Highlights
-
• The accelerated aging hypothesis states individuals with a history of traumatic brain injury (TBI) experience greater functional deterioration with age.
-
• Supporting this hypothesis, history of TBI was associated with slower saccade latency.
-
• In contrast, vision and motor skills were not significantly different between older individuals with and without a history of TBI.
Introduction
The annual incidence of traumatic brain injury (TBI) is approximately 1 in 200 for young and middle aged adults (25–64 years), with the number increasing to 1 in 50 for individuals over 75.Reference Taylor, Bell, Breiding and Xu1,Reference Wilson, Stewart and Dams-O’Connor2 Population-based studies indicate that TBI is associated with myria chronic health conditions that develop over months and years following the brain injury.Reference Corrigan and Hammond3–Reference Zaloshnja, Miller, Langlois and Selassie6 Consequently, even a single, relatively mild acute brain injury can lead to lasting impairments in an individual’s sensorimotor, cognitive and emotional function, including difficulties with social and community engagement.Reference Brown, Gordon and Spielman7,Reference Temkin, Corrigan, Dikmen and Machamer8 In the long term, a history of TBI has been linked to a greater risk of developing cognitive impairments and other neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease or frontotemporal dementia.Reference Huang, Lin and Lee9–Reference Perry, Sturm and Peterson11
Historically, TBI has been treated as a discrete event that can be successfully treated in patients to recover all functions; however, accumulating research has led to a recognition that TBI is a chronic neurological condition with potentially lifelong effects on morbidity and mortality.Reference Wilson, Stewart and Dams-O’Connor2,Reference Green12,Reference Nalder, Zabjek and Dawson13 Neuroimaging studies have shown significant cortical changes following a mild TBI (i.e., concussion). For example, the cortical thickness of an individual with a history of a brain injury was comparable to that of a healthy individual who was 10 years older.Reference Cole, Leech and Sharp14,Reference June, Williams and Huang15 This research has led to the proposal that brain injury accelerates the aging process, which is referred to as the accelerating aging hypothesis. The paradigm shift raises important questions about the effects of aging with a history of brain injury on sensorimotor and cognitive function and the corresponding association with social and community engagement. Therefore, the main objective of this study was to characterize the impact of aging with TBI on three domains: visual, visuomotor and executive functions, which together contribute to safe and efficient performance of most purposeful behaviors.
Vision provides a key sensory input for performing most of our daily activities.Reference Lamoureux, Hassell and Keeffe16,Reference Haymes, Johnston and Heyes17 Aging is associated with a reduction in vision, sensorimotor and executive function.Reference Irving, Steinbach, Lillakas, Babu and Hutchings18–Reference Yep, Smorenburg and Riek23 On the other hand, history of TBI, including concussion, is associated with significant impairments on several visual functions, with the largest deficits on tests involving the binocular vision system.Reference Alvarez, Kim, Vicci, Dhar, Biswal and Barrett24–Reference Greenwald, Kapoor and Singh28 Studies have also shown significantly lower performance on oculomotor tests following TBI, for example, longer latency and poorer accuracy for saccadic and smooth pursuit eye movements.Reference Mani, Asper and Khuu29–Reference R.Taylor, Berryhill, Mathew and G.Murray31 Although relatively fewer studies have examined eye-hand coordination, the emerging evidence indicates that young adults with a history of concussion have slower and less accurate responses when performing complex and fine motor tasks.Reference Brown, Dalecki, Hughes, Macpherson and Sergio32,Reference Benassi, Frattini, Garofalo, Bolzani and Pansell33 To date, knowledge of the effects of TBI on visual and visuomotor function in older adults is limited. Given the age-related reduction in sensorimotor and executive function,Reference Irving, Steinbach, Lillakas, Babu and Hutchings18–Reference Yep, Smorenburg and Riek23 it is important to determine whether a history of brain injury exacerbates the normal aging process.
Although various tasks could be used to evaluate visuomotor and executive functions, recording eye movements provides a sensitive assay of neurocognitive deficits following a brain injury.Reference Mani, Asper and Khuu29,Reference Cade and Turnbull34–Reference McDonald, Tayebi, McGeown, Kwon, Holdsworth and Danesh-Meyer36 Specifically, studies have demonstrated poorer performance on the antisaccade task in young adults with a history of concussion or mild TBI.Reference Ting, Schweizer, Topolovec-Vranic and Cusimano37 The antisaccade task requires inhibitory control to suppress a reflexive eye movement toward the target and requires the eyes to move in the opposite direction of the stimulus.Reference Hallett38 Accurate performance of this task relies on an extensive neural network that includes the dorsal lateral prefrontal cortex,Reference Munoz and Everling39 a brain region susceptible to head injury.Reference Munoz and Everling39–Reference Mazaharally, Stojanovski and Trossman41 Young individuals in the acute and chronic stage following a TBI have been shown to exhibit more directional errors (i.e., failure to inhibit) and longer latency for correct antisaccades.Reference Ayala and Heath42 Normal aging is also associated with a greater number of directional errors and longer latency for correct movements;Reference Yep, Smorenburg and Riek23,Reference Lin, Al Ani and Niechwiej-Szwedo43–Reference Peltsch, Hemraj, Garcia and Munoz45 however, antisaccade performance in older adults with a history of TBI has not been examined previously. Therefore, this study investigates whether older adults with a history of TBI exhibit greater deficits on the antisaccade task when compared to their peers who have not sustained a brain injury.
Like antisaccades, aging is associated with poorer performance of various sensorimotor tasks, for example, slower reaching and prolonged grasp execution.Reference Bennett and Castiello46 Older adults have been shown to rely more on vision for movement planning and online control.Reference Coats and Wann47–Reference Weir, Mallat, Leavitt, Roy and Macdonald50 Importantly, efficient performance of daily activities, such as reaching for and grasping a cup of coffee, relies on spatiotemporal eye-hand coordination, which is also altered in older individuals.Reference Rand and Stelmach51 For example, saccades to targets are delayed, and fixations on the target are longer in older compared to younger individuals. The coordination between the ocular and manual systems in older individuals with a history of TBI has not been previously examined. To shed light on this area, the current study examines eye-hand coordination while participants performed a bead threading task – a sequence task consisting of reaching for a bead and placing it precisely on a needle.Reference Niechwiej-Szwedo, Nouredanesh and Tung52 Efficient performance of the bead threading task requires good binocular vision for movement planning and execution.Reference Gonzalez and Niechwiej-Szwedo53–Reference Niechwiej-Szwedo, Colpa and Wong55 We hypothesize that older individuals with a brain injury will perform the bead threading task more slowly, and those with deficits in binocular vision will have longer grasp execution.
The chronic neurological consequences of TBI have been identified as a significant health problem across the lifespan,Reference Dams-O’Connor, Juengst and Bogner56 however, there is no research examining the effects of a brain injury on the visual and visuomotor function of older adults in the chronic phase following the injury. In addition, poor vision or difficulties with visuomotor function may impact a person’s quality of life (QoL), but the association between vision, visuomotor function and QoL of older individuals with a history of TBI has not been examined directly. Thus, the current study sought to explore this area by asking participants with a history of TBI to complete the Quality of Life after Brain Injury (QOLIBRI) instrument (QOLIBRI) and Sydney Psychosocial Reintegration Scale (SPRS). The QOLIBRI scale examines individuals’ perceptions across six domains of their health-related QoL that are commonly impacted by TBI (cognitive abilities, sense of self, daily life independence, social relationships, emotions, physical abilities). The QOLIBRI is a validated tool correlating well with the physical and emotional status of patients and other established measures of health outcomes, such as the Short-Form Health Survey-36 and the Hospital Anxiety and Depression Scale.Reference von Steinbuechel, Wilson and Gibbons57,Reference von Steinbüchel, Wilson and Gibbons58 The SPRS was developed to assess changes in a person’s lifestyle before and after a TBI across three domains of community participation – (1) occupational and leisure activities, (2) interpersonal relationships and (3) independent living skills – which provide a measure of participation restriction following a brain injury.Reference Tate, Simpson, Soo and Lane-Brown59 This tool has good psychometric properties with high reliability, internal consistency and responsiveness.Reference Tate, Simpson, Lane-brown, Soo, De wolf and Whiting60
To summarize, the current study investigates an important gap in knowledge about the effects of brain injury on visual, visuomotor and executive functions and the potential impact on QoL in older adults. Drawing on the results from studies with young individuals with a history of brain injury, we hypothesize that older individuals with a history of brain injury will have poorer binocular vision, longer antisaccade latencies, more directional errors for the antisaccade task and poorer performance on the fine motor skill task.
Methodology
This research was reviewed by the University of Waterloo Research Ethics Board and conforms with the principles and applicable guidelines for the protection of human subjects in biomedical research (ORE #41848). All participants provided written informed consent.
Participants
Participants were community-dwelling older adults (n = 27, mean age 74.6 years, SD 6.8; 14 females) recruited from the University of Waterloo Research and Aging Pool database and from the local community. A brief screening questionnaire was used to ensure the following inclusion criteria were met: ≥ 55 years old, no medical diagnoses of a neurodegenerative disease (e.g., AD, Parkinson’s disease, multiple sclerosis), no history of neurological events (e.g., stroke), no oculomotor or visual conditions (e.g., macular degeneration, glaucoma, strabismus), no neck pain or neck injury. Please see Table 1 for a detailed demographic description of the participants.
Table 1. Participant demographics depicting the mean and standard deviation (SD)
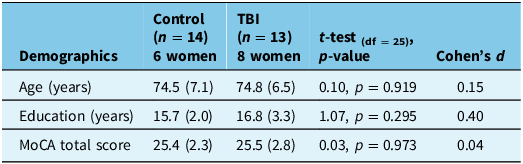
TBI = traumatic brain injury.
Of the 27 participants, 13 reported a history of a TBI resulting from motor vehicle accidents (19%), fall (38%), being struck on the head by an object (15%), sports-related concussion (8%) or other causes (19%) including nearby explosions, fights and childhood play. The history of TBI was determined using the Ohio State University TBI Identification Method (OSU TBI-ID).Reference Corrigan and Bogner61 The OSU TBI-ID consists of a structured interview to ascertain the details about the lifetime history and severity of brain injury. There were four participants who reported experiencing a single TBI event, six participants reported two TBI events, two participants with three TBI events and one who reported five TBI events (due to falling). The scoring system used to categorize the likelihood of TBI exposure is summarized in Table 2.
Table 2. Classification of lifetime history of brain injury according to the OSU TBI-ID interview
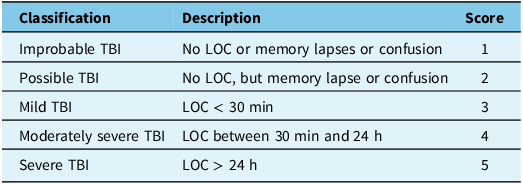
TBI = traumatic brain injury; LOC = loss of consciousness.
All participants completed the Montreal Cognitive Assessment (MoCA, version 7.1). The MoCA test is used to screen for mild cognitive impairment.Reference Koski, Xie and Finch62,Reference Nasreddine, Phillips and Bédirian63 The test consists of individual tasks, requiring verbal and drawn responses, that assess various domains of cognition including visuospatial skills, executive functions, short-term and working memory, attention, language, abstraction and orientation. Across these domains, participants are scored out of 30. Following the MoCA guidelines, scores were also increased by 1 point if the participants had reported less than 12 years of formal education, counting from grade 1 onward.Reference Koski, Xie and Finch62,Reference Nasreddine, Phillips and Bédirian63 A cutoff score of < 26 was used as an indicator of poorer cognitive function. Testing was administered by investigators (JH, AC) who completed an official online Training and Certification module and who followed the standardized instructions found on the official MoCA website (https://mocacognition.com/).
Questionnaires completed by the group with a history of TBI
A secondary aim of the study was to explore the impact of TBI on quality of life using two questionnaires: the Quality of Life after Brain Injury Instrument (QOLIBRI) and Sydney Psychosocial Reintegration Scale (SPRS). These questionnaires were designed specifically for assessing the impact of TBI; therefore, they were not completed by the participants in the control group.
The QOLIBRI was developed to assess health-related QoL after TBI. The self-reported questionnaire consists of 37 questions covering 6 dimensions including the physical, psychological and psychosocial changes that may result after a TBI. Participants’ responses regarding their level of satisfaction or concern in each dimension were encoded from one (not at all satisfied or very bothered) to five (very satisfied or not at all bothered). The total score, calculated by averaging the responses across domains, was converted to a percentage where higher values represent a better QoL (https://qolibrinet.com/scoring/).
The SPRS is a 12-item self-report scale that aims to assess the extent to which a person’s ability to engage in meaningful daily activities may have been impacted by the brain injury. The examined domains include work and leisure, interpersonal relationships and independent living skills. Part A asked the participant to respond based on the degree of change since their injury, and Part B focused on their current status (i.e., after injury). Responses were encoded on a five-point scale with questions of Part A measured from zero (extreme change) to four (no change) and Part B questions measured from zero (extremely poor) to four (very good). The analysis in the current study focused on the current status of Part B because some participants sustained their brain injury a long time ago (i.e., childhood). The total score was converted to a logit score derived from Rasch analysis.Reference Tate, Simpson, Soo and Lane-Brown59 Two participants (OSU TBI-ID score of two) did not complete the SPRS questionnaire.
Vision function assessment
All assessments were conducted in a well-lit room, while the participants wore their habitual prescription lenses. Standard vision tests measuring visual acuity and binocular vision were performed in the order outlined below. For tests that required both monocular and binocular measurements, the right eye (RE) was tested first, followed by the left eye (LE) and then binocularly (BE).
Distance visual acuity (6 M) was measured using the Bailey-Lovie visual acuity chart, and the Lighthouse Continuous Text Card was used to measure visual acuity at near (0.4 M). Acuity was defined as the line where three out of five letters were reported correctly. Contrast sensitivity was measured at 50 cm using the Mars Letter Contrast Sensitivity Test (Mars Perceptrix Corporation, New York, USA) following the publisher’s guidelines.
Near point of convergence (the point where convergence and binocular single vision are no longer maintained) was measured holding a pen starting at a distance of 0.5 M directly in the participant’s midline and moving slowly toward their nose. Distance was measured from the spectacle plant when one eye lost fixation on the target and/or the participant reported diplopia (doubled vision). The test was repeated three times and averaged.
Stereoacuity or depth perception was measured using the Randot® Stereoacuity Test (Stereo Optical Company, Chicago, USA) following the publisher’s guidelines, while the Worth 4 Dot Light Test was performed at a distance of 0.4 M and 6 M in both light and dark conditions to measure suppression. The unilateral and alternating cover test at distance (6 M) and near (0.4 M) determined the presence and amount of ocular deviation (i.e., phoria) and was measured using an accommodative target two lines above best visual acuity with the eyes in primary position.
Horizontal fusional reserves (the ability for the eyes to convergence/positive or divergence/negative based on prism demand) were measured in free space using prism bars, while viewing a 6/12 vertical line at 0.4 M. Base-in (negative) prism was presented first, followed by base-out (positive) prism. Participants were asked to keep the vertical line single as long as possible but report when the line became double (fusion break), while the prism demand increased. If the patient reported double, the prism demand decreased until the patient reported the line was single again (fusion recovery).
Lastly, vergence facility (the ability to turn eyes in/converge or turn eyes out/diverge based on the fusional demand) was performed by asking the participant to view a 20/30 vertical column at 0.4 M. A 12BO/3 BI prism flipper was interchangeably placed in front of the participant as they focused on the vertical column for a period of 1 min. The outcome measure was the number of times the participant reported that the vertical column was clear and single, which was recorded in cycles per minute (cpm). Two participants in the group with a history of TBI did not complete the vergence facility test. A two-sample t-test was used to assess between-group differences.
Visuomotor assessment
Visuomotor control was assessed using two tasks: an oculomotor task and a bead threading task, which were collected in a randomized order. Eye movements were recorded to evaluate inhibitory control using the antisaccade task, which was interleaved with the standard prosaccade task. The EyeLink II eye-tracker (SR Research, Canada) was used to record eye position at a sampling frequency of 250 Hz. Participants were seated with their chin placed in a chinrest in front of a computer monitor 80 cm away. At the start of data collection, a five-point calibration was performed and validated to ensure the error was < 1° for each point in the calibration grid. Stimulus presentation was controlled using the Experiment Builder software (ver. 1.8; SR Research, Canada). Each trial began with a fixation cross (0.25°) presented centrally on a black background for a randomized duration between 2000 and 2750 ms. The fixation stimulus was either green or red, depending on task condition: green = prosaccade; red = antisaccade. Once the fixation cross was extinguished, a peripheral target was displayed (i.e., step paradigm) for 1000 ms. The target was a white circle (0.25°), which was randomly presented 10° to the left or right of fixation along the horizontal axis. Participants were instructed to look at the peripheral target when the fixation cross was green and to move their eyes to the mirror (opposite) location when the fixation cross was red, in both cases without any head movement. There were 60 trials of the prosaccade task and 60 trials for the antisaccades. Due to technical difficulties with eye tracking, results were not recorded for one participant in the control group; thus, 13 participants were included in the control group for the saccade analysis.
Eye position data were analyzed using the eye-tracker’s Data Viewer software (ver 1.8; SR Research, Canada). First, trials were plotted and visually inspected to determine signal quality. Since saccades are conjugate (i.e., both eyes are expected to have the same latency, peak velocity and amplitude), either the left or the right eye was selected for analysis. Selection was based on signal quality. Data were excluded if a blink or loss of tracking occurred within 100 ms of target presentation, which resulted in a rejection of 7.0% of trials in the prosaccade task and 13.0% of trials in the antisaccade task. Thus, the analysis included 1554 trials in the prosaccade task and 1447 trials in the antisaccade task. The criteria used for detecting saccadic eye movements were 30°/s velocity threshold and 8000°/s2 acceleration threshold, as implemented by the Data Viewer software. The analysis focused on two outcome measures: directional errors and latency. For the antisaccade trials, directional errors were defined as movements toward the target, indicating an error in inhibition. For the prosaccades, the directional error was defined as a saccade away from the target. Only the correct trials were included in the latency analysis. Latency was defined as the interval from the time of peripheral target presentation to saccade initiation. A Shapiro–Wilk test was used to confirm that the assumption of normality was satisfied. A two-factor analysis of variance (ANOVA) was used with group (control, TBI) as the between-subject factor and task (prosaccade, antisaccade) as the within-subject factor. The dependent variables were directional errors and latency.
Eye-hand coordination was assessed using a bead threading task (for a detailed description, see Reference Niechwiej-Szwedo, Nouredanesh and Tung52). Briefly, participants were seated in front of an apparatus consisting of a vertical needle placed 15 cm in front of their midline and a bead holder which was placed 20 cm in front of the needle (i.e., 35 cm from the participant). Eye movements were recorded at 250 Hz using the Eyelink II eye-tracker (SR Research, Canada), which was calibrated using the same procedure as described for the oculomotor task. Hand movements were recorded using the Optotrak motion capture system at 250 Hz (Northern Digital, Canada). Two infrared sensors were attached to the lateral aspect of the participant’s index finger (middle phalanx) and the medial aspect of their thumb (distal phalanx), which were placed carefully such that they did not interfere with grasping. Participants practiced the task at least five times prior to data recording. Each trial began with the participant fixating on a dot presented on a computer screen while pinching the needle on the apparatus with their self-reported dominant hand. An auditory tone served as the “Go” signal. Participants were instructed to reach forward, grasp the bead, transport the bead to the needle and place the bead on the needle as fast as possible without dropping the bead. There were 15 trials recorded. Due to technical difficulties, data were not recorded for two participants, one in the control group and one in the TBI group.
Data were analyzed offline using a custom MATLAB script. The eye and hand trajectories were plotted to inspect each trial for missing data due to loss of tracking or signal artifacts. Trials with missing data (2.0%) were excluded from the analysis. The hand position data were filtered using a low-pass second-order Butterworth filter with a cutoff frequency of 10 Hz. The velocity trajectory was then used to obtain all the kinematic metrics. The bead threading task consists of four components: reach to bead, grasp, reach to needle and thread the bead. Reach initiation was defined using a velocity criterion of 30 mm/s, while reach termination was defined when the hand velocity fell below 100 mm/s. Grasping was defined as the interval between the end of the first reaching movement to the initiation of the second reaching movement after the participant picked up the bead. Threading was defined as the interval from the end of the second reaching movement to when the hand moved away from the needle after completing the task. The total time to perform the task was the interval from the “Go” signal to the end of threading. The eye position data were plotted and visually inspected to confirm the eye movements that corresponded with each reaching movement, which were analyzed to assess the temporal eye-hand coordination pattern. Two measures were calculated: the latency difference between the initiation of the eye and hand movement toward the bead and the latency difference when the hand moved toward the needle. A positive value indicates that the hand followed the eyes.
Data were assessed to ensure the assumption of normality was satisfied. Outliers on individual trials were identified as extreme data points that fell outside of the 1st or the 99th percentile of the distribution, and they were excluded from further analysis. A two-sample t-test was used to examine the difference between groups for the performance of the beads task.
Results
As shown in Table 1, participants in the control group and the group with a history of TBI were similar in age and completed education level. There was no significant difference between the groups for the MoCA test scores. Results from the OSU TBI-ID indicated a mean score of 2.6 (SD 1.0, range 2–4). The average time since the most recent injury was 25.7 years (SD 21.6) with a range of 1–61 years (i.e., childhood TBI). Six participants reported memory loss associated with the injury, which was classified as probable TBI. There were three participants who experienced loss of consciousness < 30 min (mild TBI) and four participants who experienced loss of consciousness between 30 min and 24 h, classified as a moderately severe TBI.
Vision assessment results are presented in Table 3. There were no significant differences between groups for most of the measures. The single measure that was significantly different was horizontal phoria, with the control group showing larger esophoria (inward deviation of one eye when fusion is eliminated), while the TBI group had a small magnitude exophoria (outward deviation of one eye when fusion is eliminated).
Table 3. Vision testing results depicting the mean and standard deviation (SD)
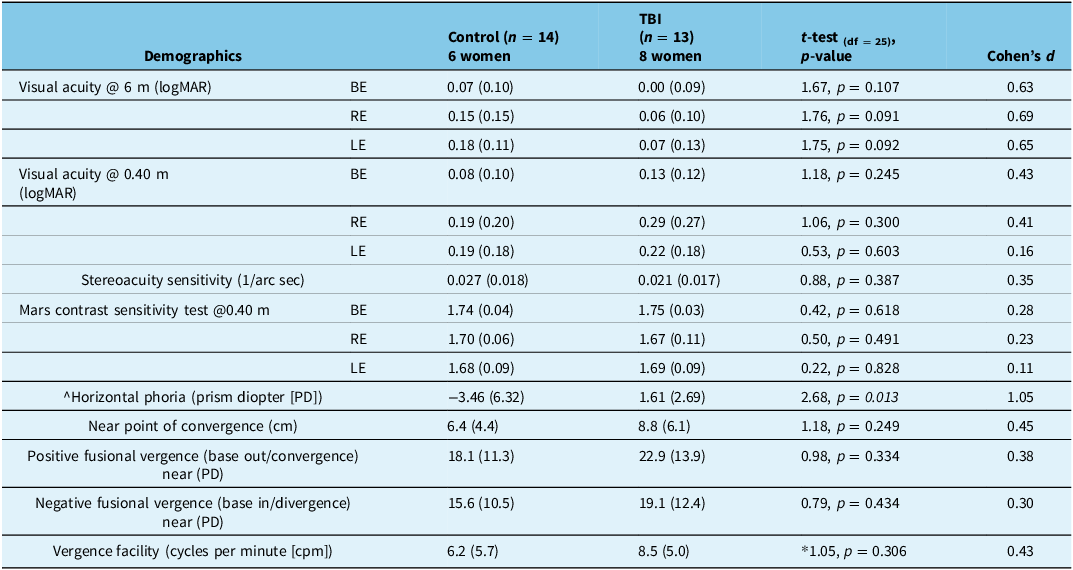
Note: ^negative value indicates esophoria. *degrees of freedom (DF) for vergence test were 23 because two participants were unable to complete this test. TBI = traumatic brain injury; BE = binocular viewing; RE = right eye viewing; LE = left eye viewing; logMAR = logarithm of the Minimum Angle of Resolution.
Results from the oculomotor test are shown in Figure 1. ANOVA analysis confirmed a significant effect of task for directional errors (Figure 1A; F1,24 = 63.08, p < 0.0001; partial eta squared ηp2 = 0.72) and latency (Figure 1B; F1,24 = 105.10, p < 0.0001; ηp2 = 0.80). As expected, there were significantly more directional errors in the antisaccade compared to the prosaccade task (27% vs 2%, respectively). Similarly, the latency for correct trials was longer in the antisaccade compared prosaccade task (382 ± 57 ms vs 282 ± 35 ms, respectively). There was a significant effect of group for latency (F1,24 = 6.03, p = 0.022; ηp2 = 0.20), but not for directional errors (F1,24 = 0.47, p = 0.498, ηp2 = 0.02). The interaction was not significant for latency (F1,24 = 0.70, p = 0.410; ηp2 = 0.03) or errors (F1,24 = 1.19, p = 0.286; ηp2 = 0.05).
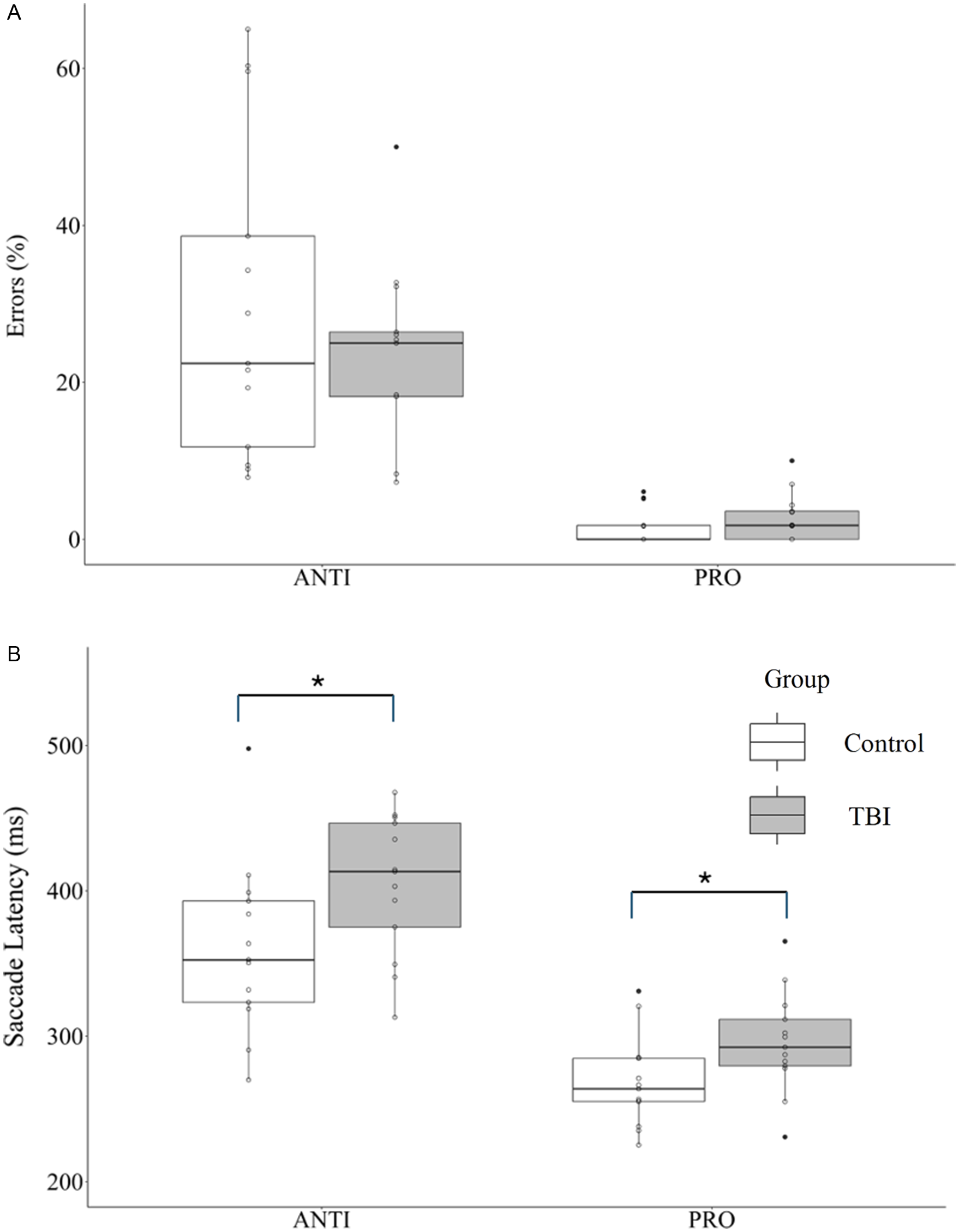
Figure 1. Performance on the prosaccade (PRO) and antisaccade (ANTI) tasks for the control and traumatic brain injury (TBI) groups depicted with boxplots. (A) Mean directional errors. (B) Mean saccade latency for correct responses. Non-filled circles represent individual data points for each participant. Significant main effect of group indicated by * = p < 0.05.
Given that six participants in the TBI group received an OSU score of 2 (i.e., probable TBI), additional analysis was conducted to compare their saccade latencies with the participants who had a more definitive TBI event based on the OSU score > 2. In these six participants with an OSU score of 2, one person reported a single TBI event, two participants reported two TBI events, two participants reported three events and one participant reported five events. Prosaccade latency was similar in the two groups (probable TBI: 301 ± 30 ms; definitive TBI: 291 ± 40 ms). Antisaccade latency was also similar (probable TBI: 422 ± 41 ms; definitive TBI: 389 ± 51 ms). These latencies were significantly different from the control group (prosaccade: 268 ± 31 ms; antisaccade: 360 ± 60 ms). Thus, multiple injuries even without LOC are associated with similar latency deficits when compared to the group with LOC, providing greater confidence that the participants with “probable TBI” suffered a significant TBI event.
The kinematic results from the bead threading task are shown in Table 4. There were no statistical differences between the groups for any of the measures. Similarly, Cohen’s d indicates that the effect size is small.
Table 4. Performance metrics (mean ± standard deviation) for the bead threading task and the corresponding statistical results
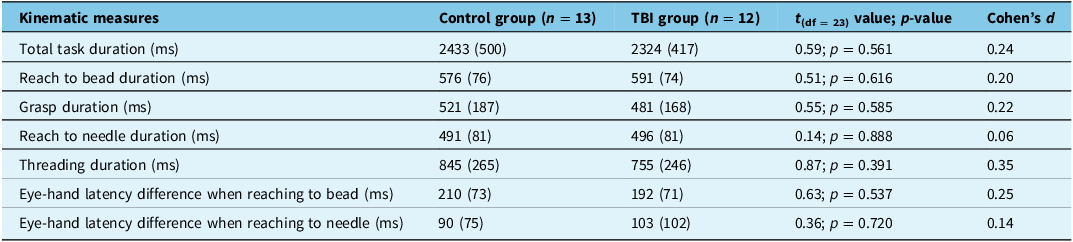
TBI = traumatic brain injury.
Participants in the TBI group completed QOLIBRI and SPRS to assess the potential impact on QoL . The average score for the QOLIBRI was 82.85 (SD 9.74, range 63.51–97.97). The average logit score for the SPRS, part B was 86.80 (SD 16.23, range 49.09–100). There was no significant association between the lifetime exposure to TBI assessed by OSU TBI-ID and scores from the QOLIBRI and SPRS questionnaires.
Given that saccade latency was significantly longer in the TBI group compared to the control group, analysis was performed to investigate the association between saccade latency and QOLIBRI and SPRS scores. After controlling for age, results from the Spearman correlation analysis revealed a significant association between antisaccade latency and SPRS scores (r9 = -0.65 [95% CI: -0.08, -0.89], p = 0.043). Given the small sample size, a Bayesian correlation analysis was conducted using JASP (version 0.18.1). Bayes factor (BF10) was 3.147, indicating a moderate strength of evidence supporting the hypothesis that antisaccade latency and SPRS scores are negatively correlated. The association was also examined in a subanalysis that included participants with a more definitive TBI (i.e., OSU score > 2) where the Spearman coefficient was -0.58. The correlation was -0.41 in the group with a probable TBI (i.e., OSU score = 2). In contrast, no significant association was found for prosaccade latency and SPRS scores (r9 = 0.28, p = 0.436, BF10 = 0.382). Similarly, there were no significant associations between the QOLIBRI scores and saccade latency (r < 0.21; all p > 0.500).
Discussion
The primary goal of this study was to investigate vision, visuomotor and executive function in older adults with a history of brain injury. In contrast to our hypothesis, visual assessment revealed no significant differences between groups, except for horizontal phoria, which was unexpectedly larger in the control group. Conversely, evaluation of visuomotor function using the bead threading task did not demonstrate any reliable between-group differences. In accordance with the hypothesis, the saccade latency was significantly longer in older adults with a history of TBI, consistent with the accelerated aging hypothesis. Moreover, there was a significant association between longer antisaccade latency and a lower measure of participation in meaningful daily activities. These findings indicate that despite comparable visual and sensorimotor function, individuals aging with a history of TBI may experience a greater decline in executive and psychosocial function when compared to their peers without a history of brain injury.
Even a mild brain injury, for example, a concussion, may have long-lasting negative consequences on the person’s cognitive and social function. The previous perspective that a brain injury is a single event with a finite recovery process has changed, and it is now widely accepted that brain injury is a chronic neurological condition.Reference Wilson, Stewart and Dams-O’Connor2,Reference Green12,Reference Nalder, Zabjek and Dawson13 It has been hypothesized that individuals with a history of brain injury may experience “accelerated aging,” which refers to a more rapid biological aging process resulting from the inflammation or other injury-related factors. Support for this comes from neuroimaging studies showing structural and functional changes across brain areas and networks.Reference Amgalan, Maher, Ghosh, Chui, Bogdan and Irimia64,Reference Dennis, Vervoordt and Adamson65 There is a dearth of studies investigating the implications of the accelerated aging hypothesis on functional measures such as vision or visuomotor function. This is an important area to investigate because healthy/normal aging has a significant impact on sensorimotor and executive function.Reference Klein, Klein, Lee, Cruickshanks and Chappell19–Reference Noiret, Vigneron, Diogo, Vandel and Laurent22 If these functions are poorer and deteriorating faster in individuals with a history of TBI, they might be at an increased risk of adverse events such as falling or other accidents.
In contrast to the study hypothesis, results demonstrated that vision function was similar in the TBI and control groups. In fact, both groups had normal corrected distance visual acuity and contrast sensitivity. Near visual acuity was slightly reduced, most likely due to presbyopia, and stereoacuity was also poorer in some participants, likely resulting from interocular acuity difference. Tests of vergence and accommodation revealed age-related reduction, which was similar in both groups. Horizontal phoria was the only measure significantly different between the groups where the control group experienced a greater magnitude of esophoria in comparison to the group with a history of TBI. This result is difficult to interpret considering the other measures of the vergence system (i.e., fusional and facility) showed no significant differences between the groups. Our results may be surprising given the literature that reported significantly poorer vergence and accommodation responses in younger individuals with TBI.Reference Green, Ciuffreda, Thiagarajan, Szymanowicz, Ludlam and Kapoor66 –Reference Thiagarajan, Ciuffreda and Ludlam68 One plausible explanation might be that both accommodation and vergence are reduced with normal aging, and brain injury has no additional effect on these functions. For example, vergence facility was < 9 cpm in both groups in this study, while a normal value for younger adults (< 35 years) is 13.5 cpm, and in symptomatic young adults with a history of TBI, vergence facility is reduced to < 10 cpm.Reference Gall, Wick and Bedell69 In all, age-related reduction in accommodation and vergence function may conceal any effects due to brain injury, which are easier to detect in younger individuals with a higher functioning visual system.
Consistent with the literature,Reference Ting, Schweizer, Topolovec-Vranic and Cusimano37,Reference Ayala and Heath42,Reference Heitger, Jones, Macleod, Snell, Frampton and Anderson70,Reference Mani, Asper, Arunachalam and Khuu71 the oculomotor task revealed differences between groups for saccade latency; however, the effects in this study were evident in both the prosaccade and antisaccade tasks. Given that visual function was similar across both groups, it is unlikely that visual processing contributed to slower information processing. Thus, the problem may arise at later stages that involve sensorimotor transformation or saccade programming.
Saccade latencies for both tasks in the control group in this study are in line with our previousReference Lin, Al Ani and Niechwiej-Szwedo43 work, while the TBI group had a 10% and 12% increase in prosaccade and antisaccade latency, respectively. The lack of interaction, combined with no significant difference in directional errors between the groups, suggests that the effects seen in the TBI group cannot be attributed solely to deficits in inhibitory control. If this were the case, we would expect an increase in directional errors in the antisaccade task. Instead, an increase in latency was found for both prosaccade and antisaccade tasks. These findings could be explained by the experimental approach adopted in the current study, which involved an interleaved design where participants initiated a prosaccade or an antisaccade based on the color of the fixation. This inherently requires task switching based on the fixation cue rather than adopting a singular task set where only one type of saccade (i.e., prosaccade or antisaccade) is required in a single block of trials. For this reason, blocked design is associated with fewer errors and shorter latencies compared to an interleaved design.Reference Ayala and Niechwiej-Szwedo72 The efficiency of inhibitory control relies on working memory,Reference Eenshuistra, Ridderinkhof and van der Molen73–Reference Magnusdottir, Faiola, Harms, Sigurdsson, Ettinger and Haraldsson75 which shows age-related decline.Reference Cohen, Marsiske and Smith76 Presumably, task switching required in the interleaved design places a greater demand on the working memory system, which manifests as longer latencies in both prosaccade and antisaccade tasks. Importantly, the current study demonstrates that the age-related increase in saccade latencies is further compounded by a history of brain injury. These results provide support for the accelerated aging hypothesis while highlighting that the specific effect is evident for tasks that engage the executive system. On the other hand, it is possible that the effects of TBI on the saccadic system arise from the original injury rather than reflecting the accumulating effects of the injury on the aging process. While this hypothesis should be examined in a longitudinal study, the results from the current investigation point toward accelerated aging. This is because longer latencies were evident in both the prosaccade and antisaccade tasks. In contrast, saccade latencies in the acute stage following a concussion or a mild TBI are longer for antisaccades but not the prosaccades.Reference Mani, Asper and Khuu29,Reference Webb, Humphreys and Heath77 Thus, the results from the current study provide preliminary evidence pointing toward the accelerated aging hypothesis.
Aging is associated with poorer sensorimotor control of fine motor skills.Reference Buckles, Stelmach and Hömberg78–Reference Seidler, Bernard and Burutolu81 Conversely, younger adults with a history of brain injury perform worse on tests of visuomotor integration and fine motor skills.Reference Benassi, Frattini, Garofalo, Bolzani and Pansell33 In contrast, the current study found no evidence to support the accelerated aging hypothesis for sensorimotor control during the bead threading task in the TBI group. Both groups performed similarly, notably, their performance was significantly longer when compared to younger adults assessed using the same task.Reference Niechwiej-Szwedo, Nouredanesh and Tung52 For example, the total duration for completing the bead threading task was almost twice as long (i.e., older adults > 2300 ms vs younger adults ∼ 1264 ms).Reference Niechwiej-Szwedo, Nouredanesh and Tung52 Older adults had a twofold increase in grasp duration and took twice as long to place the bead on the needle. Similar to the study by Rand and Stelmach,Reference Rand and Stelmach51 the current study found longer fixations on the bead prior to initiating the reaching movement, however, a history of brain injury had no further deleterious effects on task performance. It is possible that the large effect of age-related decline prevented the detection of brain injury-related effects. Alternatively, it has also been suggested that the effects of brain injury on fine motor skills could be influenced by the severity of the injury.Reference Corrigan, Wee and Collins-Praino82 The current study is not powered to evaluate this hypothesis, thus, the question remains to be explored in future studies.
An important strength of the current study is the inclusion of two questionnaires to assess quality of life and participation in the group with a history of TBI. These results provide insight into the impact of brain injury on the individual’s perception of their well-being across various domains. The intention was to characterize these measures in this cohort and to determine if the measures are associated with saccade latency, which was the only experimental measure that differentiated between the TBI and control groups. Results from the QOLIBRI showed relatively high scores (range 63–98), indicating this cohort did not report a lower quality of life than would be expected based on the reference norms, where a value of 60 is used as a threshold indicating disease-specific impairment in quality of life.Reference Gorbunova, Zeldovich and Voormolen83 There was also no significant association between the QOLIBRI score and saccade latency.
Participation in meaningful activities was assessed using the SPRS, which indicated that most participants reported little to no difficulties in participation in work or leisure, interpersonal relationships or living skills. Only two participants scored less than 36, suggesting some difficulties. It is noteworthy that a significant negative association was found between the participation score and antisaccade latency. Given our small sample size, this outcome must be interpreted with caution and should be examined in future studies. Nonetheless, the results are in line with an emerging body of research showing an association between executive dysfunction and lower community participation in people with chronic TBIReference Juengst, Wright and Vos84 and other neurological conditions.Reference Hughes, Hartoonian and Parmenter85,Reference Thomas, Snethen and Salzer86 Overall, results support the utility of the antisaccade task as a sensitive behavioral assay for the age and injury-related neurocognitive decline.
There are several limitations to this study. First, the sample size was relatively small, which precluded any statistical analysis relating injury severity to outcome measures. Moreover, the group assignment was based on an interview, which may have been influenced by recall bias. The interview was standardized using the OSU TBI-ID classification, which is a well-validated approach widely used to establish a lifetime occurrence of TBI.Reference Corrigan and Bogner61,Reference Corrigan and Bogner87 Second, the cohort was recruited from a database held at the University of Waterloo, which consists of volunteers who have been screened for major neurological conditions, such as stroke or Alzheimer’s disease. There is a high risk of selection bias because the recruited participants were well educated and highly functioning, which may have contributed to the lack of between-group effects. In addition, participants were recruited from an urban setting and had the same access to care. Adverse consequences of brain injury may be seen more distinctly in other geographical locations where access to healthcare resources is not readily available. Therefore, results from this study should not be extrapolated to cohorts that may include participants living in less affluent geographical areas or low educational attainment. Finally, the experimental tests used for assessment of visuomotor control and executive functions provide an important glimpse into these functional domains; however, other tests should be used in the future to provide a more comprehensive depiction of the chronic effects of brain injury in aging individuals.
In conclusion, this study offers the first examination of vision, visuomotor and executive function in older adults with a history of a brain injury. The results indicate that an oculomotor assessment using the interleaved antisaccade task provides a sensitive assay of executive function deficits following TBI in older individuals. Findings are consistent with the accelerated aging hypothesis; however, larger studies are necessary to establish the precise magnitude of the effect and effects of injury severity and to confirm the impact on participation in daily activities. Nonetheless, the results presented here could have important implications for clinical practice by identifying areas important for future research, as well as assessment and monitoring following a TBI.
Author contributions
ENS: Conceptualization, data curation, investigation, writing – original draft, review and editing, resources, project administration, supervision. JH: Writing – review and editing, investigation, visualization. AC: Writing – review and editing, investigation, visualization. LC: Conceptualization, Investigation, writing – review and editing, resources, project administration, supervision.
Funding statement
University of Waterloo Network for Aging Research Seed Grant.
Competing interests
The authors declare no conflict of interest.







