from 2021 suggested that brain iron concentrations, specifically in the thalamus, were lower in children with ADHD than in healthy controls [Reference Degremont, Jain, Philippou and Latunde-Dada56]. However, mixed results were reported for systemic iron level [Reference Degremont, Jain, Philippou and Latunde-Dada56, Reference Cortese, Angriman, Lecendreux and Konofal57]. In contrast, a review from 2018 concluded that low serum iron levels were associated with ADHD [Reference Tseng, Cheng, Yen, Chen, Stubbs and Whiteley58]. There was no difference in zinc levels in blood, serum, plasma [Reference Ghoreishy, Ebrahimi Mousavi, Asoudeh and Mohammadi59] or hair between children and adolescents with ADHD and healthy controls [Reference Luo, Mo and Liu60].
Long-term prognosis and life trajectories in ADHD (n = 19, Table 6)
Education and employment
ADHD was associated with lower educational attainment [Reference Christiansen, Labriola, Kirkeskov and Lund61], including failure to complete high school (OR = 3.7, 95% CI: 2.0–7.0) and failure to attend tertiary education (OR = 6.47, 4.58–9.14) [Reference Erskine, Norman, Ferrari, Chan, Copeland and Whiteford62]. Further, a negative association was found between ADHD and mathematical ability [Reference Tosto, Momi, Asherson and Malki63]. Individuals with ADHD were more prone to experience occupational challenges [Reference Christiansen, Labriola, Kirkeskov and Lund61], for example, they were more often dismissed from work (OR = 3.92, 2.68–5.74), unemployed (OR = 1.97, 1.01–3.85) [Reference Erskine, Norman, Ferrari, Chan, Copeland and Whiteford62], and more likely to receive public welfare payments [Reference Christiansen, Labriola, Kirkeskov and Lund61].
Alcohol, smoking, and substance use
Childhood ADHD was significantly associated with alcohol use disorder [Reference Di Lorenzo, Balducci, Poppi, Arcolin, Cutino and Ferri64], including the development of alcohol use disorder by early adulthood (OR = 1.35, 95% CI: 1.11–1.64) and nicotine use by middle adolescence (OR = 2.36, 1.71–3.27) [Reference Charach, Yeung, Climans and Lillie65]. Smokers with ADHD in childhood smoked significantly more cigarettes as adolescents than smokers without childhood ADHD [Reference Fond, Loundou, Guillaume, Quantin, Macgregor and Lopez66]. There was an association between ADHD and substance use disorder [Reference Di Lorenzo, Balducci, Poppi, Arcolin, Cutino and Ferri64], with an earlier meta-analysis reporting an odds ratio of 1.73(1.24–2.41) [Reference Erskine, Norman, Ferrari, Chan, Copeland and Whiteford62]. The estimated average prevalence of cocaine use in adults with ADHD was 26.0% (18.0–35.0) [Reference Oliva, Mangiapane, Nibbio, Berchialla, Colombi and Vigna-Taglianti67].
Injuries, poisoning, and suicidal spectrum behavior
Both children and adults with ADHD were at higher risk of injuries (OR = 1.96, 95% CI: 1.63–2.37) [Reference Amiri, Sadeghi-Bazargani, Nazari, Ranjbar and Abdi68], including bone fracture [Reference Seens, Modarresi, MacDermid, Walton and Grewal69] and unintentional physical injuries (OR = 1.53, 1.40–1.67) [Reference Ruiz-Goikoetxea, Cortese, Aznarez-Sanado, Magallon, Zallo and Luis70], but not for sports-related concussions [Reference Cook, Iaccarino, Karr and Iverson71]. Children and adolescents with ADHD also had a higher risk of poisoning than controls (RR = 3.14, 2.23–4.42) [Reference Ruiz-Goikoetxea, Cortese, Magallon, Aznarez-Sanado, Zallo and Luis72]. Suicidal spectrum behavior was higher in ADHD, including suicidal ideations (OR = 3.53, 2.94–4.25), suicidal plans, attempts (OR = 2.37, 1.64–3.43), and completed suicide [Reference Septier, Stordeur, Zhang, Delorme and Cortese73].
Psychotic disorders
Childhood ADHD was associated with an increased risk of subsequent psychotic disorders (OR = 4.74, 95% CI: 4.11–5.46) [Reference Nourredine, Gering, Fourneret, Rolland, Falissard and Cucherat74].
Criminal offenses and domestic violence
The pooled prevalence of ADHD among individuals in detention settings was 26.2% (95% CI: 22.7–29.6) [Reference Baggio, Fructuoso, Guimaraes, Fois, Golay and Heller75]. There was a significant association between childhood ADHD and adolescent and adulthood arrests (RR = 2.2, 1.3–3.5), convictions (RR = 3.3, 2.1–5.2), and incarcerations (RR = 2.9, 1.9–4.3) with a younger age of onset of antisocial involvement and an increased risk of criminal recidivism [Reference Mohr-Jensen and Steinhausen76]. However, there was no conclusive evidence for an association between ADHD and domestic violence [Reference Buitelaar, Posthumus and Buitelaar77].
Pregnancies and postpartum risk
Adolescent girls with ADHD had an increased risk of teenage and unintended pregnancies, and women with ADHD had a higher risk of pregnancy and birth complications, such as pre-eclampsia, infection, and cesarean section [Reference Kittel-Schneider, Quednow, Leutritz, McNeill and Reif78].
Global economic burden of ADHD
The per person annual economic burden of ADHD ranged from $US832 to 20,539 for patients with ADHD, and from $US2,670 to 4,120 for family members of patients with ADHD [Reference Chhibber, Watanabe, Chaisai, Veettil and Chaiyakunapruk79].
Table 6. Long-term prognosis and life-trajectories in ADHD

Abbreviations: DV, domestic violence; HR, hazard ratio; IPV, intimate partner violence; k, total number of included studies; LMICs, low and middle-income countries; OR, odds ratio; PD, psychotic disorder; RR, relative risk; SSB, suicidal spectrum behaviors.
a Total participants included in the systematic review and meta-analysis unless otherwise indicated.
b For findings from meta-analysis, if given effect estimates with 95% CI are presented unless otherwise indicated.
ADHD and comorbidities (n = 33, Table 7)
ADHD and other mental and neurological disorders
Children and adolescents with ADHD had significantly higher rates of autism spectrum disorder (ASD) (SMD = 1.23, 95% CI: 0.94–1.51) [Reference Hollingdale, Woodhouse, Young, Fridman and Mandy80] and pragmatic language difficulties than healthy controls [Reference Carruthers, Taylor, Sadiq and Tripp81]. ADHD in adults was strongly related to negative emotionality and low conscientious inhibition [Reference Jacobsson, Hopwood, Söderpalm and Nilsson82]. Similarly, the prevalence of bipolar disorder among adults with ADHD was 7.95% (5.31–11.06), and with 4 years earlier age of onset than in bipolar disorder without ADHD [Reference Schiweck, Arteaga-Henriquez, Aichholzer, Thanarajah, Vargas-Caceres and Matura83]. ADHD was associated with eating disorders (OR = 3.82, 2.34–6.24) [Reference Nazar, Bernardes, Peachey, Sergeant, Mattos and Treasure84], with similar findings reported in several systematic reviews [Reference Kaisari, Dourish and Higgs85, Reference Curtin, Pagoto and Mick86]. Reviews have also shown a link between ADHD and gaming disorder [Reference Dullur, Krishnan and Diaz87] and internet addiction (OR = 3.76, 2.75–5.15) in adolescents and young adults [Reference Wang, Yao, Zhou, Liu and Lv88].
Sleep
While a recent meta-analysis found no significant difference in sleep parameters between individuals with ADHD and healthy controls as measured by polysomnography [Reference Keenan, Sherlock, Bramham and Downes89], previous meta-analyses have reported impaired sleep among children with ADHD [Reference Lee, Kim and Lee90], including those measured by polysomnography [Reference Díaz-Román, Hita-Yáñez and Buela-Casal91] or actigraphy [Reference De Crescenzo, Licchelli, Ciabattini, Menghini, Armando and Alfieri92]. In adults, a meta-analysis showed longer sleep onset latency and lower sleep efficiency among adults with ADHD than without ADHD [Reference Diaz-Roman, Mitchell and Cortese93]. Clinically, adults with ADHD had an increased risk of nearly all types of sleep disorders, including insomnia and circadian rhythm disorders [Reference Instanes, Klungsoyr, Halmoy, Fasmer and Haavik94, Reference Lugo, Fadeuilhe, Gisbert, Setien, Delgado and Corrales95] and sleep bruxism [Reference Souto-Souza, Mourao, Barroso, Douglas-de-Oliveira, Ramos-Jorge and Falci96].
Obesity
Associations between ADHD and obesity have been found in children, adolescents, and adults [Reference Instanes, Klungsoyr, Halmoy, Fasmer and Haavik94, Reference Li, Xie, Lei, Li and Lei97], with one review reporting the association also after adjustments for possible confounders (children OR = 1.20, 95% CI: 1.05–1.37; adults OR = 1.55, 1.32–1.81) [Reference Cortese, Moreira-Maia, St Fleur, Morcillo-Penalver, Rohde and Faraone98]. A review on bariatric surgery showed that patients with ADHD responded equally well as patients without ADHD in terms of change in body mass index after surgery [Reference Mocanu, Tavakoli, MacDonald, Dang, Switzer and Birch99].
Somatic conditions
Associations with ADHD were reported for headache (OR = 1.98, 95% CI: 1.60–2.45) [Reference Pan, Jonsson, Şahpazoğlu Çakmak, Häge, Hohmann and Nobel Norrman100], asthma (OR = 1.52, 1.42–1.63) [Reference Kaas, Vinding, Stokholm, Bønnelykke, Bisgaard and Chawes101] and (OR = 1.53, 1.41–1.65) [Reference Cortese, Sun, Zhang, Sharma, Chang and Kuja-Halkola102], eczema (OR = 1.32, 1.20–1.45), and allergic rhinitis (OR = 1.52, 1.43–1.63) [Reference Schans, Cicek, de Vries, Hak and Hoekstra103]. Associations between ADHD and other atopic conditions have also been found [Reference Instanes, Klungsoyr, Halmoy, Fasmer and Haavik94, Reference Miyazaki, Koyama, Ota, Swa, Mlunde and Amiya104]. ADHD patients had reduced vagally-mediated heart rate variability (Hedge’s g = 0.20, 0.01–0.40) [Reference Robe, Dobrean, Cristea, Păsărelu and Predescu105], but no associations between adult ADHD and diseases of the circulatory system were found [Reference Instanes, Klungsoyr, Halmoy, Fasmer and Haavik94].
Other associated conditions
Children with ADHD had two times higher risks of having nocturnal enuresis than healthy controls. [Reference de Sena Oliveira, BdS, FCdC, MMdA, Albuquerque and Miranda106]. Reviews suggested that the findings were inconsistent for the relation between early signs of deviating motor functioning or development and later ADHD [Reference Athanasiadou, Buitelaar, Brovedani, Chorna, Fulceri and Guzzetta107, Reference Havmoeller, Thomsen and Lemcke108] and on anxiety and social functioning in children and adolescents with ADHD [Reference Bishop, Mulraney, Rinehart and Sciberras109].
ADHD in children was associated with a reduction in global retinal nerve fiber layer thickness (SMD = −0.23, 95% CI: −0.46 to −0.01) [Reference Li, Kam, Chee, Zhang, Chen and Yip110] and individuals with ADHD showed more oculomotor inhibition failure than control groups [Reference Chamorro, Betz, Philipsen, Kambeitz and Ettinger111]. Similarly, altered electrophysiological performance monitoring (i.e., reduced error-related negativity and the error positivity amplitude) during cognitive tasks, indicative of difficulties in evaluating errors in performance, have been reported both in children and adults with ADHD [Reference Bellato, Norman, Idrees, Ogawa, Waitt and Zuccolo112].
Table 7. ADHD and comorbidities
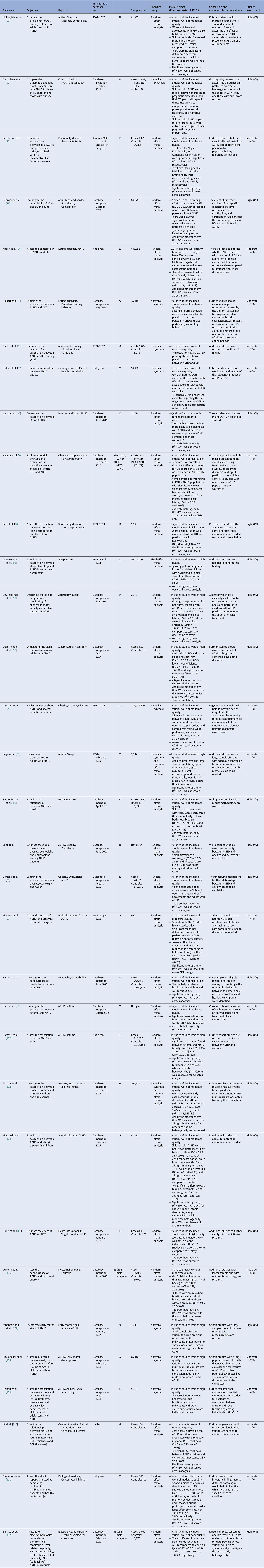
Abbreviations: ASD, autism spectrum disorder; BD, bipolar disorder; BMI, body mass index; DEB, disorder eating behavior; ED, eating disorder; ERN, error-related negativity; FRN, feedback-related negativity; GCL, ganglion cell layers; GD, gaming disorder; HRV, heart rate variability; IA, internet addiction; k, total number of included studies; OR, odds ratio; PTD, persistent tic disorder; RNFL, retinal nerve fiber layer thickness; RR, relative risk; SMD, standardized mean difference; TD, typically developing.
a Total participants included in the systematic review and meta-analysis unless otherwise indicated.
b For findings from meta-analysis, if given effect estimates with 95% CI are presented unless otherwise indicated.
Pharmacological treatment (n = 65, Table 8)
Efficacy, acceptability, and tolerability of ADHD medication
Children and adolescents
Systematic reviews have shown efficacy for pharmacological treatment of ADHD, with SMDs between 0.6 and 0.8 and consistently stronger efficacy for stimulants than nonstimulants [Reference Cerrillo-Urbina, Garcia-Hermoso, Pardo-Guijarro, Sanchez-Lopez, Santos-Gomez and Martinez-Vizcaino113, Reference Riera, Castells, Tobias, Cunill, Blanco and Capella114]. All-cause treatment discontinuation was lower with pharmacological treatment than placebo (OR = 0.68, 95% CI: 0.58–0.79) [Reference Riera, Castells, Tobias, Cunill, Blanco and Capella114].
Stimulants
In terms of efficacy, acceptability, and tolerability, evidence exists for methylphenidate (MPH) as the preferred first choice for pharmacological treatment of ADHD [Reference Cortese, Adamo, Del Giovane, Mohr-Jensen, Hayes and Carucci115–Reference Punja, Zorzela, Hartling, Urichuk and Vohra120]. For instance, in a network meta-analysis of 82 randomized controlled trials (RCTs) including more than 14,000 children and adolescents, at time point closest to 12 weeks, with clinician’s rating, MPH was found to be efficacious in reducing ADHD core symptoms as compared to placebo (SMD = –0.78,–0.93 to −0.62) [Reference Cortese, Adamo, Del Giovane, Mohr-Jensen, Hayes and Carucci115]. MPH was the only drug that had better acceptability than placebo, and with similar tolerability as placebo [Reference Cortese, Adamo, Del Giovane, Mohr-Jensen, Hayes and Carucci115]. A systematic review of four prospective or naturalistic studies and three RCTs showed MPH immediate-release (MPH-IR) as efficacious also for periods longer than 12 weeks (parent ratings for inattention and hyperactivity/impulsivity: SMD = 0.96, 0.60–1.32 and 1.12, 0.85–1.39, respectively) [Reference Maia, Cortese, Caye, Deakin, Polanczyk and Polanczyk116].
Lisdexamfetamine (LDX) was also efficacious in reducing ADHD symptoms compared to placebo [Reference Stuhec, Munda, Svab and Locatelli121, Reference Maneeton, Maneeton, Likhitsathian, Suttajit, Narkpongphun and Srisurapanont122], for example, as reported in a meta-analysis of 28 double-blind, placebo-controlled RCTs with around 4,700 participants (SMD = –1.28, −1.84 to −0.71) [Reference Stuhec, Munda, Svab and Locatelli121].
However, according to the earlier Cochrane review and meta-analyses, the efficacy of stimulants like MPH [Reference Storebø, Ramstad, Krogh, Nilausen, Skoog and Holmskov123] and amphetamine [Reference Punja, Shamseer, Hartling, Urichuk, Vandermeer and Nikles124] cannot be unequivocally established, due to methodological limitations. The authors point to small number of trials, short follow-up time, low-quality data, high risk of bias in several domains, including lack of sufficient blinding and selective outcome reporting, and heterogeneity between studies. This quite divergent conclusion is probably not due to different evidence included in the systematic reviews, but subjectively different interpretation of the evidence by the authors of different reviews.
Nonstimulants
Guanfacine was reported as safe and efficacious in treating ADHD compared to placebo, pooled (OR = 3.18, 95% CI: 2.44–4.13) [Reference Ruggiero, Clavenna, Reale, Capuano, Rossi and Bonati125]. Compared to placebo, atomoxetine (ATX) was also reported as safe and efficacious in reducing ADHD symptoms. [Reference Stuhec, Munda, Svab and Locatelli121, Reference Cheng, Chen, Ko and Ng126]. A meta-analysis from 1999 also suggested similar findings for clonidine [Reference Connor, Fletcher and Swanson127]. However, as for stimulants, meta-analyses and Cochrane review stated that the efficacy of some of the nonstimulants cannot be established due to short follow-up time, low quality, limited number of studies, sample sizes, and heterogeneity [Reference Maneeton, Maneeton, Intaprasert and Woottiluk128–Reference Matsui, Matsunaga, Matsuda, Kishi and Iwata130]. There is thus disagreement on how to interpret the literature also for nonstimulants.
Adults
Stimulants
A network meta-analysis of 52 RCTs, including more than 10,000 adults, supported amphetamines as the preferred first pharmacological choice for treatment of ADHD both regarding efficacy (clinicians’ ratings, SMD = –0.79, 95% CI: −0.99 to −0.58) and tolerability (OR = 3.26, 1.54–6.92) [Reference Cortese, Adamo, Del Giovane, Mohr-Jensen, Hayes and Carucci115]. Other reviews have also shown the efficacy of LDX, with one meta-analysis of 19 RCTs with more than 5,500 participants, reporting an SMD of −0.89 (−1.09 to −0.70) [Reference Stuhec, Lukic and Locatelli131] and another suggesting an SMD of −0.97 (−1.15 to −0.78) [Reference Maneeton, Maneeton, Suttajit, Reungyos, Srisurapanont and Martin132] for short-term treatment, compared to placebo. MPH also showed efficacy in reducing ADHD symptoms as compared to placebo (OR = 2.66, 2.12–3.33), and in terms of treatment discontinuation no significant difference was found between MPH and placebo (OR = 1.19, 0.82–1.74) [Reference Castells, Cunill and Capella133]. An indirect comparison meta-analysis of placebo-controlled trials from 2008 suggested shorter-acting stimulants, specifically IR-MPH as efficacious in reducing ADHD symptoms in adults [Reference Peterson, McDonagh and Fu134]. There was disagreement on how to interpret the original efficacy studies for adults as well. Cochrane reviews suggested that due to high risk of bias, limited sample sizes and number of studies, and heterogeneity in findings, the quality of the evidence for the efficacy of immediate-release MPH (compared to placebo or lithium) [Reference Cândido, Menezes de Padua, Golder and Junqueira135] and amphetamine (compared to placebo) [Reference Castells, Blanco-Silvente and Cunill136] was low to very-low.
Nonstimulants
In a meta-analysis of 12 RCTs with around 3,400 participants, ATX was found to have small efficacy in reducing the severity of ADHD symptoms (SMD = –0.33, 95% CI: −0.43 to −0.23) and increased rates of discontinuation compared to placebo (OR = 1.39, 1.17–1.64) [Reference Cunill, Castells, Tobias and Capellà137]. Cochrane review found low-quality evidence to conclude about the efficacy of bupropion in reducing ADHD symptoms severity and its tolerability compared to placebo [Reference Verbeeck, Bekkering, Van den Noortgate and Kramers138]. In another review, bupropion was found to be as efficacious as MPH, but due to limited number of studies, the authors suggested the findings as preliminary [Reference Maneeton, Maneeton, Intaprasert and Woottiluk128].
ADHD pharmacotherapy as a class
A network meta-analysis suggested that, despite a positive class effect of ADHD medication relative to placebo in improving clinical response, the quality of evidence was low to very low about the efficacy of different ADHD drugs on treating ADHD symptoms in adults [Reference Elliott, Johnston, Husereau, Kelly, Eagles and Charach139].
Adverse effect of ADHD medication
Cardiovascular
Patients in all age groups showed significant increases in heart rate and systolic blood pressure (SBP) from pre- to post-treatment when treated with MPH as compared to placebo (for SBP, SMD = 1.61, 95% CI: 0.81–2.41 for children and adolescents and SMD = 1.40, 0.62–2.18 for adults) [Reference Liang, Lim, Tam, Ho, Zhang and McIntyre140]. Furthermore, children and adolescents treated with ATX had a significant increase in SBP compared to those treated with MPH [Reference Liang, Lim, Tam, Ho, Zhang and McIntyre140]. However, no association was found between ADHD medication and the number of serious adverse medical events including sudden death, arrhythmia, stroke, myocardial infarction, and all-cause mortality [Reference Liu, Feng and Zhang141].
Sleep
Longer sleep latency (effect size = 0.54, 95% CI: 0.28–0.81) and shorter sleep duration (effect size = −0.59, −0.84 to –0.35) were noted in children and adolescents that used stimulants [Reference Kidwell, Van Dyk, Lundahl and Nelson142]. Similar findings were noted for children treated with MPH as measured by actigraphy for both longer sleep latency and shorter sleep duration [Reference De Crescenzo, Armando, Mazzone, Ciliberto, Sciannamea and Figueroa143].
Headache
Children and adolescents that used ADHD drugs were at increased risk of having headache during treatment period compared to placebo (for guanfacine, OR = 1.43, 95% CI: 1.12–1.82; for MPH, OR = 1.33, 1.09–1.63) [Reference Pan, Jonsson, Şahpazoğlu Çakmak, Häge, Hohmann and Nobel Norrman100].
Appetite
Short-term MPH treatment decreased appetite relative to placebo (RR = 3.66, 95% CI: 2.56–5.23) [Reference Holmskov, Storebo, Moreira-Maia, Ramstad, Magnusson and Krogh144]. This was also found for extended-release mixed amphetamine salts and ATX [Reference Chierrito de Oliveira, Guerrero de Sousa, Borges Dos Reis, Tonin, Maria Steimbach and Virtuoso145].
Pregnancy and postpartum-related side effects
No evidence of adverse offspring consequences of ADHD medication during pregnancy was found [Reference Kittel-Schneider, Quednow, Leutritz, McNeill and Reif78, Reference Li, Sujan, Butwicka, Chang, Cortese and Quinn146, Reference Jiang, Zhang, Jiang and Fu147]. MPH was reported as safe to use during breastfeeding, while the reported stimulating effect of guanfacine on prolactin secretion was considered to affect breastfeeding negatively [Reference Kittel-Schneider, Quednow, Leutritz, McNeill and Reif78].
Adverse events of MPH treatment
A meta-analysis showed low quality and reliability of evidence for adverse events of both short and long-term MPH treatment in children and adolescents and young adults [Reference Storebø, Pedersen, Ramstad, Kielsholm, Nielsen and Krogh148].
Efficacy of medication on ADHD symptoms in patients with comorbid conditions
Seven systematic reviews reported about the efficacy of medication on ADHD symptoms in patients with comorbid disorders [Reference Rodrigues, Lai, Beswick, Gorman, Anagnostou and Szatmari149–Reference Tsujii, Usami, Naya, Tsuji, Mishima and Horie156]. For example, both MPH and ATX were efficacious in reducing ADHD symptoms also in youth with ASD [Reference Rodrigues, Lai, Beswick, Gorman, Anagnostou and Szatmari149]. MPH was further found to decrease ADHD symptoms compared to placebo in children and adolescents with borderline intellectual functioning or intellectual disability (ID) (Hedges’ g = 0.87, 95% CI: 0.61–1.14) [Reference Sun, Tseng, Wu, Li, Chen and Stubbs150]. However, another review stated that the quality of evidence to conclude MPH as efficacious in treating ADHD symptoms in ID as poor [Reference Tarrant, Roy, Deb, Odedra, Retzer and Roy151]. No sufficient evidence was found to conclude about the effect of either amphetamine [Reference Thomson, Maltezos, Paliokosta and Xenitidis157] or risperidone [Reference Thomson, Maltezos, Paliokosta and Xenitidis158] in the treatment of ADHD symptoms in people with ID.
Efficacy of medication on comorbidity and other consequences of ADHD
In general, pharmacotherapy for ADHD was found to be efficacious (SMD = 0.40, 95% CI: 0.25–0.55) in reducing both ADHD symptoms and substance use in patients with both ADHD and substance use disorder [Reference Fluyau, Revadigar and Pierre159].
ADHD medications generally lowered the risk of injury (rate ratio = 0.76, 0.61–0.93) in individuals with ADHD, based on within-individual analysis [Reference Man, Ip, Chan, Law, Leung and Ma160]. However, a recent meta-analysis revealed higher risk of bone fracture among individuals treated with nonstimulants for ADHD (OR = 1.37, 1.20–1.58) whereas lower, but nonsignificant risk was observed for individuals treated with stimulants [Reference Zhang, Shen and Yan161].
Youths treated with stimulants had lower smoking rates than untreated youths with ADHD (OR = 0.51, 0.32–0.80) [Reference Schoenfelder, Faraone and Kollins162]. Further, long-term use (>90 days) of stimulants among ADHD individuals was associated with lower risk of suicide attempt (RR = 0.75, 0.66–0.84), based on within-individual analysis [Reference Liu, Mao, Hu, Song, Jiang and Zhang163].
In-school assignment was increased by 15% and on-task behavior by 14% or more among ADHD children treated with stimulants [Reference Prasad, Brogan, Mulvaney, Grainge, Stanton and Sayal164]. Likewise, MPH improved instrumental learning in children with ADHD [Reference Hulsbosch, De Meyer, Beckers, Danckaerts, Van Liefferinge and Tripp165]. Compared to placebo adult ADHD patients using MPH scored better on several neurocognitive measures and tests of driving ability (Hedge’s g = 0.17, 0.05–0.28) [Reference Pievsky and McGrath166].
Effect modification of comorbid conditions and drug effects on cooccurring symptoms
Comorbid anxiety did not change the efficacy of ATX on ADHD in children, adolescents, and adults [Reference Villas-Boas, Chierrito, Fernandez-Llimos, Tonin and Sanches167]. While ADHD medications like MPH and ATX showed smaller effects, LDX was moderately efficacious in treating emotional dysregulation in adults with ADHD (SMD = –0.50; 95% CI: −0.80–0.21) [Reference Lenzi, Cortese, Harris and Masi168]. Compared to placebo, stimulants were efficacious in reducing both overt and covert aggression in children and adolescents with ADHD [Reference Connor, Glatt, Lopez, Jackson and Melloni169]. Similarly, risperidone reduced aggressive behavior in youth with ADHD [Reference Pringsheim, Hirsch, Gardner and Gorman170].
Pharmacogenetics and animal models
While noradrenergic gene polymorphisms were associated with improved MPH response in children with ADHD [Reference Yuan, Zhang, Huang, Wang, Jiao and Huang171], low-quality evidence exists regarding the impact of SLC6A3 polymorphisms in response to MPH in children with ADHD [Reference Soleimani, Salehi, Soltanipour, Hasandokht and Jalali172]. No effect was found for MPH on hypertensive rats as an animal model of ADHD [Reference Leffa, Panzenhagen, Salvi, Bau, Pires and Torres173].
Pharmacological treatment rate
The first systematic review on undertreatment, overtreatment, and misuse of ADHD medication reported that 19.1% (95% CI: 11.5–29.9) of school-aged children and adolescents having ADHD were treated with medication for the disorder, and 0.9% (0.5–1.7) of individuals without the diagnosis used medication for ADHD. Their findings indicated both overtreatment and misuse of drugs in individuals without ADHD, and undertreatment of ADHD drugs in youths with the disorder [Reference Massuti, Moreira-Maia, Campani, Sônego, Amaro and Akutagava-Martins174].
Table 8. Pharmacological treatment

Abbreviations: AMP, amphetamine; ASD, autism spectrum disorder; ATX, atomoxetine; CVD, cardiovascular disease; d-MPH, dex-methylphenidate; dex-AMP, dex-amphetamine; ED, eating disorder; HR, heart rate; IR-MPH, immediate-release methylphenidate; k, total number of included studies; LDX, lisdexamphetamine; MPH, methylphenidate; NA, not applicable; OR, odds ratio; OROS, osmotic release oral system; SBP, systolic blood pressure; SMD, standardized mean difference; SUD, substance use disorder; WMD, weighted mean difference.
a Total participants included in the systematic review and meta-analysis unless otherwise indicated.
b For findings from meta-analysis, if given effect estimates with 95% CI are presented or otherwise specified.
Nonpharmacological treatment (n = 42, Table 9)
Children and adolescents
Behavioral interventions, including social and academic skills training, cognitive behavioral therapy (CBT), parent coaching on social skills, and stress management, were found to decrease the child’s ADHD symptoms and conduct problems and improve social skills, academic performance, and positive parenting when reported by proximal observers [Reference Daley, van der Oord, Ferrin, Danckaerts, Doepfner and Cortese175]. Effects on parenting and children’s conduct problems persisted when assessment was blinded [Reference Daley, van der Oord, Ferrin, Danckaerts, Doepfner and Cortese175]. Parental training was an efficacious intervention for reducing ADHD symptoms in preschool children (Hedges’ g = 0.51, 95% CI: 0.33–0.65) and negative parenting style, as based on parents’ rating [Reference Rimestad, Lambek, Zacher Christiansen and Hougaard176].
In a school-based setting, combined interventions, including social skills training, behavior modification technique, study and organizations skills training, were found to improve ADHD symptoms, as rated by both teachers and parents [Reference Moore, Russell, Matthews, Ford, Rogers and Ukoumunne177]. Daily behavior report cards were associated with reductions in teacher-rated ADHD symptoms [Reference Iznardo, Rogers, Volpe, Labelle and Robaey178] and improvement in academic outcomes [Reference Moore, Russell, Matthews, Ford, Rogers and Ukoumunne177]. Peer-inclusion interventions led to moderate improvements in pre-post measure of social functioning (Hedges’ g = 0.58, 0.45–0.70) among those receiving treatment [Reference Cordier, Vilaysack, Doma, Wilkes-Gillan and Speyer179].
Results were mixed for the efficacy of cognitive interventions in reducing ADHD symptoms and for improving working memory performance [Reference Chen, Yu, Zhang, Zhang, Zhang and Wang180–Reference Cortese, Ferrin, Brandeis, Buitelaar, Daley and Dittmann182]. Virtual reality-based interventions were more effective than other nonpharmacological interventions or no intervention in improving sustained attention in children and adolescents with ADHD [Reference Romero-Ayuso, Toledano-González, Rodríguez-Martínez, Arroyo-Castillo, Triviño-Juárez and González183]. Interventions based on mind–body therapies [Reference Barranco-Ruiz, Etxabe, Ramirez-Velez and Villa-Gonzalez184] and few-foods diet, that is diet elimination of many foods and additives, have shown positive effects on ADHD core symptoms [Reference Pelsser, Frankena, Toorman and Rodrigues Pereira185]. However, homeopathy did not show positive effects in reducing ADHD symptoms [Reference Heirs and Dean186].
Several reviews suggested that despite their wide applications, significant knowledge gaps exist regarding the effectiveness of various nonpharmacological interventions. These include mindfulness [Reference Oliva, Malandrone, di Girolamo, Mirabella, Colombi and Carletto187–Reference Zhang, Diaz-Roman and Cortese189], neurofeedback [Reference Sampedro Baena, Fuente, Martos-Cabrera, Gómez-Urquiza, Albendín-García and Romero-Bejar190–Reference Sonuga-Barke, Brandeis, Cortese, Daley, Ferrin and Holtmann193], behavioral interventions [Reference Sonuga-Barke, Brandeis, Cortese, Daley, Ferrin and Holtmann193], cognitive training [Reference Sonuga-Barke, Brandeis, Cortese, Daley, Ferrin and Holtmann193], dietary interventions [Reference Händel, Rohde, Rimestad, Bandak, Birkefoss and Tendal194–Reference Gan, Galer, Chen and Xiong201], herbal interventions [Reference Bruton, Nauman, Hanes, Gard and Senders202, Reference Anheyer, Lauche, Schumann, Dobos and Cramer203], parent and teacher training [Reference Zwi, Jones, Thorgaard, York and Dennis204, Reference Montoya, Colom and Ferrin205], social skills training [Reference Storebø, Elmose Andersen, Skoog, Joost Hansen, Simonsen and Pedersen206], school-based interventions [Reference Richardson, Moore, Gwernan-Jones, Thompson-Coon, Ukoumunne and Rogers207], equine-assisted therapies [Reference Helmer, Wechsler and Gilboa208, Reference Perez-Gomez, Amigo-Gamero, Collado-Mateo, Barrios-Fernandez, Munoz-Bermejo and Garcia-Gordillo209], video modeling [Reference Wilkes-Gillan, Cordier, Chen, Swanton, Mahoney and Trimboli210], acupuncture [Reference Lee, Choi, Kim, Kim and Ernst211], and physical exercise [Reference Cerrillo-Urbina, García-Hermoso, Sánchez-López, Pardo-Guijarro, Santos Gómez and Martínez-Vizcaíno212].
Adults
In adults, long-term, that is at least 12 months follow-up of psychotherapies (CBT, dialectical behavioral therapy, mindfulness-based cognitive therapy) showed greater improvement in self-reported total ADHD symptoms (SMD = 0.71, 95% CI: 0.22–1.21), inattention, and hyperactivity/impulsivity in intervention than control groups [Reference López-Pinar, Martínez-Sanchís, Carbonell-Vayá, Fenollar-Cortés and Sánchez-Meca213]. One systematic review suggested that CBT might improve the core symptoms of ADHD, but the evidence was of low quality [Reference Lopez, Torrente, Ciapponi, Lischinsky, Cetkovich-Bakmas and Rojas214]. CBT was also efficacious in treating comorbid-internalizing symptoms in adults with ADHD [Reference Lopez-Pinar, Martinez-Sanchis, Carbonell-Vaya, Sanchez-Meca and Fenollar-Cortes215]. Mind–body therapies including meditation were efficacious in reducing ADHD core symptoms compared to, for example, treatment as usual, although the evidence was of low quality [Reference Poissant, Mendrek, Talbot, Khoury and Nolan216].
Evidence for behavioral intervention to improve driving skills in young train novice drivers with ADHD was inconclusive [Reference Bruce, Unsworth and Tay217]. Transcranial direct current stimulation might have some effect on neuropsychological and cognitive deficits, but there was no evidence to suggest that it decreases ADHD symptoms [Reference Salehinejad, Wischnewski, Nejati, Vicario and Nitsche218].
Table 9. Nonpharmacological treatment

Abbreviations: CBT, cognitive behavioral therapy; DBRC, daily behavior report cards; ED, emotional dysregulation; EQS, equine-assisted services; k, total number of included studies; MBI, mindfulness-based interventions; NF, neurofeedback; PUFA, polyunsaturated fatty acid; SMD, standardized mean difference; tDCS, transcranial direct current stimulation.
a Total participants included in the systematic review and meta-analysis unless otherwise indicated.
b For findings from meta-analysis, if given effect estimates with 95% CI are presented unless otherwise indicated.
Pharmacological versus nonpharmacological treatment (n = 7, Table 10)
Two meta-analyses have reported the efficacy of stimulant treatment compared to nonstimulant or other interventions [Reference Yang, Lane, Chang and Tzang219, Reference Catala-Lopez, Hutton, Nunez-Beltran, Page, Ridao and St-Gerons220]. A network meta-analysis of 190 RCTs found stimulants to be superior compared to nonpharmacological treatment in children and adolescents with ADHD [Reference Catala-Lopez, Hutton, Nunez-Beltran, Page, Ridao and St-Gerons220]. While medications like extended release-MPH, amphetamine formulations, ATX, and extended release-guanfacine improved ADHD symptoms, psychosocial treatments were beneficial for academic and organizational skills in adolescents [Reference Chan, Fogler and Hammerness221]. Results from head-to-head trials comparing MPH and neurofeedback were too inconsistent to conclude [Reference Yan, Wang, Yuan and Zhang222]. Similarly, findings for the efficacy of MPH versus traditional Chinese medicine were suggested by earlier review [Reference Lan, Zhang and Luo223].
A recent systematic review revealed that stimulant treatment appeared to be cost-effective compared to other treatments or no treatment for ADHD in children and adolescents [Reference Dijk, Wessels, Constanti, van den Hoofdakker, Hoekstra and Groenman224]. Similar findings for pharmacotherapy as a whole were suggested by earlier review [Reference Wu, Hodgkins, Ben-Hamadi, Setyawan, Xie and Sikirica225].
Table 10. Pharmacological versus nonpharmacological interventions for ADHD
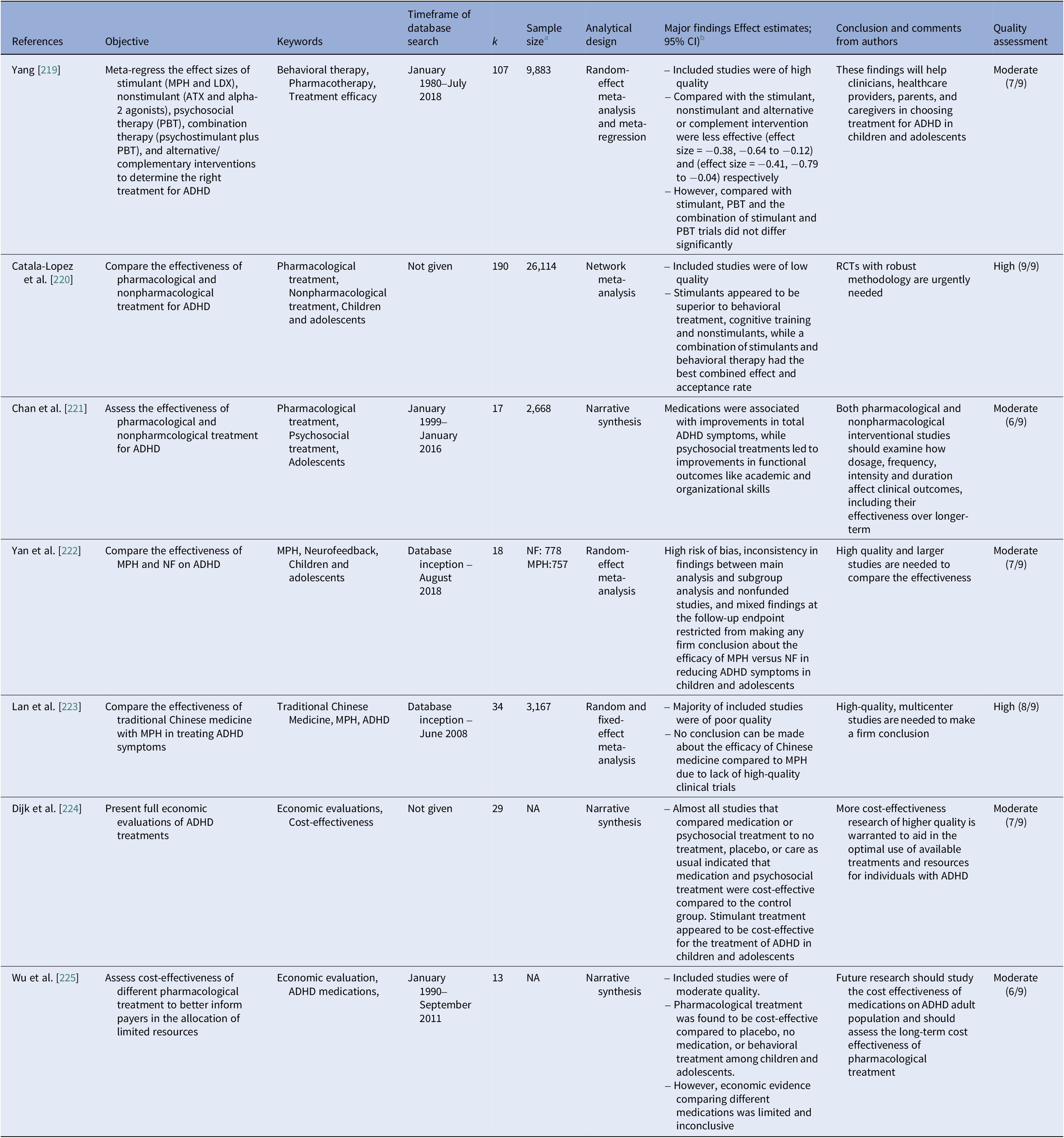
Abbreviations: k, total number of included studies; LDX, lisdexamphetamine; MPH, methylphenidate; NA, not applicable; NF, neurofeedback; PBT, parental behavioral therapy; RCTs, randomized controlled trials.
a Total participants included in the systematic review and meta-analysis unless otherwise indicated.
b For findings from meta-analysis, if given effect estimates with 95% CI are presented or otherwise specified.
Patients’ and caregivers’ experience of ADHD beyond symptoms (n = 13, Table 11)
Impact on quality of life
Of the two included reviews on health-related quality of life of children and adolescents with ADHD, one found that the parents reported significantly worse health-related quality of life of their children than that reported by the children with ADHD themselves [Reference Lee, Yang, Lee and Teng226], while the other review suggested no such differences [Reference Lee, Yang, Chen, Lee, Teng and Lin227].
Experience with ADHD, pharmacological and nonpharmacological interventions
Individuals with ADHD experience poor academic functioning, pressure to fit in with societal rules and expectations, fear of stigma, and difficulties in being part of the workplace and performing work tasks [Reference Eccleston, Williams, Knowles and Soulsby228–Reference Bjerrum, Pedersen and Larsen230]. However, they may also recognize the positive and rewarding aspects of having ADHD [Reference Eccleston, Williams, Knowles and Soulsby228–Reference Bjerrum, Pedersen and Larsen230].
Patients with ADHD and their caregivers experience medication as a last resort [Reference Rashid, Lovick and Llanwarne231] or as a coping strategy [Reference Bjerrum, Pedersen and Larsen230]. Some experience that starting medication trigger off an identity crisis [Reference Eccleston, Williams, Knowles and Soulsby228, Reference Rashid, Lovick and Llanwarne231]. There were reports of concerns about the long-term side effects of medication and financial costs, and the decision of taking medication was based on a process of “pro and con” considerations [Reference Eccleston, Williams, Knowles and Soulsby228, Reference Rashid, Lovick and Llanwarne231]. Educators, children, and adolescents with ADHD, and their parents felt that school-based nonpharmacological interventions can lead to stigma, but also improve relationships and attitudes [Reference Moore, Gwernan-Jones, Richardson, Racey, Rogers and Stein232].
Parenting a child with ADHD was felt as exhausting and emotional journey filled with feelings of guilt, frustration, although “not all bad” [Reference Wong, Hawes, Clarke, Kohn and Dar-Nimrod229, Reference Laugesen and Groenkjaer233]. The most commonly used coping strategy of parents seemed to be avoidant-focused coping and was linked to distress and depression [Reference Martin, Papadopoulos, Chellew, Rinehart and Sciberras234, Reference Craig, Savino, Fanizza, Lucarelli, Russo and Trabacca235].
Societal and familial barriers to ADHD treatment
Reviews of qualitative studies found perceived stigma as a barrier for acknowledging ADHD by primary care practitioners [Reference French, Sayal and Daley236] and for implementing nonpharmacological intervention in schools [Reference Gwernan-Jones, Moore, Cooper, Russell, Richardson and Rogers237]. There was no sufficient evidence to conclude if poverty moderates psychosocial treatment efficacy for ADHD [Reference Ogle, Frazier, Helseth, Cromer and Lesperance238].
Table 11. Patients’ and caregivers’ experience of ADHD beyond symptoms

Abbreviations: HRQOL, health related quality of life; k, total number of included studies.
a Total participants included in the systematic review and meta-analysis unless otherwise indicated.
b For findings from meta-analysis, if given effect estimates with 95% CI are presented unless otherwise indicated.
Discussion
To the best of our knowledge, this is the first systematic meta-review that summarizes the main findings of 231 existing systematic reviews and meta-analyses on ADHD of moderate to high quality.
Different from earlier narrative reviews of evidence-based conclusions about ADHD [Reference Faraone, Banaschewski, Coghill, Zheng, Biederman and Bellgrove239–241], we have pre-registered the protocol, adhered to the PRISMA and JBI guidelines to perform a systematic quality assessment of the included reviews. However, our review has some limitations that should be considered. Importantly, this meta-review is not an overview of all published literature on ADHD but limited to academic publications on ADHD covered by systematic reviews and meta-analyses of moderate to high quality, indexed in the five most relevant databases or found in reference lists. Grey literature publications were not covered in our search. Secondly, our inclusion criteria and threshold for quality assessment were quite strict, thus excluding some of the pertinent systematic reviews on topics including genetics [Reference Liu, Dai, Wu, Yuan, Gu and Chen242–Reference Franke, Vasquez, Johansson, Hoogman, Romanos and Boreatti-Hümmer249], neurobiology [Reference Kaiser, Schoemaker, Albaret and Geuze250, Reference Lei, Du, Wu, Chen, Huang and Du251], prevalence including young adults [Reference Polanczyk, Willcutt, Salum, Kieling and Rohde252–Reference Bonvicini, Faraone and Scassellati254], comorbidities like dyslexia [Reference Lonergan, Doyle, Cassidy, MacSweeney Mahon, Roche and Boran255, Reference McGrath and Stoodley256], speech disorder [Reference Machado-Nascimento, Melo and Lemos257], borderline personality disorder [Reference Ditrich, Philipsen and Matthies258] and nonpharmacological interventions in adults [Reference Nimmo-Smith, Merwood, Hank, Brandling, Greenwood and Skinner259].
Nevertheless, this meta-review will aid both researchers and clinicians to get an update on the main conclusions from ADHD research. Importantly our review may also be used as a bird’s eye view by identifying areas where there is sufficient evidence, insufficient evidence, and systematic weaknesses in reviews in various fields of ADHD. In the following, we will summarize some of our key findings with emphasis on weaknesses in the literature of systematic reviews on ADHD.
Prevalence of ADHD
According to included meta-analyses, the prevalence of ADHD is 7.2% in children [Reference Thomas, Sanders, Doust, Beller and Glasziou12] and 2.5% in adults [Reference Song, Zha, Yang, Zhang, Li and Rudan18]. However, there is considerable variation in the reported prevalence between the original studies included in these reviews. This strong variation is not fully understood, although probably due to other factors than true phenotype differences within or between populations studied. More plausible explanations for the variation in prevalence might include: (i) There are differences in research design, applied diagnostic instruments, and source of information which may cause bias [Reference Polanczyk, Willcutt, Salum, Kieling and Rohde252]. (ii) There may be variation in provider-preference, that is clinician-dependent variation in assessment and diagnostic decisions [Reference Mykletun, Widding-Havneraas, Chaulagain, Lyhmann, Bjelland and Halmøy260]. This variation may be caused by variation in clinicians’ attitudes toward ADHD diagnosis and medications, for example, from a liberal to a restrictive position, even within uniform health systems [Reference Lyhmann, Widding-Havneraas, Zachrisson, Bjelland, Chaulagain and Mykletun261]. (iii) There may be variation in supply [Reference Patel, Maj, Flisher, De Silva, Koschorke and Prince262–Reference Madsen, Ersbøll, Olsen, Parner and Obel264] and demand [Reference Johnson and Stukel265] of health services, which in turn ultimately will affect variation in rates of diagnosed ADHD. (iv) Finally, the rate of diagnosed ADHD has increased over the last decades, both in children [Reference Thomas, Sanders, Doust, Beller and Glasziou12, Reference Kazda, Bell, Thomas, McGeechan, Sims and Barratt17, Reference Xu, Strathearn, Liu, Yang and Bao266] and adults [Reference Song, Zha, Yang, Zhang, Li and Rudan18]. This may reflect a previous under-recognition of ADHD or increasing over-diagnosis [Reference Kazda, Bell, Thomas, McGeechan, Sims and Barratt17]. Hence, this has left the field of ADHD research with the question of how certain are we of the prevalence of ADHD? The uncertainty is not merely a question of narrowing the confidence interval in meta-analyses by including more studies. More sophisticated reviews are needed, addressing the issues of various forms of bias in original studies.
Risk factors for ADHD: Correlations versus causality
There is evidence of a whole range of ADHD “risk factors,” including for example biological [Reference Ronald, de Bode and Polderman20], maternal [Reference Li, Lagerberg, Chang, Cortese, Rosenqvist and Almqvist21–Reference Schwartz, Reyes, Meschke and Kintziger28, Reference Zhu, Gan, Huang, Li, Qu and Mu30, Reference Franz, Bolat, Bolat, Matijasevich, Santos and Silveira31, Reference Min, Li and Yan35], environmental [Reference Asarnow, Newman, Weiss and Su39, Reference Daneshparvar, Mostafavi, Zare Jeddi, Yunesian, Mesdaghinia and Mahvi41, Reference Caye, Petresco, de Barros, Bressan, Gadelha and Goncalves46], social [Reference Russell, Ford, Williams and Russell47], and nutritional factors [Reference Del-Ponte, Quinte, Cruz, Grellert and Santos49, Reference Farsad-Naeimi, Asjodi, Omidian, Askari, Nouri and Pizarro50]. However, this literature is mostly based on research designs precluding conclusions on causality due to problems with confounding, and reverse causality. Adjustment for confounders usually makes a difference in such correlational studies, indicating residual confounding due to a lack of information on potential confounders or the reliability of measured confounders. As an example, maternal smoking has, and still is, consistently associated with offspring ADHD in classical epidemiological studies. However, later years’ research, combining different approaches, has shown that this association is not causal, but mainly due to genetic confounding [Reference Rice, Langley, Woodford, Davey Smith and Thapar267–Reference Havdahl, Wootton, Leppert, Riglin, Ask and Tesli269]. Hence, we need more original studies that integrate results from different methodological approaches [Reference Lawlor, Tilling and Davey Smith270], allowing for causal inference.
Long-term prognosis of ADHD
The included reviews indicate a rather bleak prognosis over months and years of follow-up for young patients with ADHD in terms of criminal behavior [Reference Baggio, Fructuoso, Guimaraes, Fois, Golay and Heller75, Reference Mohr-Jensen and Steinhausen76], school dropout [Reference Christiansen, Labriola, Kirkeskov and Lund61, Reference Erskine, Norman, Ferrari, Chan, Copeland and Whiteford62], vocational challenges [Reference Christiansen, Labriola, Kirkeskov and Lund61, Reference Erskine, Norman, Ferrari, Chan, Copeland and Whiteford62], injuries [Reference Amiri, Sadeghi-Bazargani, Nazari, Ranjbar and Abdi68–Reference Ruiz-Goikoetxea, Cortese, Aznarez-Sanado, Magallon, Zallo and Luis70], comorbidities [Reference Hollingdale, Woodhouse, Young, Fridman and Mandy80, Reference Schiweck, Arteaga-Henriquez, Aichholzer, Thanarajah, Vargas-Caceres and Matura83, Reference Nazar, Bernardes, Peachey, Sergeant, Mattos and Treasure84], and welfare dependency [Reference Christiansen, Labriola, Kirkeskov and Lund61]. However, ADHD is correlated with a whole range of “risk factors” which themselves may be causally linked to challenging life trajectories. Studies of prognosis in ADHD may thus be confounded, and overestimate the negative prognosis of ADHD.
Since ADHD is a very heterogeneous condition, studies focusing more on predictors for different prognostic trajectories would be more informative and fruitful both for increasing our understanding of underlying mechanisms, targeting treatment, and preventing negative outcomes. Future systematic reviews should specifically address issues of publication bias, confounding, and residual confounding, and aim to highlight studies allowing for causal inference.
Pharmacological treatment of ADHD
There is strong evidence for ADHD symptom reduction during weeks or even months of stimulant use [Reference Cerrillo-Urbina, Garcia-Hermoso, Pardo-Guijarro, Sanchez-Lopez, Santos-Gomez and Martinez-Vizcaino113, Reference Cortese, Adamo, Del Giovane, Mohr-Jensen, Hayes and Carucci115, Reference Stuhec, Munda, Svab and Locatelli121, Reference Stuhec, Lukic and Locatelli131] and also some evidence for nonstimulant medication [Reference Stuhec, Munda, Svab and Locatelli121, Reference Cunill, Castells, Tobias and Capellà137] in both children, adolescents, and adults. There is, however, an interesting controversy on how solid the trial evidence on ADHD medication efficacy is. Several reviews on both children and adults, and for both stimulant and nonstimulant pharmacotherapy, conclude with caution as to conclusions on efficacy [Reference Storebø, Ramstad, Krogh, Nilausen, Skoog and Holmskov123, Reference Punja, Shamseer, Hartling, Urichuk, Vandermeer and Nikles124, Reference Maneeton, Maneeton, Intaprasert and Woottiluk128, Reference Matsui, Matsunaga, Matsuda, Kishi and Iwata130, Reference Cândido, Menezes de Padua, Golder and Junqueira135, Reference Elliott, Johnston, Husereau, Kelly, Eagles and Charach139, Reference Thomson, Maltezos, Paliokosta and Xenitidis157, Reference Thomson, Maltezos, Paliokosta and Xenitidis158].
The follow-up time in trials is generally in terms of weeks, and the focus has mainly been on symptom scales rather than real-life outcomes [Reference Cerrillo-Urbina, Garcia-Hermoso, Pardo-Guijarro, Sanchez-Lopez, Santos-Gomez and Martinez-Vizcaino113, Reference Cortese, Adamo, Del Giovane, Mohr-Jensen, Hayes and Carucci115]. We need systematic review evidence on the efficacy of ADHD pharmacotherapy to mitigate the negative prognosis in ADHD. Can we prevent criminal behavior, school dropout, poor academic performance, vocational challenges, accidents, suicide, comorbidities, and welfare dependency, during years of follow-up, with pharmacological therapies? This is perhaps the most pressing question in the ADHD community, and it is yet to be answered. It should be noted that RCTs due to ethical and practical reasons, and conventional epidemiological studies due to the issue of residual confounding, have not been able to address this evidence gap [Reference Mykletun, Widding-Havneraas, Chaulagain, Lyhmann, Bjelland and Halmøy260]. We need evidence for efficacy based on large-scale, population-based studies with research designs allowing for causal inference as to the efficacy of ADHD medication in mitigating the rather bleak life trajectory in ADHD. These studies need to reach beyond symptom relief over weeks follow-up, but rather address outcomes regarding life-trajectories with over several years follow-up as to criminal behavior [Reference Baggio, Fructuoso, Guimaraes, Fois, Golay and Heller75, Reference Mohr-Jensen and Steinhausen76], school dropout [Reference Christiansen, Labriola, Kirkeskov and Lund61, Reference Erskine, Norman, Ferrari, Chan, Copeland and Whiteford62], vocational challenges [Reference Christiansen, Labriola, Kirkeskov and Lund61, Reference Erskine, Norman, Ferrari, Chan, Copeland and Whiteford62], injuries [Reference Amiri, Sadeghi-Bazargani, Nazari, Ranjbar and Abdi68–Reference Ruiz-Goikoetxea, Cortese, Aznarez-Sanado, Magallon, Zallo and Luis70], comorbidities [Reference Hollingdale, Woodhouse, Young, Fridman and Mandy80, Reference Schiweck, Arteaga-Henriquez, Aichholzer, Thanarajah, Vargas-Caceres and Matura83, Reference Nazar, Bernardes, Peachey, Sergeant, Mattos and Treasure84], and welfare dependency [Reference Christiansen, Labriola, Kirkeskov and Lund61, Reference Li, Chang, Sun, Jangmo, Zhang and Andersson271].
Nonpharmacological treatment of ADHD
The evidence on the efficacy of nonpharmacological treatment for ADHD is more mixed than that of pharmacotherapies. Mixed evidence exists for almost all type of nonpharmacological interventions: behavioral intervention for children and adolescents [Reference Daley, van der Oord, Ferrin, Danckaerts, Doepfner and Cortese175, Reference Moore, Russell, Matthews, Ford, Rogers and Ukoumunne177, Reference Sonuga-Barke, Brandeis, Cortese, Daley, Ferrin and Holtmann193] and for adults [Reference López-Pinar, Martínez-Sanchís, Carbonell-Vayá, Fenollar-Cortés and Sánchez-Meca213, Reference Lopez, Torrente, Ciapponi, Lischinsky, Cetkovich-Bakmas and Rojas214], parental training [Reference Daley, van der Oord, Ferrin, Danckaerts, Doepfner and Cortese175, Reference Rimestad, Lambek, Zacher Christiansen and Hougaard176, Reference Zwi, Jones, Thorgaard, York and Dennis204, Reference Montoya, Colom and Ferrin205], dietary interventions [Reference Händel, Rohde, Rimestad, Bandak, Birkefoss and Tendal194–Reference Gan, Galer, Chen and Xiong201], mindfulness [Reference Barranco-Ruiz, Etxabe, Ramirez-Velez and Villa-Gonzalez184, Reference Oliva, Malandrone, di Girolamo, Mirabella, Colombi and Carletto187–Reference Zhang, Diaz-Roman and Cortese189], and other interventions. One reason may be that these interventions are more complex both to deliver and study, for example, therapies being less standardized than drugs, and also that it is more difficult to design blinded and “placebo”-controlled conditions for these more complex intervention. Despite this mixed evidence, ADHD treatments beyond pharmacotherapies are commonly administered and recommended in clinical guidelines [Reference Razzak, Ghader, Qureshi, Zafar, Shaijan and Al Kuwari7].
Conclusion
In this meta-review, we found a large number of reviews that have reasonably well elucidated the evidence for different topics on ADHD. However, when summarizing the findings from the included reviews we still see some important knowledge gaps, for example on prevalence and risk factors. The most pressing knowledge gap is probably that of the efficacy of current ADHD treatments in mitigating the rather bleak life trajectory in ADHD. Hence, future systematic reviews and meta-analyses should address the identified knowledge gaps related to ADHD. To some extent, the lack of systematic review and meta-analysis evidence reflects lack of relevant original studies on ADHD.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1192/j.eurpsy.2023.2451.
Author contribution
Conceptualization: A.C., A.M., I.L., A.H., I.B., and T.W.-H.; Protocol: A.C. and A.M.; Search strategy: A.C. in consultation with A.M., I.L., T.W.-H., A.H., and I.B.; Screening: A.C., I.L., O.N. (for screening of abstract and full text published on 2021), and A.M.; Quality assessment: A.C. and I.L.; Data extraction: A.C., I.L., and O.N. (for articles published in 2021); Writing – original draft preparation: A.C. and I.L.; Writing – review and editing: A.H., A.M., I.B., T.W.-H., and O.N.; Supervision: A.M., A.H., and I.B.
Financial support
This work was supported by the Research Council of Norway (RCN) under the program FRIMEDBIO (project number 288585). The funders have not been involved in the creation or carrying out of the study.
Competing interest
The authors declare no conflicts of interest.
















Comments
No Comments have been published for this article.