Depression is a major contributor to the global disease burden(Reference Herrman, Kieling and McGorry1,Reference Liu, He and Yang2) . With an estimated worldwide prevalence of 31·53 million cases (95 % CI: 27·2, 36·5), depression impacts physical and mental well-being, leading to socio-economic consequences, reduced quality of life and an increased burden on healthcare systems(Reference Santomauro, Mantilla Herrera and Shadid3). As approximately 70 % of adult mental health disorders, including depression, emerge in childhood or early adolescence(4), the public health impact of adolescent-onset depression may be even greater, as depression is both an independent source of impairment as well as a risk factor for other chronic health conditions later in life (e.g. obesity, type two diabetes mellitus, ischaemic heart disease)(Reference Copeland, Alaie and Jonsson5–Reference Hulvershorn7). Despite advances in treatments, many children and adolescents do not respond to first-line depression treatment(s), and treatment resistance occurs in 30–40 % of cases(Reference Zhou, Michael and Liu8). As highlighted by the 2018 Lancet Commission on Psychiatry and the 2021 State of the World’s Children Report, there is an urgent need for innovative therapeutic interventions for children and adolescents with depression(Reference Holmes, Ghaderi and Harmer9,Reference Keeley10) .
Diet modification offers promise in improving both mental and physical health. Lifestyle factors such as healthy eating have been shown to play a pivotal role in the treatment and prevention of numerous non-communicable chronic diseases, and their impact on mental health is being increasingly recognised(Reference Di Daniele11,Reference Firth, Solmi and Wootton12) . Some of the physiological pathways hypothesised to be affected by diet in relation to depression include oxidative stress(Reference Liu, Zhong and Liao13), inflammation(Reference Galecki and Talarowska14,Reference Colasanto, Madigan and Korczak15) , mitochondrial function(Reference Allen, Caruncho and Kalynchuk16), the gut microbiome (via the gut–brain axis)(Reference Capuco, Urits and Hasoon17) and disruptions of tryptophan–kynurenine metabolism(Reference Pu, Liu and Zhang18). Research in adults has demonstrated an association between greater adherence to healthy diets, such as the Mediterranean diet and reduced depressive symptoms(Reference Lassale, Batty and Baghdadli19–Reference Psaltopoulou, Sergentanis and Panagiotakos22). In addition, greater adherence to healthy dietary patterns is associated with lower depressive symptoms among both clinical and community-based samples of adolescents(Reference Altun, Brown and Szoeke21,Reference Orlando, Savel and Madigan23,Reference Campisi, Zasowski and Shah24) and adults(Reference Altun, Brown and Szoeke21,Reference Swainson, Reeson and Malik25,Reference Gianfredi, Dinu and Nucci26) . Moreover, among adolescents with major depressive disorder (MDD), lower dietary quality is associated with higher depressive symptoms(Reference Korczak, Perruzza and Chandrapalan27).
As adolescence is a developmental period during which dietary habits become entrenched(Reference Birch and Fisher28), and as unhealthy dietary patterns track between adolescence and adulthood(Reference Chong29,Reference Appannah, Murray and Trapp30) , the period of adolescence represents a potential window of opportunity for intervention to improve eating behaviours lifelong. However, factors that influence adolescent food choices are complex and are also nested in family factors, such that interventions targeting adolescent dietary patterns must also be multifaceted. Thus, nutrition interventions should encompass the adolescent food environment and include elements that address nutrition literacy(Reference Yurtdas Depboylu, Kaner and Suer31), family eating habits and practices(Reference Hendrie, Sohonpal and Lange32,Reference Fleary and Ettienne33) , peer influences(Reference Contento, Williams and Michela34), school environments(Reference Wordell, Daratha and Mandal35) as well as broader cultural and societal norms(Reference Stok, Renner and Clarys36,Reference Neufeld, Andrade and Ballonoff Suleiman37) .
The overarching objective of this study was to assess the feasibility of a novel personalised nutrition intervention for adolescents with MDD using a mixed-methods study design. The secondary objective was to gather preliminary data to inform a larger, appropriately powered study to test the personalised nutrition intervention’s effectiveness in improving dietary intake and depressive symptoms.
Methods
Study design
This 8-week open, single-arm study assessed the feasibility of implementing a personalised nutrition intervention, based on the principles of a Mediterranean diet, among adolescents with MDD. The study combined quantitative and qualitative data to more completely evaluate the intervention and the study protocol(Reference Molina-Azorin38).
This study was approved by The Hospital for Sick Children Research Ethics Board #1000079300 on March 20, 2022. Recruitment and intervention delivery occurred from May 2022 to July 2023. All adolescents and their guardians provided written informed consent. The study was registered at the US National Institutes of Health (ClinicalTrial.gov) #NCT06175052.
Participants
The study recruited ten adolescent–parent dyads. Adolescents were recruited from the Children’s Integrated Mood and Body (CLIMB) depression program at The Hospital for Sick Children (SickKids), a tertiary care children’s hospital in Toronto, Canada. The CLIMB programme receives referrals from primary care clinicians in the community via the centralised intake programme in the outpatient psychiatry department at SickKids. Adolescents referred to the CLIMB programme undergo semi-structured diagnostic interviews by a trained staff member using the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS)(Reference Nishiyama, Sumi and Watanabe39), and psychiatric diagnoses, including MDD, are confirmed following a review with a child and adolescent psychiatrist. Dyads who expressed interest in participating in the current study provided their consent and completed screening questionnaires to determine their eligibility. Participants received usual clinical care during the study. Details regarding the CLIMB programme have been published elsewhere(Reference Korczak, Perruzza and Chandrapalan40).
Study inclusion criteria were the following: adolescent age 11–17 years; diagnosis of MDD as defined by the Diagnostic and Statistical Manual for Mental Disorders 5th edition (DSM-5)(Reference Vahia41); access to the internet and a computer or smartphone and a parent willing to participate. Adolescents were excluded if they already adhered to a high-quality diet (i.e. a score ≥ 8 on the KIDMED(Reference Altavilla and Caballero-Perez42)); met the criteria for an eating disorder based on the K-SADS(Reference Nishiyama, Sumi and Watanabe39) semi-structured interview; were currently participating in other dietary programs or studies or were actively attempting to increase or decrease body weight; had a chronic medical condition not including MDD or had an unstable psychiatric disorder (e.g. manic symptoms, active suicidal ideation). Parents and/or legal guardians of adolescents were eligible to participate if they lived with the adolescent at least half of the time and had access to the internet and a computer or smartphone.
Sample size
This study, designed to assess the feasibility of a dietary intervention for adolescents with depression, employed a sample of ten participants. Other feasibility studies conducted among adolescents with depression use similar sample sizes ranging from 10 to 13 participants(Reference Dopp, Mooney and Armitage43–Reference Cotter, Hornack and Fotang45). This feasibility stage focussed on gathering initial data regarding participant recruitment, intervention adherence and data collection procedures. A sample size of ten enabled evaluation of these aspects and will inform the design of a future pilot study with a more robust sample size capable of yielding statistically significant results.
Study procedure
Following enrollment, adolescents and parents completed independent pre-intervention interviews. Adolescents completed baseline questionnaires about depression symptoms, dietary intake, nutrition knowledge and attitudes, parental food modelling as well as perceived intervention acceptability and feasibility. Parents completed baseline questionnaires about the family food environment and perceived intervention acceptability and feasibility.
Following the assessment, participants began the 8-week personalised nutrition intervention. Nutrition counselling session feedback was obtained via a questionnaire after each session. In Week 5 and Week 9 (1 week post-intervention), adolescent self-report and parent-report measures were repeated. Adolescents and parents completed independent post-intervention interviews following the completion of the intervention to elicit further feedback about their experiences. The study procedure is further detailed in Fig. 1.

Fig. 1. Schedule of study assessments and activities.
Personalised nutrition intervention
The personalised nutrition intervention included four virtual nutrition counselling sessions, provided every 2 weeks for 8 weeks. The nutrition counselling sessions followed a structured five-step approach known as the 5A’s (Assess, Advise, Agree, Assist and Arrange)(Reference Sherson, Yakes Jimenez and Katalanos46). At each nutrition counselling session, adolescent–parent dyads and the nutrition counsellor collaboratively set one nutrition goal (i.e. eating two or more servings of fruit per day) and one eating behaviour goal (i.e. eating breakfast regularly). A 1-week personalised menu plan was then co-created based on the principles of the Mediterranean diet (i.e. higher intake of fruit, vegetables, grains, legumes and lean protein and lower intake of red meats and processed foods) for use over the following 2 weeks, using ‘That Clean Life’ menu planning software(47). Participants also received weekly grocery deliveries containing food items from the menu plan and weekly eHealth messages by email. The eHealth messages were tailored to reinforce the principles of a high-quality diet. An additional grocery delivery was sent to each dyad after completing the post-intervention assessments.
Quantitative measures
Quantitative measures were administered using REDCap(Reference Harris, Taylor and Minor48), a secure web-based platform for data collection, with the exception of the 24-h dietary record, which was accessed via the secure website link(Reference Conway, Ingwersen and Vinyard49).
Adolescent depression
Depression symptoms were assessed using the Centre for Epidemiological Studies Depression Scale for Children (CES-DC), a validated twenty-item self-report measure(Reference Radloff50). Participants rated their symptoms on a four-point Likert scale, with higher scores indicating more severe depression symptoms and a maximum total score of 60.
Dietary assessment
Adolescents’ adherence to the personalised nutrition intervention was assessed using the KIDMED questionnaire, a sixteen-item self- or interviewer-administered measure(Reference Altavilla and Caballero-Perez42). Items related to lower adherence are assigned a value of –1 (4 items), and those related to higher adherence are assigned a value of +1 (12 items). Scores range from 0 to 12. A total score of ≤ 3 indicates poor adherence; a score of 4–7 indicates moderate adherence and a score of ≥ 8 indicates high adherence.
The intended method for assessing diet quality was the Healthy Eating Index-2015 (HEI-2015).(Reference Krebs-Smith, Pannucci and Subar51) Two 24-hour dietary recalls at each time point were obtained using the National Cancer Institute’s Automated Self-Administered 24-hour (ASA-24) Dietary Assessment, a validated instrument that employs the Automated Multiple-Pass Method(Reference Conway, Ingwersen and Vinyard49). The ASA24 instrument has been shown to be a valid measure of dietary quality that is sensitive to change in studies of short-term (4–8 weeks) dietary interventions in adolescents and adults.(Reference Hall52–Reference Chiu, Siri-Tarino and Bergeron56) The HEI-2015 is a scoring system based on density rather than quantity, with values ranging from 0 to 100. Total scores over 80 are categorised as good quality diets, those with scores 51–79 as needing improvement and those with scores ≤ 50 as being of poor quality. The HEI-2015 includes thirteen subgroups categorised into adequacy components and moderation components. Adequacy components are foods and nutrients that are encouraged, and higher scores reflect higher intakes. Moderation components are reverse-scored since they include foods and nutrients with recommended limits. Higher moderation scores reflect lower intakes since lower intakes better align with recommendations.
Anthropometry
Pre-intervention BMI data were obtained from standardised assessments during clinic visits, as detailed elsewhere(Reference Korczak, Perruzza and Chandrapalan40). To evaluate the feasibility of including body weight data within the study protocol, participants were instructed to weigh themselves while wearing light clothing with a parent present, using their home scales. Self-reported weights were measured in duplicate and submitted electronically via REDCap.
Nutrition attitudes and knowledge
We assessed nutrition attitudes and knowledge using a Nutrition Attitudes and Knowledge (NAK) questionnaire, designed to evaluate children’s understanding of the 2019 Canada Food Guide(Reference Franco-Arellano, Brown and Froome57). The questionnaire consists of four five-point Likert scale questions to measure positive attitudes about nutrition, as well as twenty multiple-choice and true/false questions related to Canada Food Guide food groups (drinks, whole grain foods, vegetables and fruit and protein foods).
Parent food modelling
Parental food modelling was evaluated with five items adapted from Cullen’s scale, each rated on a four-point Likert scale from ‘Never’ to ‘Always,’ which has been used to predict diet outcomes in adolescent samples(Reference Loth, MacLehose and Larson58).
Family food environment
Parents used the Family and Nutrition and Physical Activity questionnaire, a twenty-item tool on a four-point Likert scale, to assess their family’s food environment and practices. Higher scores indicate healthier home food environments(Reference Ihmels, Welk and Eisenmann59).
Acceptability and feasibility of intervention measures (AIM and FIM)(Reference Weiner, Lewis and Stanick60)
The AIM and FIM are self-reported four-item measures that are scored using a five-point Likert scale ranging from ‘completely disagree’ (1) to ‘completely agree’ (5) with higher scores indicating greater acceptability or feasibility. While there are no established cut-off scores for interpreting AIM and FIM data, most research evaluates the intervention’s reception by examining mean scores(Reference Mettert, Lewis and Dorsey61). The maximum score achievable for each measure is 20. Higher mean scores for each item (closer to 5) indicate greater acceptability and feasibility.
Satisfaction with the menu planning and nutrition counselling was rated by parents and adolescents independently following each counselling session using a five-point Likert scale ranging from ‘very dissatisfied’ (1) to ‘very satisfied’ (5).
Qualitative interviews
Semi-structured interviews were conducted using an interview guide and recorded virtually by members of the research team. The pre-intervention interview probed reasons for study participation and potential concerns, perceptions of the personalised nutrition intervention, participants’ expectations of the study and motivation for enrollment. The post-intervention interview asked participants about their perspectives of the intervention as a whole and its individual components, including the nutrition counselling sessions, grocery delivery and eHealth messages. Participants were also asked about the factors that either hindered or facilitated their adherence to the personalised nutrition intervention.
Feasibility measures
Feasibility was evaluated based on the following key focus areas as delineated by Bowen et al. (Reference Bowen, Kreuter and Spring62) – demand, acceptability, implementation, adaptation and limited efficacy testing. Qualitative interviews with youth and parents were integrated to enrich quantitative data for a more robust overall feasibility evaluation(Reference Pearson, Naylor and Ashe63).
1. Demand, the extent to which the intervention would be accessed by adolescents with MDD, was measured quantitatively with the recruited rate. The recruitment rate was defined as the number of dyads enrolled in the study divided by the number of dyads approached to participate in the study. Demand was also assessed through pre-intervention qualitative interviews.
2. Acceptability, the extent to which the intervention was judged to be suitable to dyads, and fit with daily life activities, was assessed using the AIM. Acceptability was also assessed using post-intervention qualitative interviews. Satisfaction with the menu planning and nutrition counselling sessions was assessed using the quantitative satisfaction scores, and through post-intervention qualitative interviews.
3. Implementation, the degree to which the intervention and questionnaires can be delivered as proposed, was assessed using the FIM and by intervention adherence, participant attrition and the completion rate of the quantitative measures. Adolescent dietary adherence was assessed using the KIDMED questionnaire. The attrition rate was defined as the number of dyads who started the intervention divided by the number of dyads who completed the study, expressed as a percentage. The degree of missingness examined the proportion of missing data (i.e. potentially indicative of questionnaire fatigue or objectionable/unclear items).
4. Adaptation, the need to make intervention or procedural changes, was assessed through the qualitative interviews conducted post-intervention.
5. Limited efficacy testing, considered whether the intervention led to changes in depression symptoms at the mid-point and post-intervention. Limited efficacy was also assessed for secondary outcomes, including adolescent nutrition attitudes and practices, parent food modelling and the family food environment.
Data analysis
The analyses were descriptive and exploratory. Quantitative continuous data were reported as means and standard deviations; categorical data were reported as frequencies and proportions. Qualitative data obtained through audio-recorded, semi-structured interviews were transcribed verbatim, de-identified and subject to inductive thematic analysis using NVivo12 software(Reference Bowen, Kreuter and Spring62,Reference Pearson, Naylor and Ashe63) . The thematic analysis procedure followed the guidelines outlined by Braun and Clarke (2006)(Reference Clarke and Braun64). Initially, each member of the research team conducted an independent review of the transcripts to immerse themselves in the data and gain a comprehensive understanding. Subsequently, individual team members created initial codes, which were later collectively compared and synthesised to form overarching themes through collaborative discussions. Upon achieving consensus on the most representative themes and subthemes, excerpts were selected from the transcripts to support and illustrate each theme.
Limited efficacy testing was conducted through reported means, standard deviations (sd), effect sizes (i.e. Cohen’s d) and 95 % CI. As this current study was not powered for hypothesis testing, effect sizes are reported to communicate the size and the direction of the potential treatment effect(Reference Lee, Whitehead and Jacques65). We used the guidelines by Cohen to interpret the magnitude of the effect sizes, namely small (0·2), moderate (0·5) and large (0·8)(Reference Cohen66). All quantitative analyses were performed using R statistical software(67).
Results
Forty-three adolescent–parent dyads were approached to participate in the study, twenty were not interested or did not respond to the invitation and eight did not meet the eligibility criteria. Fifteen dyads consented to participate in the study. Two dyads withdrew prior to commencing the study, and three dyads withdrew after completing one session: one found the diet too challenging and two withdrew for unrelated reasons (Fig. 2). Participating adolescents (n 10) had a mean age of 15·3 (sd = 1·4) years and were 100 % female. Participant characteristics are presented in Table 1. The study was well-tolerated with no adverse events reported.
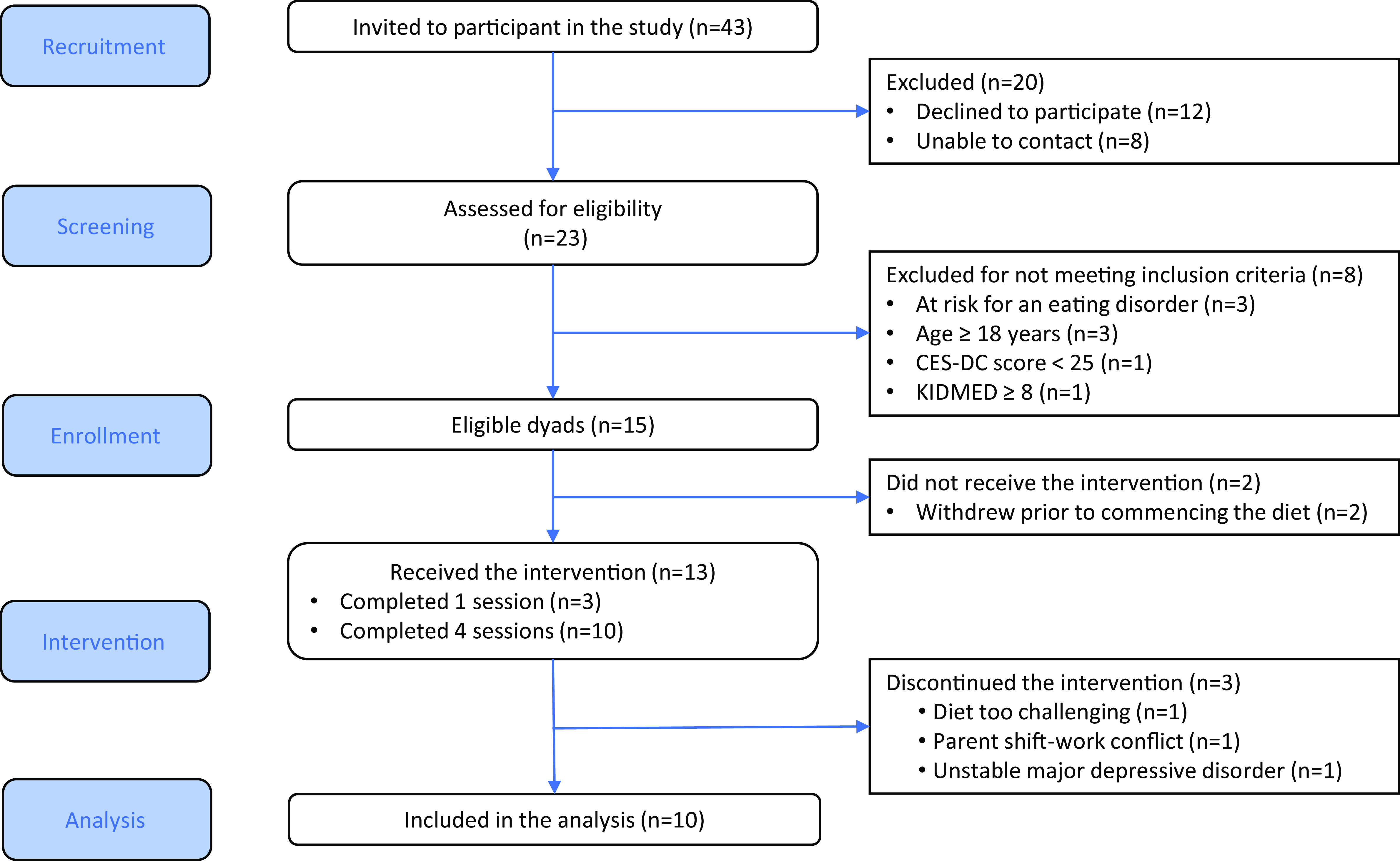
Fig. 2. CONSORT participant flow diagram.
Table 1. Participant characteristics (Mean values and standard deviations; Numbers and percentages)

CAD, Canadian Dollar.
Feasibility outcomes
Demand
Of the forty-three adolescent–parent dyads that were approached to be part of the study, fifteen consented to be part of the study, resulting in a recruitment rate of 40 % (see flow diagram, Fig. 2). Pre-intervention interviews offered further insight into reasons for participating in the study and anticipated barriers to study participation (Table 2).
Table 2. Pre-Intervention qualitative interview themes, mentions and illustrative quotes, n 10

Motivations for study participation were categorised into six subcategories (labelled 1·1–1·6). Firstly (1·1), parents (n 3) and adolescents (n 2) highlighted the perceived benefits for the entire family, emphasising the belief that involvement in the study would bring positive changes not only to adolescents but also to the well-being of the family as a whole. Secondly (1·2), parents (n 1) and adolescents (n 4) expressed hope for potential improvements in their adolescents’ eating habits and considered study participation as a means to positively influence adolescents’ dietary choices. Thirdly, parents (n 3) and adolescents (n 1) reported that another motivating factor for study participation was their desperation to improve depression symptoms (1·3). Parents (n 4) also expressed a strong desire for adolescents to become more self-aware of their eating habits (1·4) and saw the study as an opportunity to foster greater self-awareness and healthier eating choices. Some adolescents (n 3) and parents (n 1) reported more altruistic motives, expressing a desire for their participation to benefit not only themselves but also others who might be in a similar situation in the future (1·5). Lastly, adolescents (n 3) expressed genuine interest in learning about nutrition (1·6), highlighting curiosity and eagerness to expand their knowledge in this area.
Participants also identified anticipated barriers to intervention adherence, which were categorised into two subcategories (labelled 2.1 and 2.2). The first subcategory (2·1), anticipated barriers to intervention adherence, encompassed a range of potential practical difficulties. Parents (n 5) and adolescents (n 4) were concerned about managing fluctuations in the adolescent’s mood, potential distractions from friends, deviation from current eating habits, intervention duration and potential scheduling conflicts with family activities such as summer holidays during the summer months, or extracurricular engagements during the school year. The second subcategory, dietary preferences (2·2), centred only on adolescents’ concerns (n 2) that they may not like the food that comprised the nutrition intervention, due to their self-professed pickiness as eaters.
Acceptability
Acceptability was determined using the AIM and qualitative interviews. All adolescents and their parents completed the AIM questionnaires pre- and post-intervention. The AIM remained relatively consistent for both parents and adolescents pre- and post-intervention (AIM parent mean pre-intervention scores 17·90 (sd = 2·08) v. post-intervention scores 17·90 (sd = 1·91); AIM adolescent mean pre-intervention scores 15·78 (sd = 1·92) v. post-intervention scores 16·0 (sd = 2·11)
Parents expressed a high level of satisfaction with menu planning and nutrition counselling, while adolescents demonstrated an adequate level of satisfaction with menu planning and nutrition counselling (Fig. 3).

Fig. 3. Satisfaction scores for menu planning and nutrition counselling for adolescents and parents, by session (n 10).
Post-intervention interviews were coded into three categories to facilitate the interpretation of acceptability data: (1) perceived benefits of study participation; (2) factors contributing to study satisfaction and (3) challenges to study participation. Table 3 summarises interview categories and representative quotes.
Table 3. Post-Intervention qualitative interview theme, mentions and illustrative quotes, n 10

In the first category, ‘Benefits to Study Participation,’ participants recognised several advantages (labelled 1·1–1·3). Within the first subcategory (1·1), parents (n 4) and adolescents (n 4) recognised that the study provided an opportunity for adolescents to try new foods. This allowed them not only to expand their dietary choices but also to incorporate new food into their eating habits (1·2). Parents (n 5) and adolescents (n 4) expressed the intention to continue eating new food they tried during the intervention emphasising the sustainability of these positive changes beyond the study period. Lastly, one parent and one adolescent mentioned that the study involved family members outside the dyads outside the recruited dyads (1·3).
Within the second category, ‘Factors Influencing Study Satisfaction’, five subcategories emerged, elucidating the factors contributing to study satisfaction (labelled 2.1–2.5). One key aspect highlighted was the flexibility of the intervention’s pace (2·1), as both parents (n 4) and adolescents (n 4) appreciated the ability to adapt the intervention to their individual goals and schedules. Another factor that emerged as contributing to adolescent satisfaction was the wide variety of acceptable foods that were available for them to select (2·2), which was mentioned by parents (n 3) and adolescents (n 3). This increased the feasibility of the intervention for adolescents in that they were able to meet their food goals while still enjoying their meals. Parents (n 1) and adolescents (n 4) also commended the menu planning software for its personalisation features, including grocery lists, menu plans and recipes (2·3). This feature not only simplified meal planning but also added a level of personalisation that resonated with participants, enhancing their overall experience. The virtual nature of the nutrition counselling sessions (2·4) was another factor positively influencing study satisfaction. Both parents (n 4) and adolescents (n 4) found virtual sessions convenient and appreciated the accessibility of nutrition counselling from the comfort of their homes. Lastly, grocery deliveries received positive feedback from adolescents (n 2) and parents (n 3) (2·5). This service streamlined the process of obtaining the novel ingredients for the intervention and allowed adolescents to explore new foods they may have not tried otherwise.
In the third category, ‘Challenges to Study Participation,’ two subcategories emerged and provided insights into the difficulties that participants faced during the study (labelled 3.1–3.2). Parents (n 2) and adolescents (n 2) thought that the time required for meal preparation was too lengthy (3·1). Parents (n 1) and adolescents (n 1) also raised implementation concerns about the intervention timing with respect to the time of year (3·2), suggesting that external factors could impact participation and adherence. Of note, however, participant perspectives regarding the optimal time of year in which to implement a new dietary intervention were discrepant, with some participants suggesting the stress-free summer time would be a better time, while others suggested that the more routinised academic year would allow for easier implementation.
Implementation
Implementation feasibility was determined using the FIM and by intervention adherence, participant attrition and completion rate of measures. Mean FIM scores for both parents and adolescents showed relative stability across pre- and post-intervention assessments. Parents’ scores averaged 15·80 (sd = 2·78) pre-intervention and increased slightly to 16·60 (sd = 2·84) post-intervention. Similarly, adolescents’ scores remained steady at 16·33 (sd = 2·06) pre-intervention and 16·00 (sd = 1·89) post-intervention. Over the 8-week intervention, adherence to the diet, as assessed by the KIDMED, demonstrated improvement with a large effect size between baseline and midpoint (Cohen’s d = 0·80, 95 % CI (0·24, 2·41)), and a moderate effect size between baseline and post-intervention (Cohen’s d = 0·64, 95 % CI (0·15, 1·33))). Of the thirteen dyads that initiated the study, ten completed the study for an attrition rate of 23 %. Reasons for withdrawing from the study included personal (non-intervention) reasons (n 2) and a challenging diet (n 1) (Fig. 2).
While all REDCap measures achieved 100 % completion across all three timepoints, dietary data collection using the ASA-24 had suboptimal completion rates. Six participants completed at least one ASA-24 recall at baseline and mid-point, including two incomplete baseline recalls with implausibly low daily caloric intake (less than 500 kcal). At the final time point, four participants completed a single ASA-24; one participant provided an incomplete recall with a similarly low caloric intake. The low completion rate prevented the assessment of diet quality using the HEI-2015. Completion of the anthropometric data was 90 % at the midpoint and 70 % at the end of the study time point.
Adaptation
Participant feedback from post-intervention interviews highlighted some procedures requiring adaptation. Participants also made several suggestions with respect to adaptation of the intervention for the study team to consider: adolescents (n 6) reported that they either did not receive or did not read the eHealth messages; one adolescent suggested making the menu planning software accessible to both the nutritionist and participants, to allow adolescents to explore recipes at their own pace; two parents expressed interest in having more frequent (weekly instead of biweekly sessions) to discuss study progress; three parents expressed interest in receiving more information about the principles of the Mediterranean Diet during the initial nutrition counselling session and two parents recommended providing paper copies of menu plans and recipes for their convenience.
Limited efficacy testing
Effect sizes for adolescent and parent-reported outcome scores are presented in Table 4. Small effects on depressive symptoms were observed (Cohen’s d = 0·36, 95 % CI (–0·24, 3·36)). We also observed improvements in parent food modelling (Cohen’s d = 0·24, 95 % CI (–0·43, 1·16)), adolescent nutrition attitudes (Cohen’s d = 0·36, 95 % CI (–0·25, 1·33)) and substantial enhancements in the family food environment (Cohen’s d = 0. 61. 95 % CI (–0·04, 2·61)). Adolescents’ nutrition knowledge was average at 74 % (14·8/20) pre-intervention and 78 % (15·5/20) post-intervention, corresponding to a negligible increase (Cohen’s d = 0·11, 95 % CI (–1·10, 0·53)).
Table 4. Effect size (Cohen’s d) for outcome measures at pre-intervention, mid-point and, post-intervention, n 10 (standard deviations and 95 % CI)

CES-DC, Center for Epidemiological Studies Depression Scale for Children; PMQ, Parent Modelling Questionnaire; FNPA, family and nutrition and physical activity; NA, not assessed. ¶pre-intervention v. midpoint; †pre-intervention v. post-intervention.
Discussion
The current study examined the feasibility of implementing a novel 8-week personalised nutrition intervention among adolescents with MDD. We assessed the feasibility of this intervention across various dimensions, encompassing demand, acceptability, implementation and a preliminary exploration of its efficacy.
In this study focusing on a new intervention for adolescents with MDD and their parents, we achieved a recruitment rate of 43 %. This is similar to a dietary intervention study for young adults (mean age of 19·6 years) with depression (40 %)(Reference Francis, Stevenson and Chambers68) and a trial of a dietary intervention for adults with MDD (42 %)(Reference Jacka, O’Neil and Opie69). Among studies of adults, younger age, male gender and lower socio-economic status have been associated with decreased research participation(Reference Gul and Ali70,Reference Patel, Doku and Tennakoon71) . However, fewer studies have examined factors affecting the recruitment of children and adolescents for dietary intervention studies, and we are not aware of any research that has examined correlates of research participation of adolescent and parent dyads in this field. Among the broader literature regarding child and adolescent research recruitment, no significant associations were observed between age or gender; however, higher parental educational level has been associated with higher enrollment rates(Reference Robinson, Adair and Coffey72). Further investigation into factors affecting recruitment, particularly for dietary inventions, is warranted.
The pre-intervention interviews provided insight regarding motivations and barriers to study participation and offered potential areas for adjustment to the recruitment strategy. Parents’ reasons for study participation aligned with some adolescents’ reasons but not always to the same degree. For example, both parents and adolescents were hopeful that the intervention would improve depression symptoms, with parents also sharing their own feelings of desperation with respect to finding an effective treatment for their child’s depression. More adolescents than parents expressed altruistic motivations for participating in the current study, which aligns with adolescent motivation in similar research(Reference Saarijarvi, Wallin and Moons73). Both parents and adolescents foresaw challenges when it came to incorporating a higher-quality diet into their existing lifestyles. These challenges included practical obstacles, such as the perceived time and effort required, as well as managing mood fluctuations related to adolescent depression symptoms. Additionally, maintaining adherence to the personalised nutrition intervention was seen as a potential hurdle, influenced by individual food preferences and dislikes. Finally, juggling busy schedules, including school, extracurricular activities and holiday plans, posed yet another obstacle to successful diet integration. However, only adolescents mentioned selective food preferences as a barrier to intervention adherence. Common reasons for declining research participation in the current study (e.g. parent was too busy, might dislike included foods) also align with those of previous research(Reference van der Wurff, Meyer and de Groot74). This suggests that addressing potential barriers to recruitment proactively, by highlighting the personalised and adaptable nature of the dietary intervention, will be important in future research to improve recruitment rates.
Convergence of quantitative and qualitative data including responses to validated questionnaires and themes generated from parent and adolescent interviews in the current study suggests that a personalised nutrition intervention is acceptable among adolescents with depression and their parents. The high level of intervention acceptability and satisfaction among adolescents and parents indicates that the programme was generally well-received. Furthermore, satisfaction with menu planning and nutrition counselling was generally high, with parents reporting slightly higher satisfaction than adolescents. The attrition rate of 23 % indicates moderate success in retaining participants throughout the intervention and is similar to other adolescent lifestyle interventions (i.e. general nutrition and/or exercise) for depression (15–35 %)(Reference Jelalian, Jandasek and Wolff75–Reference Egilsson, Bjarnason and Njardvik77). Post-intervention interviews revealed that participants recognised several benefits of study participation, such as trying new foods, expanding dietary choices and involving family members in the process. Other factors contributing to the acceptability and satisfaction with the intervention included the flexibility of the dietary components, the availability of acceptable food choices, personalised menu planning software, virtual nutrition counselling and the convenience of grocery deliveries.
The ASA-24, a self-reported dietary intake measure, had the lowest completion rates among the study measures, and many reports contained missing data. To address this issue, more support may be required when completing this measure in future trials. For example, ASA-24 assessments could be conducted during nutrition counselling sessions, or alternate dietary measures could be incorporated to collect self-report data via REDCap or employ novel, specialised, food photography apps to document participants’ dietary intake.
This study offers preliminary evidence for the effectiveness of the intervention in reducing depressive symptoms among adolescents with MDD. Additionally, the improvement in diet quality (KIDMED score) suggests a positive impact on dietary habits. These preliminary results are consistent with the only other study that has conducted a dietary intervention aimed at reducing depression symptoms among young people with depression, which reported lower self-reported depression symptoms using the Center for Epidemiologic Studies Depression Scale – Revised and high dietary compliance(Reference Francis, Stevenson and Chambers68). Furthermore, the current study provides early evidence for improvements in parent food modelling, adolescent nutrition attitudes and the family food environment. The negligible change in adolescent nutrition knowledge may be attributed to the already reasonably high levels of participants’ nutrition knowledge at baseline and the lack of formal nutrition education during counselling sessions, which may have limited the potential for significant improvement.
Limitations
The interpretation of the efficacy of the intervention should be considered with caution, as the focus of this study was on feasibility. Moreover, the absence of a control group makes it challenging to determine whether the observed positive impacts can be attributed solely to the intervention or if other variables (i.e. the non-specific benefits of meeting regularly with an interested health professional) also contributed to the improvements in depressive symptoms observed. Additionally, as the study period was reasonably brief (8 weeks), whether the dietary and depression changes found in the current study will be sustained in the longer term is not known(Reference Black, Prentice and Goldberg78–Reference Podsakoff, MacKenzie and Podsakoff80). Data regarding the effect of the intervention on dietary intake should be interpreted with caution, given both the small initial sample size and the incomplete data, as three participants did not complete the intervention. It is also possible that social desirability bias may have affected self-reported dietary intake and health outcomes; however, adolescents with MDD have been shown to report their energy intake at levels comparable to that of other adolescents, indicating similar potential effects of social desirability bias on dietary intake reporting irrespective of MDD diagnosis(Reference Campisi, Cost and Korczak81–Reference Brener, Billy and Grady83). The potential effect of medication on appetite was not explicitly explored in this pilot study. Future trials with larger sample sizes should address this limitation by including an examination of the intervention’s potential effects on underlying appetite trends. This examination should consider the potential influence of disease course and medications used in clinical care. Finally, FIM and AIM scores reflect data from participants who completed the entire study. Three participants who withdrew after the first session are therefore excluded from the presented FIM and AIM results.
Conclusions
This study found that a newly developed personalised nutrition intervention was feasible according to the criteria outlined by Bowen et al. (Reference Bowen, Kreuter and Spring62) for adolescents with MDD and their families. Future studies should consider recruitment strategies that mitigate hesitation about dietary change by proactively highlighting the flexible and customisable nature of the personalised nutrition intervention that accommodates individual food preferences. These insights lay the groundwork for future research examining the potential effectiveness of family-based, personalised nutrition interventions in the treatment of adolescent depression.
Acknowledgements
The authors would like to acknowledge the participants of the study. We would also like to acknowledge Jacky Au, Logan Wilkinson and Hemantika Mahesh, who were part of the initial protocol development and assisted with data collection. We would like to thank Enid Selkirk for her qualitative analysis input and Isabella Rodrigues who assisted with the thematic qualitative analysis.
This work was supported by the Psychiatry Endowment Fund Seed Grant by SickKids’ Hospital Psychiatry Association and the Garry Hurvitz Centre for Brain & Mental Health. Susan C Campisi was supported in part by the SickKids RestraComp Postdoctoral Award and by the Canadian Institutes of Health Research (CIHR) grant number 409 491. The funders had no role in the study.
S. C. C. conceptualised the study design, secured funding for the study, conducted the nutrition counselling sessions, developed the analysis plan, conducted the analysis and wrote and revised all drafts of the manuscript. M. L. conducted the qualitative analysis and reviewed the manuscript. S. J. A. and E. D. conceptualised the study design, developed the analysis plan, conducted the analysis and revised all drafts of the manuscript. D. J. K. conceptualised the study design, secured funding for the study, developed the analysis plan, revised all drafts of the manuscript and provided senior supervision for all aspects of the study. All authors assisted in data interpretation and critically reviewed and approved the final manuscript.
There are no conflicts of interest.










